Abstract
The chemical stability of uranyl arsenates of rare-earth elements with the general formula MIII(AsUO6)3⋅16H2O (MIII–La–Lu) in aqueous solutions has been studied in a wide range of acidity. The acid–base ranges of the existence of these compounds in aqueous solutions were established, the transformation products formed outside these ranges were identified, and the solubility of MIII(AsUO6)3⋅16H2O was determined. Based on the experimental data obtained, the solubility products, Gibbs free energies of the formation of the rare-earth elements uranyl arsenates were calculated, the solubility curves of the studied compounds were computed, and the speciation diagrams of uranium(VI), arsenic(V), and rare-earth elements in saturated aqueous solutions and equilibrium solid phases were constructed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This study focuses on rare-earth elements (REE) uranyl arsenates, that belong to a large family of uranium(VI) compounds with a general formula Mk(AsUO6)k⋅nH2O, where Mk represents a wide array of chemical elements in different oxidation states k. A number of mineral species of this family, such as abernathyite КAsUO6⋅4H2O, novacekite Mg(AsUO6)2⋅12H2O, uranospinite Ca(AsUO6)2⋅10H2O, heinrichite Ba(AsUO6)2⋅10H2O, kahlerite Fe(AsUO6)2⋅(10–12)H2O, zeunerite Cu(AsUO6)2⋅(10–16)H2O, lodevite Zn(AsUO6)2⋅10H2O, are found in nature. A range of compounds Mk (AsUO6)k⋅nH2O, where Mk represents chemical elements in oxidation states of + 1, + 2 and + 3 was synthesized in laboratory conditions [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16].
All Mk (AsUO6)k⋅nH2O compounds have a similar layered structure, where negatively charged layers [AsUO6]2∞δ− are comprised of uranium square bipyramids and arsenic tetrahedra [1,2,3, 11,12,13, 16]. Cations Mk+ and H2O molecules occupy the interlayer space, link uranium-arsenic layers and determine their arrangement. Molecular water in the structure of uranyl arsenates serves the role of ligand deficiency compensator for interlayer cations hence its amount is determined by the coordination number of interlayer atoms and the hydration energy of their ions. REE uranyl arsenates contain the largest amount of molecular water in the Mk (AsUO6)k⋅nH2O family of compounds. Similar characteristics of REE allow of the same number of water molecules n = 16 in the composition of all studied compounds MIII(AsUO6)k⋅nH2O (MIII–La–Lu).
Any way of usage of uranium compounds requires data on their chemical stability and speciation in aqueous media. Despite the successes in gas and melt technologies in the nuclear industry, most stages of the nuclear fuel cycle are conducted in aqueous solutions. Research into chemical stability and solubility of uranyl arsenates is crucial to solving many technological and ecological tasks. Despite all of this, there are few scientific papers devoted to the solubility of uranyl arsenates of alkaline [17, 18], alkaline earth [19] and transition elements in aqueous solutions [11,12,13, 19, 20].
REE uranyl arsenates are the least studied among Mk (AsUO6)k⋅nH2O compounds and there are no publications devoted to their solubility and chemical stability in aqueous solutions. At the same time, data on their chemical stability is of scientific and technological importance due to REE predominantly forming during the fission of uranium nuclei and their close contact with uranium species during post-reactor stages of the nuclear fuel cycle [21]. Interaction of uranium compounds with rare-earth elements is also possible in natural conditions and the environment [22, 23].
Therefore this paper presents the results of a comprehensive study of the state of REE uranyl arsenates in water and aqueous solutions of perchloric acid and sodium hydroxide at 25 °C.
Experimental
Synthesis of M III (AsUO 6 ) 3 ⋅16H 2 O (M III –La–Lu) compounds
REE uranyl arsenates were obtained via an ion-exchange reaction between crystalline HAsUO6⋅4H2O and 0.1 mol/l solutions of MIIIAn3 (An–Cl−, NO3−) [14]:
To push the equilibrium point towards the increased formation of the product, the REE salt solution that was in contact with the precipitate was refreshed multiple times. The increase in temperature led to a similar effect. The completeness of the ionic exchange was determined through the measurement of pH of the mother liquor. The synthesis was considered to be complete if the pH value was the same for 0.5–1 h and was equal to the pH value of 0.1 mol/l solution of MIIIAn3. The precipitates were rinsed with distilled water and air-dried.
Uranyl arsenic acid HAsUO6⋅4H2O, that was used in the uranyl arsenates synthesis, was obtained via precipitation [4]:
The temperature conditions of the reaction and the acidity of the mother liquor were under close consideration. Only acidic medium and boiling facilitate sufficiently pure product. The synthesis of HAsUO6⋅4H2O was conducted in a round-bottom flask with a volume of 0.5 l. 100 ml of 0.15 mol/l solution of H3AsO4 was put into the flask and stirred while 60 ml of 0.25 mol/l solution of UO2(NO3)2 was added drop by drop. Then the flask containing the working solution was connected to a reflux condenser and heated on sandbath for three days. The precipitate HAsUO6⋅4H2O was separated from solution via centrifugation, rinsed with distilled water and air-dried at 18–25 °C.
Phase individuality of synthesized compounds and absence of crystalline impurity were established via X-ray powder diffraction. The resulting diffraction patterns were compared to reported data [14]. The mass fraction of H2O was found gravimetrically by calcining the samples at a temperature of 600 °C for five hours. Contents of uranium, arsenic and REE were determined via X-ray fluorescence analysis, using the fundamental parameter method with correction sensitivity coefficients calculated with data from standard samples. The standard samples were prepared by mixing UO3, As2O5 and MIII2O3 in molar ratios of 3:1.5:0.5 and homogenising the mix. The oxides were calcined tentatively to remove excess moisture. The found amounts of the elements in the solid phase have coincided with theoretical values within a margin of 0.5% (Table 1).
Study of the M III (AsUO 6 ) 3 ⋅nH 2 O (M III –La–Lu) state in aqueous solution
In order to investigate the state of REE uranyl arsenates in aqueous solutions, sample weights (~ 100 mg) were placed into 100 ml of solutions of HClO4 or NaOH (with molar concentration of 0.1, 0.01, 0.001 and 0.0001 mol/l) or distilled water. The experiments were conducted in plastic isolated containers with the minimal free volume of air (less than 1–2 ml) to minimize contact between solutions and CO2. The solutions with precipitates were shaken periodically and pH values were measured for several months. After the constant pH values were reached, the precipitate was separated from the solution, rinsed with distilled water, air-dried at room temperature and studied via X-ray fluorescence and powder diffraction analysis. The saturated aqueous solutions were analyzed via spectrophotometry to determine total concentrations of uranium(VI), arsenic(V) and REE(III). Nephelometry and turbidimetry were used to demonstrate the absence of suspended and colloidal particles in the studied solutions.
Spectrophotometric study of aqueous solutions
Arsenic(V) concentration in aqueous solution was determined via spectrophotometric method using the absorption of the reduced form of arsenic-molybdenum heteropolyacid (λmax = 900 nm, reducing agent–ascorbic acid) [24]. The stock solution of arsenic(V) was prepared by dissolving NaH2AsO4 in distilled water. The uranium(VI) concentration was determined using the absorption of its adduct with Arsenazo III (λmax = 650 nm, pH 3 for uranium concentration more than 10−4 mol/l and λmax = 675 nm, using concentrated HCl after reduction of U(VI) to U(IV) with metallic zinc for uranium concentration less than 10−5 mol/l) [25]. The stock solution of uranium(VI) was prepared by dissolving UO2(NO3)2·6H2O in distilled water. REE concentrations in aqueous solutions were determined via a spectrophotometric titration with 10−3 mol/l solution of disodium EDTA in the presence of xylenol orange (λmax = 570 nm, pH 5.5, detection limit is 1 ⋅ 10−6 mol/l) [26]. The equivalence point was determined as the point of intersection of linear sections of spectrophotometric curves.
Calculation
Solubility products calculation
The solubility products were calculated on the basis of solubility data for REE uranyl arsenates in 1 ⋅ 10−3 mol/l HClO4. The following equation represents the heterogeneous reaction:
The equilibrium constant KSP of this reaction is called solubility product and is represented by the following equation:
To calculate ionic activities for Eq. (4), uranium(VI), arsenic(V) and M(III) were assumed to exist in aqueous solutions as a collection of species, presented in Table 2 [27,28,29]. The activity coefficients of ions were calculated using Debye–Huckel equation which considered the theory of specific ionic interaction [29]:
I—ionic strength of an aqueous solution, ε(z±,m,I)—coefficient of ionic interaction of z ± ion with counterions [29], Cm—molar concentration of ion m.
The values of M3+, UO22+, AsO43– activity coefficients in 1 ⋅ 10−3 mol/l HClO4 solutions used for the calculations of solubility products are shown in Table 3.
The activity coefficients of molecular species were assumed to be equal to 1.0.
Solubility curves and speciation diagrams calculation
To simulate the behaviour of REE uranyl arsenates in aqueous solutions, the formation of various secondary solids was assumed in studied heterogeneous systems. With that in mind, the following system of equations was devised.
m0(MIII(AsUO6)3⋅16H2O)—the mass of the primary REE uranyl arsenate phase; V—the volume of the primary solution of HClO4, NaOH or water; M—molar mass; mL—the mass of the forming solid phase component; L—MIII(AsUO6)3⋅16H2O, HAsUO6⋅4H2O, (UO2)3(AsO4)2⋅12H2O, UO3⋅2.25H2O, MIII(OH)3, Na2U2O7; ωAs,L, ωU,L, ωM,L—the mass fraction of arsenic(V), uranium(VI) and REE(III) in L; Kw—ionic product of water.
The system of combined Eqs. (4, 6–16) allows us to simulate not only the existence of MIII(AsUO6)3⋅16H2O in the solid phase, but also the formation of several known secondary solids, such as HAsUO6⋅4H2O, ((UO2)3(AsO4)2⋅12H2O, UO3⋅2.25H2O, MIII(OH)3, Na2U2O7 and a few others. In the system above, Eqs. (4, 6–10) denote the equilibrium constants for heterogeneous reactions between primary and secondary compounds in the solid phase and equilibrium solutions. Equations (11–13) consider homogeneous equilibria between different species of uranium(VI), arsenic(V) and REE(III) in aqueous solutions, and Eqs. (14–16) correspond to the total amounts of U(VI), As(V) and M(III) in the solid phase and solution. Overall, the proposed system of Eqs. (4, 6–16) allows us to simulate and predict different parameters of studied heterogeneous systems «MIII(AsUO6)3⋅16H2O(cr)—aqueous solution» in a wide interval of solution acidity. Thus, ionic activities a(UO22+), a(AsO43−), a(M3+), total concentrations CU, CAs, CM, primary and secondary solids mass m(MIII(AsUO6)3⋅16H2O), m(HAsUO6⋅4H2O), m((UO2)3(AsO4)2⋅12H2O), m(UO3⋅2.25H2O), m(MIII(OH)3), m(Na2U2O7) were calculated based on set equilibrium pH values, the initial volume V and the mass of primary REE uranyl arsenate in the solid phase. The secondary solids mass were used to plot speciation diagrams and determine pH ranges of the existence of the solid phase components. Calculated concentrations were used to plot solubility curves. Ionic activities were used to calculate speciation diagrams of U(VI), As(V), M(III) in saturated aqueous solutions.
The calculation of Gibbs energies of M III (AsUO 6 ) 3 ⋅16H 2 O formation
Obtained solubility product values were used to calculate standard Gibbs free energies of formation of studied compounds. The following equations were used:
ΔGf—Gibbs energy (298 K) of formation of ions or molecules [27,28,29]; ΔGr(298)—Gibbs energy (298 K) of reaction (3).
Calculated ΔGf of REE uranyl arsenates and known ΔGf of secondary compounds [27,28,29] were used for thermodynamic evaluation of the possibility of transformation reactions in studied heterogeneous aqueous-salt systems at 298 K. The following equation was used for the calculation:
q and r—stoichiometric numbers.
Equipment and reagents
Diffraction patterns were recorded with a Shimadzu XRD-6000 diffractometer at the CuKα line. The elemental composition of the samples was determined using Shimadzu EDX-900 HS energy dispersive X-ray fluorescence spectrometer. The spectrophotometric measurements were performed using Shimadzu UV-1650 spectrophotometer. Scattered radiation intensity was measured using NPM (Russia) nephelometer. The solid phases and saturated solutions were separated using CLN-2 centrifuge (Russia) at 10,000 rpm. The pH values were measured with a pH-meter pH-410 Aquilon and a glass electrode (ESK-10601/7). Distilled water free of CO2 was used in all experiments [26, 27]. The sodium hydroxide solutions free of CO2 were prepared as described in [27]. All reagents were of the “chemically pure” grade. Mathematical simulation of the heterogeneous system and the prediction of its state were performed using Mathcad 8.0.
Results and discussion
Acid–base ranges of REE uranyl arsenates existence and transformation of M III (AsUO 6 ) 3 ⋅16H 2 O (M III –La–Lu) into other compounds
The behaviour of all REE uranyl arsenates in aqueous solutions obeys a general pattern. The acidity of the medium has the biggest impact on the state of studied heterogeneous aqueous-salt systems. The pH value of the equilibrium solution largely determines the composition and structure of solid compounds, solubility of uranyl arsenates and their transformation products, the dominant species of uranium(VI), arsenic(V) and REE in aqueous solution. Uranyl arsenates retain their composition and structure upon contact with solutions in a certain acidity range from pH of 1.8–2.0 to pH of 8.4–9.8 (Table 4). The width of this acid–base range changes slightly in the series of REE uranyl arsenates due to the analogous structure of the studied compounds and similar properties of interlayer atoms MIII.
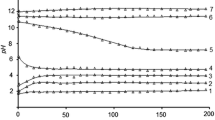
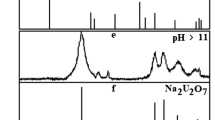
Solid-phase speciation diagrams for all REE uranyl arsenates in equilibrium heterogeneous systems are almost identical. The dependence of the mass fraction of solid compounds on pH values for neodymium uranyl arsenate is presented in Fig. 1 as an example. Shaded regions denote the formation of various secondary compounds in the solid phase. The acidity range of studied uranyl arsenate existence, presented in Table 2, is the range of pH in which the solid phase contains more than 97%w of crystalline compound MIII(AsUO6)3⋅16H2O. This is confirmed by powder diffraction analysis of equilibrium solid phases (Fig. 2a, b).
Outside the given existence pH ranges the structure of REE uranyl arsenates in aqueous solutions deteriorates. The nature of transformation products is also determined by the acidity. Solid-phase REE uranyl arsenates transform into uranyl arsenic acid in acidic medium:
This transformation causes changes in solid-phase diffraction patterns—the reflections of corresponding REE uranyl arsenates are replaced by the reflections of crystalline HAsUO6⋅4H2O (Fig. 2 (c, d)). Intensive band δ(H3O+) at 1737 cm−1 in IR-spectra of solids convincingly indicates the formation of HAsUO6⋅4H2O. X-ray fluorescent analysis confirms the lack of rare-earth elements in precipitate under these conditions. Described transformation is facilitated by both analogous layered structure of studied compounds MIII(AsUO6)3⋅16H2O and forming acid HAsUO6⋅4H2O and a large concentration of hydrogen ions in the aqueous solution. In terms of equilibrium thermodynamics, the ion-exchange reaction between the solid phase and the solution (20) is spontaneous and can happen in studied systems under standard conditions, which is proven by negative values of standard Gibbs free energies of these reactions for all compounds from MIII(AsUO6)3⋅16H2O series. The values of standard Gibbs free energies of the transformation reaction are presented in Table 5.
REE uranyl arsenates are less stable in alkaline solutions than reported earlier [18] alkaline elements derivatives. Their structure starts deteriorating at pH values large than 8–9 and new solid phases, that are rich with uranium and MIII element, begin to form. In all likelihood, it is caused by a greater hydrolysis affinity of rare earth element ions, which leads to the formation of stable hydroxy-complexes in aqueous solutions and their precipitation in the form of insoluble compounds The low crystallinity of the solid-phase transformation products at pH 9–11 and the limited information on uranium compounds with REE in the literature [32,33,34,35] do not allow their reliable identification. Thermodynamic simulation shows that the formation of uranyl oxide hydrate phases is possible in this pH range such as UO3⋅2.25H2O (schoepite), its dehydrated forms and products of interaction with REE [36,37,38,39,40,41,42,43]. The formation of largely crystalline Na2U2O7 and REE hydroxides with various degree of crystallinity MIII(OH)3 occurs for all REE uranyl arsenates in highly alkaline media with C (NaOH) > 0.1 mol/l (Fig. 2e, f) [44]. The transformation process of REE uranyl arsenates can be presented as the following equations in accordance with obtained data:
Spontaneousness of these reactions in heterogeneous aqueous-salt systems for studied REE uranyl arsenates is also confirmed by the thermodynamic calculations. Table 5 demonstrates that the Gibbs energies of the transformation of all studied REE compounds are sufficiently large negative values at the temperature 298 K.
Solubility of M III (AsUO 6 ) 3 ⋅16H 2 O (M III –La–Lu) compounds in aqueous solutions
Concentrations of uranium(VI) CU, arsenic(V) CAs and rare-earth elements CM in saturated aqueous solutions of MIII(AsUO6)3·16H2O are presented in Table 6. The analysis of presented data leads to the conclusion that the aqueous solution acidity has the most influence of the concentration of uranium(VI) and other structure-forming elements in heterogeneous aqueous-salt systems of studied compounds. Not only the absolute values of CU, CAs and CM change depending on the pH, but their ratio changes as well, which correlates well with supposed uranyl arsenates transformation process. In the wide range of pH values from 1.8–2.0 to 8.4–9.8, wherein studied compounds retain their structure, uranium and arsenic concentrations are equal within the experimental error margins. Under the same conditions, rare earth element concentration is approximately three times smaller than CU and CAs. These ratios are consistent with stoichiometry of MIII(AsUO6)3·16H2O compounds and are evidence of congruent dissolution of uranyl arsenates and, consequently, an equivalent transition of uranium(VI), arsenic(V) and M(III) into the solution. In acidic medium, wherein the transformation of the studied compound into uranyl arsenic acid takes place, hydrogen ions fully replace MIII in the solid phase and CM becomes constant. Under these conditions, uranium(VI) and arsenic(V) concentrations are the same and are determined by the solubility of the forming HAsUO6⋅4H2O. In the alkaline media determined CU and CAs differ by a factor of 10 and more, which is explained by the deterioration of uranyl arsenates structure and the formation of secondary solids, rich in uranium and rare earth element. The solubility of these solids determines the concentrations of U(VI) and M(III) in the solution in that case. Arsenic(V) is not retained in the solid phase under these conditions and is leached into the solution. All described patterns are similar to the ones exhibited by alkaline elements uranyl arsenates and consistent with theoretical assumptions about the composition and structure of Mk (AsUO6)k·nH2O compounds [18].
The REE uranyl arsenates solubility can only be discussed in the range of pH values from 1.8–2.0 to 8.4–9.8, wherein the compounds retain their composition and structure. Solubility of MIII(AsUO6)3·16H2O compounds is related to concentrations of structure-forming elements in saturated aqueous solutions by the following ratio S = CM = CU / 3 = CAs / 3. The dependence of uranyl arsenates solubility on pH in the range of their chemical stability is parabolic with the minimum in the neutral solutions, wherein S is equal to 10−7–10−6 mol/l (Fig. 2). The concentration of various species in saturated solutions increases correspondingly with increases in acidity or alkalinity and reaches 10−4–10−2 mol/l at the boundaries of the pH range of existence. At the same pH values, the solubility changes very little depending on the interlayer atom and varies in the range (1.1–1.5)⋅10−7 mol/l at pH 7.
Solubility products of M III (AsUO 6 ) 3 ⋅16H 2 O (M III –La–Lu)
Solubility products of studied uranyl arsenates were calculated using experimental data on the solubility of the compounds and the results are presented in Table 7.
General structural likeness of REE uranyl arsenates and similarity in properties of rare-earth elements explain the insignificant influence of the nature of interlayer element on solubility and chemical resistance of all MIII(AsUO6)3⋅16H2O compounds.
Ionic-molecular composition of saturated aqueous solutions in the system «M III (AsUO 6 ) 3 ⋅16H 2 O (cr) –aqueous solution»
Ionic-molecular composition of saturated aqueous solutions of studied uranyl arsenate systems correlates well with processes of transformation and dissolution of crystalline compounds. Heterogeneous reactions in the system «MIII(AsUO6)3⋅16H2O(cr)—aqueous solution» are a consequence of complex ionic-molecular interactions between various species of uranium(VI), arsenic(V) and rare earth element M(III) in liquid phase. Those reactions cause the deterioration of the structure of studied uranyl arsenates, their transformation into compounds with different composition and structure, and changes in solubility of uranium compounds. Thus, to gain insight into the dissolution process, composition of aqueous solutions in the studied heterogeneous systems is closely examined. Speciation diagrams for uranium(VI), arsenic(V) and rare earth element in saturated solutions of Nd(AsUO6)3⋅16H2O(cr) as an example are presented in Fig. 3. They facilitate the evaluation of the state of structure-forming elements in aqueous solutions, the fraction of different dominant species at different pH values and their influence on general state of the heterogeneous system and indicate a rather complex composition of saturated aqueous solutions of uranyl arsenates. Cationic species of rare-earth elements M3+ and uranium(VI) with arsenic(V) UO2H2AsO4+ and are dominant in the solution in acidic media. The latter, in all likelihood, facilitates the formation of HAsUO6⋅4H2O acid. High chemical resistance of uranyl arsenic layer allows for the preservation of anionic layer [AsUO6]∞δ− and the existence of solid with given composition despite highly acidic medium usually causing the deterioration of the structure of many uranium compounds [38, 45,46,47].
Slightly acidic media facilitate the hydrolysis of uranyl ions, leading to the formation of stable uranyl hydroxy-complexes of various composition, that are the dominant species in neutral and alkaline solutions. Under these conditions, the described hydroxy-complexes are the reason for the formation of insoluble uranyl oxide hydrate phases of complex composition and structure. However, the formation of ionic-molecular species of uranium(VI) with a high degree of condensation, leading to the formation of colloidal particles, is not observed in the studied systems, which can be proven via nephelometry and turbidimetry. In highly alkaline media non-condensed products of uranyl ions hydrolysis, containing a large amount of OH− per each UO22+ moiety, are dominant, which leads to the formation of a crystalline uranate of constant composition in the solid phase.
The minimal solubility of uranyl arsenates is observed in neutral media, which correlates well with ionic-molecular composition of saturated aqueous solutions. Under these conditions, the least charged species of uranium(VI), arsenic(V) and element M(III) are dominant in the solution, which facilitates the least influence of electrostatic forces on the polar structure of crystalline solids. In acidic and alkaline solutions more charged species of structure-forming elements are dominant, which, as well as the presence of H+ and OH− ions, leads to an increase in solubility of studied compounds.
Conclusion
The chemical stability of uranyl arsenates of rare-earth elements with the general formula MIII(AsUO6)3⋅16H2O (MIII–La–Lu) in aqueous solutions has been studied in a wide range of acidity. Rare-earth elements uranyl arsenates retain their composition and structure upon the contact with aqueous solutions in the range of pH values from 1.8–2.0 to 8.4–9.8. Outside this range, they transform into compounds with different composition and structure, such as HAsUO6⋅4H2O, UO3⋅nH2O, MIII(OH)3, Na2U2O7. The solubility of MIII(AsUO6)3⋅16H2O compounds has the least value (10−7 mol/l) in neutral solutions and increases up to 10−4–10−2 mol/l in acidic or alkaline solutions. Ionic-molecular composition of saturated aqueous solutions of studied uranyl arsenate systems correlates well with processes of transformation and dissolution of crystalline compounds. The dependence of solubility product logarithm lgKSP on interlayer atom ionic radius r(M3+) is typical for REE compounds and displays the characteristic gadolinium break. However, general structural likeness of studied compounds and similarity in properties of interlayer atoms MIII lead to a small spread of lgKSP values and analogous influence of different factors on all MIII(AsUO6)3⋅16H2O compounds chemical resistance in aqueous solutions.
References
Locock AJ, Burns PC, Duke MJM, Flynn TM (2004) Structures and synthesis of layered and framework amine-bearing uranyl arsenates and phosphates. Can Mineral 177(8):2675–2684. https://doi.org/10.1016/j.jssc.2004.03.045
Locock AJ, Burns PC (2003) Crystal structures and synthesis of the copper-dominant members of the autunite and meta-autunite groups: torbernite, zeunerite, metatorbernite and metazeunerite. Can Mineral 41:489–502. https://doi.org/10.2113/gscanmin.41.2.489
Locock AJ, Burns PC (2003) Structures and synthesis of framework Rb and Cs uranyl arsenates and their relationships with their phosphate analogues. J Solid State Chem 175(2):372–379. https://doi.org/10.1016/S0022-4596(03)00383-9
Chernorukov NG, Karyakin NV, Suleimanov EV, Chernorukov GN (1994) Synthesis and study of MIAsUO6⋅nH2O compounds. Russ J Inorg Chem 1:23–26
Chernorukov NG, Karyakin NV, Suleimanov EV, Belova YS (1998) Synthesis and study of Mg(PUO6)2⋅nH2O and Mg(AsUO6)2⋅nH2O compounds. Russ J Inorg Chem 3:380–383
Chernorukov NG, Karyakin NV, Suleimanov EV, Belova YS (1997) Synthesis and study of Ba(PUO6)2⋅nH2O and Ba(AsUO6)2⋅nH2O compounds. Russ J Inorg Chem 5:693–697
Chernorukov NG, Karyakin NV, Suleimanov EV, Belova YS (1996) Synthesis and study of Sr(PUO6)2⋅nH2O and Sr(AsUO6)2⋅nH2O compounds. Radiochemistry 5:729–732
Chernorukov NG, Suleimanov EV, Jabarova ST (1998) Synthesis and study of Ni(PUO6)2⋅nH2O and Ni(AsUO6)2⋅nH2O compounds. Russ J Inorg Chem 7:1090–1095
Chernorukov NG, Suleimanov EV, Jabarova ST (1999) Synthesis and study of Co(PUO6)2⋅nH2O and Co(AsUO6)2⋅nH2O compounds. Russ J Inorg Chem 5:782–787
Chernorukov NG, Suleimanov EV, Jabarova ST, Barch SV (2000) Synthesis and study of AII(BVUO6)2⋅nH2O (AII—Mn, Fe Co, Ni, Cu; BV—P, As) compounds. Radiochemistry 1:15–32
Vochten R, De Grave E, Pelsmaekers J (1986) Synthesis, crystallographic and spectroscopic data, solubility, and electrokinetic properties of metakahlerite and its Mn analogue. Am Mineral 71(7–8):1037–1044
Vochten R, Piret P, Goeminne A (1981) Synthesis, crystallographic data, solubility and electrokinetic properties of copper-, nickel- and cobalt˗uranylphosphate. Bull Minéral 104:457–467. https://doi.org/10.3406/bulmi.1981.7496
Vochten R, Goeminne A (1984) Synthesis, crystallographic data, solubility and electrokinetic properties of meta-zeunerite, meta-kirchheimerite and nickel-uranylarsenate. Phys Chem Miner 11:95–100. https://doi.org/10.1007/BF00308011
Chernorukov NG, Suleimanov EV, Barch SV, Alekseev EV (2001) Synthesis and study of lanthanides and yttrium uranyl arsenates. Radiochemistry 1:9–16
Suleimanov EV, Chernorukov NG, Golubev AV (2001) Synthesis, structure and physico-chemical properties of Pb(BVUO6)2⋅nH2O (BV—P, As, V) compounds. Radiochemistry 5:412–417
Chernorukov NG, Nipruk OV, Pykhova YP (2010) On the role of interlayer atoms and molecular water in formation of crystalline structure of salts of uranyl arsenic acid HAsUO6⋅4H2O. Russ J Inorg Chem 55(2):190–194. https://doi.org/10.1134/S0036023610020099
Chukhlantsev VG, Sharova AK (1956) Solubility products of uranyl arsenates. Russ J Inorg Chem 1:36–42
Nipruk OV, Chernorukov NG, Elipasheva EV, Klinshova KA, Bakhmetev MO (2020) State of uranyl arsenates MIAsUO6·nH2O (MI–H+, Li+, Na+, K+, Rb+, Cs+, NH4+) in aqueous solution. J Radioanal Nucl Chem 324(1):233–244. https://doi.org/10.1007/s10967-020-07062-3
Zhiltsova IG, Polupanova LI, Shamriovich EM, Perlina SA (1987) Physico-chemical conditions of formation of ore uranyl arsenate mineralization. Lithol Miner 3:44–54
Chernorukov NG, Nipruk OV, Pykhova YP, Godovanova NS (2012) Study of the state of uranoarsenates MII(AsUO6)2⋅nH2O (MII = Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Pb2+) in aqueous solutions. Russ J Gen Chem 82(8):1348–1356. https://doi.org/10.1134/S107036321208004X
Baranovskaya NV, Ageeva EV, Soktoev BR, Narkovich DV, Denisova OA, Matkovskaya TV (2020) Rare earth and radioactive (Th, U) elements in the components of the environment on the territory of Tomsk region. Bull Tomsk Polytech Univ 331(2):17–28. https://doi.org/10.18799/24131830/2020/2/2477
Gob S, Guhring JE, Bau M, Markl G (2013) Remobilization of U and REE and the formation of secondary minerals in oxidized U deposits. Am Mineral 98(4):530–548. https://doi.org/10.2138/am.2013.4275
Zhang W, Chen WT, Gao JF, Chen HK, Li JH (2019) Two episodes of REE mineralization in the Qinling Orogenic Belt, Central China: in-situ U-Th-Pb dating of bastnasite and monazite. Miner Deposita 54(8):1265–1280. https://doi.org/10.1007/s00126-019-00875-7
Nemodruk AA (1976) Analytical chemistry of arsenic. Science, Moscow
Savvin SB (1964) Analytical Applications of Arsenazo III. Part II: determination of thorium, uranium, protactinium, neptunium, hafnium and scandium. Talanta. https://doi.org/10.1016/0039-9140(64)80003-5
Ryabchikov DI, Ryabukhin VA (1966) Analytical chemistry of rare earth elements & yttrium. Science, Moskow
Guillaumont R, Fanghänel T, Fuger J et al (2003) Update on the chemical thermodynamics of uranium, neptunium, and plutonium. Elsevier, Amsterdam
Yungman VS (ed), Glushko VP, Medvedev VA, Gurvich LV (1999) Thermal constants of substances. Wiley, USA
Grenthe I, Fuger J, Koning R et al (2004) Chemical thermodynamics of uranium. North-Holland, Amsterdam
Korostelev PP (1964) Preparation of solutions for chemical and analytical works. Science, Moscow
Kiseleva EK, Suslennikova VM (1959) Reference guide for the preparation of titrated solutions and determining their titres. Typolithography LKVVIA named after A.F. Mozhaisky, Leningrad
Tang M, Holliday KS, Jiang C, Valdez JA, Uberuaga BP, Dickerson PO, Dickerson RM, Wang Y, Czerwinski KR, Sickafus KE (2010) Order-to-disorder phase transformation in ion irradiated uranium—bearing delta—phase oxides RE6U1O12 (RE=Y, Gd, Ho, Yb, and Lu). J Solid State Chem. https://doi.org/10.1016/j.jssc.2010.01.020
Venkata Krishnan R, Jena H, Govindan Kutty KV, Nagarajan K (2010) Heat capacity and thermal expansion coefficient of rare earth uranates RE6UO12 (RE–Nd, Gd and Eu). J Therm Anal Calorim. https://doi.org/10.1007/s10973-009-0618-y
Krishnan RV, Babu R, Panneerselvam G, Ananthasivan K, Antony MP, Nagarajan K (2012) Thermophysical properties of Dy6UO12. Ceram Int. https://doi.org/10.1016/j.ceramint.2012.02.044
Shukla B, Sanjay Kumar NR, Sekar M, Chandra Shekar NV, Jena H, Asuvathraman R (2016) Stability of Dy6UO12 under high pressure and high temperature. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2016.02.202
Nipruk OV, Knyazev AV, Chernorukov GN, Pykhova YP (2011) Synthesis and study of hydrated uranium(VI) oxides, UO3⋅nH2O. Radiochemistry. https://doi.org/10.1134/S1066362211020044
Finch RJ, Hawthorne FC, Miller ML, Ewing RC (1997) Distinguishing among schoepite, [(UO2)8O2(OH)12](H2O)12, and related minerals by X-ray powder diffraction. Powder Diffr 12(4):230–238. https://doi.org/10.1017/S0885715600009799
Chernorukov NG, Nipruk OV, Arova MI, Blazhenova DV (2013) Synthesis and study of polyuranates MIIIU3O10.5⋅6H2O (MIII = La, Ce, Pr, Nd, Sm). Russ J Gen Chem 83(4):642–645. https://doi.org/10.1134/S1070363213040051
Chernorukov NG, Nipruk OV, Arova MI, Chaplieva KA (2014) Preparation and properties of the polyuranates MIIIU2O7.5 (MIII = Y, Tb, Dy, Ho, Er, Tm, Yb, or Lu). Russ J Gen Chem 84(1):6–8. https://doi.org/10.1134/S1070363214010022
Nipruk OV, Chernorukov NG, Chaplieva KA (2017) Synthesis and study of hexauranates MIII[(UO2)6O4.5(OH)6]⋅7H2O (MIII—Nd, Sm, Eu, Gd, Dy). J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-017-5462-0
Zhang Y, Aughterso R, Karatchevtseva I, Kong L, Tran TT, Čejka J, Aharonovich I, Lumpkin GR (2018) Uranyl oxide hydrate phases with heavy lanthanide ions: [Ln(UO2)2O3(OH)]⋅0.5H2O (Ln = Tb, Dy, Ho and Yb). New J Chem 42(15):12386–12393. https://doi.org/10.1039/c8nj01376d
Zhang YJ, Aughterson RD, Zhang ZM, Wei T, Lu K, Cejka J, Karatchevtseva I (2019) Syntheses, crystal structures, and spectroscopic studies of uranyl oxide hydrate phases with La(III)/Nd(III) ions. Inorg Chem 58(16):10812–10821. https://doi.org/10.1021/acs.inorgchem.9b01102
Lu KT, Zhang YJ, Wei T, Cejka J, Zheng RK (2020) Layer-structured uranyl-oxide hydroxy-hydrates with Pr(III) and Tb(III) ions: hydroxyl to oxo transition driven by interlayer cations. Dalt Trans 49(18):5832–5841. https://doi.org/10.1039/d0dt00526f
Kovba LM (1972) Crystal structure of sodium diuranate. Radiochemistry 14:727–730
Nipruk OV, Chernorukov NG, Zakharycheva NS, Kostrova EL (2017) State of rare earth elements uranyl germanates in aqueous solutions. J Radioanal Nucl Chem 311(1):519–529. https://doi.org/10.1007/s10967-016-5044-6
Nipruk OV, Chernorukov NG, Godovanova NS, Kostrova EL (2013) Behavior of uranosilicates MIII(HSiUO6)3·10H2O (MIII = La–Lu, Y) in aqueous solutions. Radiochemistry 55(1):63–71. https://doi.org/10.1134/S1066362213010128
Nipruk OV, Chernorukov NG, Eremina AA, Kostrova EL, Chaplieva KA (2014) Behavior of rare earth uranovanadates in aqueous solutions. Radiochemistry 56(4):392–399. https://doi.org/10.1134/S106636221404006!
Acknowledgements
The work was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation, Project No. 0729-2020-0039.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nipruk, O.V., Chernorukov, N.G., Klinshova, K.A. et al. Chemical stability of rare-earth elements’ uranyl arsenates with general formula MIII(AsUO6)3·16H2O (MIII–La–Lu) in aqueous solution. J Radioanal Nucl Chem 328, 739–751 (2021). https://doi.org/10.1007/s10967-021-07692-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07692-1