Abstract
This study aimed to evaluate the prognostic value of interim positron emission tomography (iPET) in diffuse large B-cell lymphoma (DLBCL). Among 53 enrolled patients, 39 had iPET-negative (iPET−) and 14 had iPET-positive (iPET+) scans. The objective response rate was 94.3%. The 3-year progression-free survival was 65.7% and the 3-year overall survival was 79.9%. The iPET− patients had significantly higher 3-year PFS rate (78.1% vs. 34.3%) and improved OS (87.1% vs. 62.3%) than iPET+ patients. In the univariate analysis, iPET− was the sole independent prognostic factor for PFS. In conclusion, PET/CT has a good prognostic value in patients with advanced-stage DLBCL.
Trial registration: ClinicalTrials.gov, NCT 01804127. Registered on March 5th, 2013, https://www.clinicaltrials.gov/ct2/show/NCT01804127?term=01804127&rank=1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin’s lymphoma (NHL) in adults and is associated with an aggressive clinical course. Treatment failure remains a significant challenge in DLBCL as the 3-year progression-free survival (PFS) of DLBCL patients is approximately 60–70% when RCHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone)-like treatment was used as the first-line strategy [1, 2].
The interim 18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) scan (iPET) during first-line therapy has been considered as the strongest prognostic tool in advanced Hodgkin’s lymphoma (HL), even better than the traditional International Prognostic Index (IPI) [3]. In patients with advanced HL, better survival and life quality can be achieved when treated with iPET-driven strategy. As iPET-negative (iPET−) indicates good prognosis, doctors may downgrade the treatment, such as removing bleomycin, to avoid toxicity and second primary tumor [3]. In contrast, iPET-positive (iPET+) suggests poor outcomes; therefore, early therapy intensification in response to positive iPET may improve the survival of patients. However, compared to conventional ABVD regimen, intensified treatments such as BEACOPP and high-dose chemotherapy with autologous stem cell transplantation may result in great toxicities [4]. As the effect of cytotoxic drugs is limited, not all iPET+ patients can achieve complete response (CR) after intensified chemotherapy [5, 6].
In DLBCL, it is common to perform an iPET after 2 to 4 cycles of first-line chemotherapy. Whether iPET+ patients should receive a more intensive regimen as an immediate salvage treatment is still a topic of debate. In this study, we performed an open-label, non-randomized, single arm, phase II study of a cohort of DLBCL patients to examine the prognostic value of iPET in DLBCL.
Methods
Ethical approval
From April 2013 to September 2015, we performed a large prospective trial of patients with newly diagnosed DLBCL. Here we reported a subset analysis of patients at advanced stages. The study was approved by the institutional review board of Fudan University Shanghai Cancer Center. The trial was registered with ClinicalTrials.gov (number NCT 01804127). All patients provided written informed consent.
Inclusion and exclusion criteria
Patients who were diagnosed with stage III–IV DLBCL according to the Ann Arbor staging system [7, 8] and aged between 18 and 80 years were eligible for this study. All patients had Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 and adequate hepatic, renal, and hematologic functions. Patients had at least one measurable target lesion. Patients with left ventricular ejection fraction less than 50%, which was evaluated by echocardiogram at baseline, were excluded. Patients with a history of severe heart disease, uncontrolled hemorrhage, or infection were also excluded.
Treatment and response evaluation
All patients underwent a baseline PET within 2 weeks before the treatment and showed positive and measurable lesions in PET. Then, they were initially treated with 4 cycles of RCHOP (rituximab 375 mg/m2 d1; cyclophosphamide 750 mg/m2 d2; doxorubicin 50 mg/m2 d2; vincristine 1.4 mg/m2 [maximum 2 mg] d2; prednisone 100 mg orally daily d2–6). RCHOP was administered every 3 weeks.
The iPET scan was performed after 4 cycles of RCHOP in all cases on cycle 4 (day 18–20). The Lugano criteria [9] was used for the evaluation of therapy response. The response criteria were based on the Deauville 5-point scale (1–2: negative; 3–5: positive). Patients with no metabolic response or progressive metabolic disease on PET were recorded as stable disease (SD) and progressive disease (PD), respectively.
Patients received 2 additional cycles of RCHOP when achieved iPET CR (6 in total). Patients who achieved iPET partial response (PR) received 4 additional cycles of RCHOP (8 in total), and a final PET (fPET) scan was performed on cycle 8 (day 18–20). Patients who had SD or PD on iPET were treated by salvage chemotherapy and discontinued the clinical trial, but included in efficacy analysis.
Patients were followed every 3 months for the first 2 years, and then every 6 months for 3 years after the therapy.
Statistical analysis
We hypothesized that personalized treatment cycles according to iPET could show similar efficacy with previous studies [1, 2]. Therefore, we expected that the 3-year PFS of patients with I–IV stages was 70–80%. At least 164 patients were needed to be enrolled when a significance was set at a two-sided 5% type I error and at least 90% power. Accounting for a 20% dropout rate, the final number of patients that were needed was 196. Here we only reported a subset analysis with the patients with advanced-stage DLBCL.
The primary endpoint was 3-year PFS, and secondary endpoints included 3-year overall survival (OS) and objective response rate (ORR). PFS was defined as the interval between initiation of RCHOP treatment and disease progression or the last follow-up visit in remission. OS was calculated from the date of initiation of RCHOP treatment to the date of death from any cause or last follow-up.
Categorical variables are expressed as frequencies. Chi square test or Fisher’s exact test were applied to detect differences between groups. PFS and OS were calculated using Kaplan–Meier analysis, with differences between groups compared using a log- rank test and a difference of P < 0.05 was considered significant. All statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
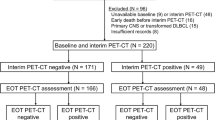
A total of 198 patients (stage I–IV) were enrolled in this study. Here, we reported a subset analysis of patients with advanced stage (III–IV) DLBCL (n = 55). Finally, 53 patients with baseline and interim PET/CT scans were analyzed for efficacy. The reasons for exclusion were disease progression (n = 1) and serious adverse event (n = 1) before interim response assessment.
A total of 24 women (45.3%) and 29 men (54.7%) were included. The mean age was 50 years (range 23–76 years). In total, 32 patients (60.4%) had stage IV disease, and 14 patients (26.4%) exhibited B symptoms. Based on the IPI scores, 62.3% of patients were within intermediate-high or high risk of relapse parameters. The baseline clinical characteristics of the patients are summarized in Table 1.
Thirty-nine patients had iPET-negative and 14 patients had iPET-positive scans. Among the 14 iPET+ patients, 11 had PR and 3 had PD. The ORR was 94.3%. All 53 patients were included in efficacy analysis. At a median follow-up time of 36.4 months (range 3.8–63.6 months), the 3-year PFS was 65.7% and the 3-year OS was 79.9% for the entire cohort (n = 53). The median PFS and OS were not yet reached.
Thirty-two patients with iPET− (39 in total) did not relapse during the period of observation. iPET− patients had a significantly higher 3-year PFS rate (78.1%) than iPET+ patients (34.3%) (P < 0.01). iPET− patients also had significantly improved 3-year OS than iPET+ patients (87.1% vs. 62.3%, P = 0.03) (Fig. 1a, b).
a Progression free survival curve between iPET negative and positive patients. b Overall survival curve between iPET negative and positive patients. c Progression free survival curve between iPET CR and fPET CR patients. d Overall survival curve between iPET CR and fPET CR patients. CR complete response
All 11 patients who had iPET PR received another 4 cycles RCHOP, and 10 of them underwent fPET. Six patients had fPET-negative (fPET−) and four patients had fPET-positive (fPET+) scans. Among those six patients with fPET−, four patients did not relapse during the period of observation. The 3-year PFS or OS did not significantly differ between the iPET− and fPET− patients (78.1% vs. 62.5%, P = 0.64, 87.1% vs. 83.3%, P = 0.81) (Fig. 1c, d).
In addition, among all iPET+ patients, patients with a Deauville score of 3 had a significantly longer PFS and OS than those with a Deauville score of 4 and 5 (P < 0.01, Fig. 2a; P < 0.01, Fig. 2b).
In the univariate analysis, iPET negative was the sole independent prognostic factor for PFS in patients with DLBCL treated with RCHOP. No other baseline clinicopathological factors, including age, disease stage, gender, molecular subtype, B symptoms, ECOG PS, elevated lactate dehydrogenase (LDH), IPI score, or extra-nodal involvement, were predictive for PFS in the entire cohort. For this reason, multivariate analysis was not performed.
In the iPET− group (n = 39), seven patients relapsed during the period of observation. The log-rank test showed that patients with more than one extra-nodal involvement sites or poor ECOG status were associated with worse outcomes with a borderline P value of 0.058 and 0.065, respectively. Other variables, such as age, disease stage, B symptoms, IPI score, or molecular subtype, did not significantly affect the risk of disease progression.
Discussion
PET/CT is currently used for staging, assessment of remission and recurrence, and evaluation of therapeutic efficacy of patients with DLBCL [10]. Researchers have been focusing on whether PET can guide treatment escalation in poor responders to improve remission rates in NHL [11]. To date, PET predicts response in DLBCL, but more intensive chemotherapy has failed to improve the outcomes for patients with iPET+ scans [11, 12]. Several large prospective studies, such as the PETAL and LYSA trials, demonstrated that treatment intensification, such as the Burkitt-type approach or autologous stem cell transplantation, failed to prevent iPET+ patients from having a higher risk of relapse compared to iPET− patients [5]. RCHOP-like chemotherapies have been proven to be effective in DLBCL for many years. Any alternative options will be considered only after being shown to be superior to the ongoing treatment. In this study, patients who had iPET PR were treated with 4 additional RCHOP cycles. Patients who achieved fPET CR had similar good outcomes as iPET− patients. Only patients with a fPET+ scan had inferior PFS and OS. This study demonstrated no inferiority of continuation of the first-line regimen in patients with iPET PR. Therefore, when aiming to maximize cure while minimizing toxicity, there is no need to escalate the treatment of iPET+ patients.
In this study, patients with a Deauville score of 3 on iPET had significantly higher PFS and OS than patients with a Deauville score of 4 and 5. Our data are in agreement with those reported by Cheson et al. [9] Based on their findings, a score of 3 on iPET might be a good outcome predictor. However, in trials involving PET where de-escalation was investigated, it might be preferable to consider a score of 3 as inadequate response (to avoid undertreatment) [9].
Over the past decades, the IPI has become the most commonly used prognostic index in DLBCL patients [13, 14]. The IPI differentiates DLBCL patients into distinct risk groups for the survival after RCHOP. Recent evidence suggests a high predictive value of iPET in HL. It has been validated as a strongest prognostic tool in advanced HL, even better than traditional IPS. iPET− could indicate a good prognosis, while iPET+ could indicate a poor outcome, regardless of gender, stage, age, or count of hemoglobin and lymphocyte [5, 15]. However, many studies focusing on the role of iPET in PFS prediction have shown conflicting results [16,17,18,19,20,21,22]. From these studies, it can be concluded that phenotypic and genotypic heterogeneity of DLBCL, heterogeneity in patient populations, therapy regimens, PET scanners, and timing and interpretation criteria of iPET scans made it hard to clarify the accuracy of iPET to predict the clinical outcomes of DLBCL patients.
In our study, the difference in PFS may be related to ECOG status and extra-nodal involvement in iPET− patients. But the statistical analysis showed that they were not independent predictors of disease progression (P > 0.05). The difference in PFS may be underestimated, given our small sample size and subsequent limited power to calculate the difference in survival.
Recently, several studies have shown that PET/CT was a more valid prognosticator of survival for patients with DLBCL than traditional clinicopathologic factors, such as the IPI score [23,24,25,26,27]. Our results confirmed that only iPET was a significant independent indicator for the outcomes of patients with DLBCL in the rituximab era. iPET− patients had significantly higher PFS rate and OS rate than iPET+ patients.
There are some limitations to this study. First, this study was a single-arm, small-scale clinical trial. Randomized, large-scale, prospective trials are needed to determine whether iPET+ patients should continue with the same treatment or switch to an intensified treatment. Second, the follow-up time was not long. Future studies with long-term follow-up are needed to assess the 5-year PFS and 5-year OS.
Conclusion
PET/CT has a good prognostic value in patients with advanced-stage DLBCL. Univariate analysis showed that iPET− identified good outcome regardless of B symptoms, LDH, IPI score, and molecular subtypes. There was little significant benefit to intensifying chemotherapy if the iPET scan was positive. Another 4 cycles of the first-line regimen (RCHOP) was acceptable as fPET− patients could also have a good PFS.
Availability of data and materials
The dataset of the current study were available from the corresponding author on reasonable request.
Abbreviations
- DLBCL:
-
Diffuse large B-cell lymphoma
- NHL:
-
Non-Hodgkin’s lymphoma
- PFS:
-
Progression-free survival
- 18F-FDG:
-
18F-Fluoro-2-deoxy-d-glucose
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- iPET:
-
Interim PET
- HL:
-
Hodgkin’s lymphoma
- ECOG:
-
Eastern Cooperative Oncology Group
- PS:
-
Performance status
- RCHOP:
-
Rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone
- CR:
-
Complete response
- PR:
-
Partial response
- fPET:
-
Final PET
- PD:
-
Progressive disease
- OS:
-
Overall survival
- ORR:
-
Objective response rate
- IPI:
-
International Prognostic Index
- iPET−:
-
iPET-negative
- iPET+:
-
iPET-positive
- fPET−:
-
fPET-negative
- fPET+:
-
fPET-positive
- LDH:
-
Lactate dehydrogenase
References
Zelenetz AD (2014) Guidelines for NHL: updates to the management of diffuse large B-cell lymphoma and new guidelines for primary cutaneous CD30+ T-cell lymphoproliferative disorders and T-cell large granular lymphocytic leukemia. J Natl Compr Cancer Netw 12(5 Suppl):797–800
Bolshinsky M, Nabhan C (2016) Interim PET scans in diffuse large B-cell lymphoma: is it ready for prime time? Clin Lymphoma Myeloma Leuk 16(12):655–661
Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A et al (2016) Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med 374(25):2419–2429
Zinzani PL, Broccoli A, Gioia DM, Castagnoli A, Ciccone G, Evangelista A et al (2016) Interim positron emission tomography response-adapted therapy in advanced-stage Hodgkin lymphoma: final results of the phase II part of the HD0801 study. J Clin Oncol 34(12):1376–1385
Le Gouill S, Casasnovas RO (2017) Interim PET-driven strategy in de novo diffuse large B-cell lymphoma: do we trust the driver? Blood 129(23):3059–3070
Press OW, Li H, Schoder H, Straus DJ, Moskowitz CH, LeBlanc M et al (2016) US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose–positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol 34(17):2020–2027
Kwak YK, Choi BO, Kim SH, Lee JH, Kang DG, Lee JH (2017) Treatment outcome of diffuse large B-cell lymphoma involving the head and neck: two-institutional study for the significance of radiotherapy after R-CHOP chemotherapy. Medicine (Baltimore) 96(25):e7268
Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC et al (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 7(11):1630–1636
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068
Barrington SF, Kirkwood AA, Franceschetto A, Fulham MJ, Roberts TH, Almquist H et al (2016) PET-CT for staging and early response: results from the response-adapted therapy in advanced Hodgkin lymphoma study. Blood 127(12):1531–1538
Hertzberg M, Gandhi MK, Trotman J, Butcher B, Taper J, Johnston A et al (2017) Early treatment intensification with R-ICE and 90Y-ibritumomab tiuxetan (Zevalin)-BEAM stem cell transplantation in patients with high-risk diffuse large B-cell lymphoma patients and positive interim PET after 4 cycles of R-CHOP-14. Haematologica 102(2):356–363
Voltin CA, Mettler J, Grosse J, Dietlein M, Baues C, Schmitz C et al (2020) FDG-PET imaging for Hodgkin and diffuse large B-cell lymphoma—an updated overview. Cancers (Basel) 12(3):601
Ruppert AS, Dixon JG, Salles G, Wall A, Cunningham D, Poeschel V et al (2020) International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood 135(23):2041–2048
Alamer F, Alamir A, Alqahtani A, Alkabli AM, Alshabib H, Damlaj M (2020) Validation of the international prognostic index and subsequent revisions for diffuse large B-cell lymphoma in patients from the Middle East and North Africa Region. Cureus 12(8):e9620
Evens AM, Kostakoglu L (2014) The role of FDG-PET in defining prognosis of Hodgkin lymphoma for early-stage disease. Blood 124(23):3356–3364
Zinzani PL, Gandolfi L, Broccoli A, Argnani L, Fanti S, Pellegrini C et al (2011) Midtreatment 18F-fluorodeoxyglucose positron-emission tomography in aggressive non-Hodgkin lymphoma. Cancer Am Cancer Soc 117(5):1010–1018
Yang DH, Ahn JS, Byun BH, Min JJ, Kweon SS, Chae YS et al (2013) Interim PET/CT-based prognostic model for the treatment of diffuse large B cell lymphoma in the post-rituximab era. Ann Hematol 92(4):471–479
Huntington SF, Nasta SD, Schuster SJ, Doshi JA, Svoboda J (2015) Utility of interim and end-of-treatment [(18)F]-fluorodeoxyglucose positron emission tomography-computed tomography in frontline therapy of patients with diffuse large B-cell lymphoma. Leuk Lymphoma 56(9):2579–2584
Yoo C, Lee DH, Kim JE, Jo J, Yoon DH, Sohn BS et al (2011) Limited role of interim PET/CT in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol 90(7):797–802
Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL (2011) 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med 52(3):386–392
Pregno P, Chiappella A, Bello M, Botto B, Ferrero S, Franceschetti S et al (2012) Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 119(9):2066–2073
Moskowitz CH, Schoder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS et al (2010) Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol 28(11):1896–1903
Fan Y, Zhang Y, Yang Z, Ying Z, Zhou N, Liu C et al (2017) Evaluating early interim fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography with the SUVmax-liver-based interpretation for predicting the outcome in diffuse large B-cell lymphoma. Leuk Lymphoma 58(9):1–9
Kong Y, Qu L, Li Y, Liu D, Lv X, Han J (2016) Predictive significance of a new prognostic score for patients with diffuse large B-cell lymphoma in the interim-positron emission tomography findings. Medicine (Baltimore) 95(6):e2808
Mamot C, Klingbiel D, Hitz F, Renner C, Pabst T, Driessen C et al (2015) Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol 33(23):2523–2529
Carr R, Fanti S, Paez D, Cerci J, Gyorke T, Redondo F et al (2014) Prospective international cohort study demonstrates inability of interim PET to predict treatment failure in diffuse large B-cell lymphoma. J Nucl Med 55(12):1936–1944
Nols N, Mounier N, Bouazza S, Lhommel R, Costantini S, Vander BT et al (2014) Quantitative and qualitative analysis of metabolic response at interim positron emission tomography scan combined with international prognostic index is highly predictive of outcome in diffuse large B-cell lymphoma. Leuk Lymphoma 55(4):773–780
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JJ and YL were responsible for data collection and drafted the manuscript; XH, JC and YG participated in the design of the study. JJ, QZ, KX and ZX performed statistical analysis and data interpretation; JJ and FL designed the study and revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Ethics Committee of Fudan University Shanghai Cancer Center. Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jin, J., Liu, Y., Zhang, Q. et al. Interim PET/CT result is the sole prognostic factor of survival in patients with advanced-stage diffuse large B-cell lymphoma: a subset analysis of a prospective trial. J Radioanal Nucl Chem 327, 353–358 (2021). https://doi.org/10.1007/s10967-020-07511-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07511-z