Abstract
Quinoline Silicate Lewatit Composite and activated Lewatit were prepared and tested for uranium removal from sulfate solution. Uranium sorption capabilities of the tested adsorbents was estimated under different conditions; uranium initial concentration, pH, contact time, temperature, adsorbent dose and interfering ions. Experimental data obeyed Langmuir isotherm model with 69.44 mg/g and 217.39 mg/g theoretical capacity for AL and QSLC, respectively. Thermodynamic studies indicated an exothermic behavior with a decrease in randomness. Kinetics studies showed that the adsorption process obeyed pseudo-second order model. Optimum conditions were carried out for uranium recovery from a rock sample, producing uranium concentrate with 93.33% purity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many types of ion exchange resins have been tried for separation of uranium. So far, strong base anion exchange resins (SBA) are widely used across the world to extract natural uranium (both type I and type II) [1,2,3,4]. Though some authors advocate different performance of these two resin types, there wasn’t any observed meaningful difference between them [5, 6].
In recent years, a number of strong base anion Lewatit commercial resins, such as Lewatit MP 62 (WBA) [7], Lewatit DW 630 [8], Lewatit S4528 [9], Lewatit K6362 [10], Lewatit K6267 [11], Lewatit MP-64 (WBA) [12, 13], Lewatit MonoPlus M 500 [14,15,16], Lewatit MonoPlus M 800 [5] and Lewatit MonoPlus M 600 [17] have been used for heavy metals uptake.
Lewatit MonoPlus M 500 is a strongly basic anion exchange resin containing styrene/divinylbenzene copolymer coupled with quaternary ammonium groups. The effective ion exchange capacity is due to the presence of positively charged quaternary amine groups within the polymer skeleton. It is a typical microporous anion exchanger having about 8% DVB cross-linking that provides both mechanical strength and easy diffusion of exchangeable ions [18]. Its primary structure can be represented by the following formula:

However, the exact structural formula of the resin is cross-linked, irregularly assembled and of much complicated stereostructure [11].
In the following study, the author examines the possibility of integrating the chelating capabilities of quinoline and sodium metasilicate with adsorptive capabilities of Lewatit MonoPlus M 500. The selective separation of uranium from aqueous acidic solution using a new synthesized Lewatit modified with quinoline and sodium metasilicate was studied extensively.
High-capacity composites obtained by modifying Lewatit ion-exchange resins can possess almost two fold the absorption kinetics on them [19]. Adsorption of uranium using quinoline anchored on polymeric resins has been recently studied. The synthesized chelating polymeric sorbent affinity for U (VI) was superior [20]; showing a maximum sorption capacity of 120.30 mg/g. In A study to test the efficiency of adsorption of uranyl ions by cross-linked chitosan resins modified with quinoline-8-ol moiety [21]. The retention capacity found for uranium (VI) was 218 mg/g by the modified resin.
Sodium silicate has shown excellent potential in the safe disposal of radioactive waste due to its special structure and excellent performance [22]. Unfortunately, sodium metasilicate Na2SiO3·5H2O is soluble in aqueous solution and be easily lost within the medium during batch adsorption experiments. Sodium silicate was successfully integrated with polyacrylic acid as well as tartaric acid to produce novel adsorbents tested and applied for efficient uranium uptake [23]. The advantages of silicate based adsorbents are due to the presence of various chemical entities that can be used for ion-exchange. Its low cost, availability, and environment-friendly nature gave rise to increasing interest in the development of silicate-based adsorbents of ultimate adsorption capacity [24]. Such adsorbents exhibit a quick and highly efficient adsorption behavior toward heavy metal ions [25].
This work focuses on the selective separation of uranium from aqueous acidic solution using a new synthesized composite of Lewatit modified with quinoline and sodium metasilicate. The obtained results were applied to adsorption isotherms and kinetics models. The results demonstrate that the synthesized composite has high uranium removal efficiency and can be physically separated from the treated aqueous solution.
Experimental
Instrumentation
The absorbance of uranium, SiO2, Al2O3, TiO2, and P2O5 was measured using Metertech SP-8001 UV–visible spectrophotometer. Na+ and K+ were determined by Sherwood 410 flame photometer. CaO, MgO and total iron content were determined volumetrically [26]. Trace elements and the resulted uranium concentrate from Gattar leach liquor were detected using ICP-OES [27].
The molecular functional groups of AL and QSLC were characterized using Thermo Scientific NICOLET IS10 FTIR spectrometer, before and after uranium adsorption. Scanning electron microscope (SEM) was used illustrate the surface morphology of AL and QSLC before and after uranium adsorption. AL and QSLC were characterized after uranium adsorption by CHNS elemental analysis. The stoichiometry of the constituents was determined using Philips sequent 2400 XRF; Solid samples were ground to very fine powders then mixed with polyvinyl methacrylate as a binder to facilitate the pressing process. The mixture was pressed in aluminum sample holder of 40 mm diameter by a pressing machine at 20 psi. The concentrations of Na, Mg, Al, Si, P, S, Cl, K, Ca, Fe, Rh, Zr, U and Pb were measured according to Super-Q quantitative application program.
Chemicals and reagents
Lewatit Mono-Plus M500 anion exchange resin was used to synthesize AL and QSLC. Its activity is gained from quaternary –N (CH3)+3 groups. The resin contains exchangeable Cl− ions. The large fixed porosity of the resin bead structure permits the high adsorptive capacity for large molecules. It has excellent regeneration efficiency based on cross-linked polystyrene, excellent physical stability and high operating capacity. The resin characteristics were illustrated in (Table 1).
All chemicals and reagents used are of analytical grade. HCl, H2SO4 and HNO3 were obtained from POCH S.A. N-phenyl anthranilic acid as well as Arsenazo III were obtained from Merck. Ammonium vanadate, bromine, Urea, KBr and FeSO4·7H2O were obtained from Scharlau Chemie.
Preparation of standard stock solution
A standard stock solution of 1000 mg/L of U (VI) was prepared by dissolving UO2SO4 crystals in distilled water. Several stock solutions of 1000 mg/L of possible interfering ions were prepared.
Preparation of gattar leach liquor
Studied granitic ore sample assaying 1400 mg/Kg of uranium was obtained from Gattar area, NE Desert, Egypt. Leaching factors were; time 2 h, 3 M H2SO4, − 200 mesh particle size, 25 °C and solid to liquid ratio 1:3 to verify best uranium leaching efficiency (95%). The final leach liquor contained approximately 443 mg/L of uranium. 3L of leach liquor were stirred with suitable weights of AL and QSLC. Major oxides content was determined spectrophotometrically, while trace elements were detected by ICP-OES. All data are shown in (Tables 2, 3 and 4).
Synthesis of adsorbents
Synthesis of activated lewatit AL
20 g of Lewatit Mono-Plus M500 were treated with (1:1) ethanol–water solution then 2 mL of HCl (2 M) were added for 10 h to remove any persisting monomers or impurities. The treated Lewatit was washed with distilled water and dried at 50 °C. The dried Lewatit was placed into a beaker containing 0.5 M oxalic acid and stirred for 4 h then filtered and dried at room temperature for 2 days.
Synthesis of quinoline silicate lewatit composite QSLC
After preparation of activated Lewatit, 10 g of AL were mixed with 10 g of sodium meta-silicate in 250 ml conical flask containing 50 mL of 2 M quinoline dissolved in toluene and stirred for 4 h. The obtained composite was filtered and washed with 3 M H2SO4 then dried at room temperature for 2 days.
Adsorption procedures
Factors affecting U (VI) adsorption were studied by two adsorbents AL and QSLC; pH, contact time, initial uranium concentration, adsorbent dose, temperature and interfering ions. In each experiment, 25 mL of synthetic uranium solution containing uranium concentration 50–700 mg/L were stirred at 150 rpm with 0.05 g of each adsorbent for a definite time from 5 to 60 min at different temperatures. Uranium uptake capacity (qe, mg/g) was calculated from the following relation:
where Co and Ce are the initial and uranium concentration at equilibrium (mg/L), respectively. V is volume of the aqueous solution (L) containing uranium and m is the adsorbent weight (g). The distribution coefficient Kd is calculated using Eq. (2), where; V is the volume of the aqueous phase (mL):
Batch elution procedures
Different eluting agents were studied for uranium recovery from loaded adsorbents. In each experiment, 0.05 g of loaded adsorbent was shaken with 25 mL of eluting agent of different concentrations for 30 min at 25 °C.
Uranium (VI) precipitation
The eluted solution was subjected to precipitation by adding 40% NaOH solution till reaching pH 7. Uranium was then precipitated as sodium diuranate (Na2U2O7).
Analytical procedures
Uranium (VI) was analyzed in solution by UV spectrometer using Arsenazo III as indicator at 650 nm [28]. Results were confirmed by modified Davies and Grey oxidometric titration against ammonium meta-vanadate [29, 30].
Results and discussion
Effect of initial uranium concentration
Uranium adsorption efficiency was found to be constant from 50 to 150 mg/L; (Fig. 1). It is obvious that at low uranium concentrations, the available uranyl ions are less than the number of active sites on the surface of the adsorbent. The adsorption efficiency decreased beyond 150 mg/L due to the gradual saturation of active sites. As for QSLC, uranium adsorption remained constant till 450 mg/L and then decreased gradually.
The maximum value of uranium uptake for AL was 69 mg/g at conc. 150 mg/L of U, while the maximum uptake for QSLC was 207 mg/g at conc. 450 mg/L of U (VI).
Effect of pH
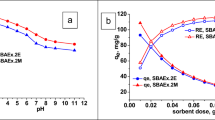
The pH of the aqueous solution can influence the aqueous chemistry of uranium [31]. The mobility of present ions in the medium is affected by concentration of H+ ions. Moreover, it also affects interest and capacity of adsorbents for target species. There is always a competition between H+ ions and other ionic species in the medium. If there are polyanionic or polycationic species in solution, interactions are fairly affected by the pH of the medium. The effect of initial solution pH was investigated by preparing a series solution having a different pH within the range 0.5 to 6, adjusted by using either 0.5 M H2SO4 and 0.5 M NaOH. 0.05 g of each adsorbent were allowed to contact with 25 mL of aqueous solution of U (VI) concentration 150 mg/L with AL and 450 mg/L with QSLC at 25 °C. Results shown in (Fig. 2a) clarifies that uranium uptake increases from pH 1 to 2.5 (69 mg/g for AL and 207 mg/g for QSLC). Beyond pH 2.5, the adsorption efficiency starts decreasing with pH elevation. The optimum pH for adsorption is 2.5.Bivalent [UO2 (SO4)2]2− anionic species and neutral uranium sulfate complex [UO2SO4] were found to be dominant lower pH using Hydra-Medusa software as shown in Fig. 2b. Uranium anionic complexes are adsorbed on the resin and their interaction may be represented as follows;
where R represents the ion-exchange sites on the resin, and X− for exchangeable chloride ions.
Effect of contact time
The effect of contacting time was investigated through 5–60 min, (Fig. 3). 0.05 g of each adsorbent was mixed with 25 mL of uranium solution at pH 2.5. From the obtained results, uranium uptake increases with increasing contact time for the first 30 min (69 mg/g for AL and 207 mg/g for QSLC), thereafter remaining almost constant.
A contact time of 30 min. was found sufficient to establish equilibrium and used in all subsequent studies.
Adsorption kinetic modeling
Kinetic parameters are helpful for the prediction of adsorption rate. The mechanism of uranium adsorption by both adsorbents was investigated according to pseudo-first order and pseudo-second order models [32,33,34]. The pseudo-first order kinetic model is represented by the following equation:
where K1 is the rate constant, qe is the amount of metal adsorbed per unit mass at equilibrium and qt is the amount adsorbed per unit time. Plotting log (qe– qt) versus t gives a straight line as shown in (Fig. 4), which provide K1 and qe from its slope and intercept, respectively. The plot diagram suggested the applicability of the pseudo-first order kinetic model to fit the practical data as given in (Table 5). The calculated value of qe was found 123.73 mg/g for AL and 346.57 mg/g for QSLC, which is far from the experimental uptake capacity (69 mg/g for AL and 207 mg/g for QSLC). The experimental data did not agree with the first order kinetic model which is not suitable for explaining the studied system.
The pseudo-second order kinetic model is represented by the following equation:
where K2 is the rate constant. The straight line of plot t/qt versus t whose slope = 1/qe and the intercept = 1/k2.q2e . Figure 5, suggested the applicability of pseudo-second order kinetic model to fit the experimental data as shown in (Table 5). The calculated value of qe was found to be 69.44 mg/g for AL and 217.39 mg/g for QSLC. The calculated values according to second order kinetic model are in agreement with experimental data and therefore it is suitable for describing the studied system.
Adsorption isotherm modeling
The Langmuir model is based on the assumption that maximum adsorption occurs as saturated monolayer of adsorbate molecules on the adsorbent surface and that the energy of adsorption is constant, with no trans-migration of adsorbate on the surface of adsorbent [35]. Langmuir isotherm model is represented by the following equation:
where Ce is the concentration at equilibrium, qe is the amount of uranium adsorbed at equilibrium; qo and b are Langmuir constants related to maximum uptake capacity and adsorption energy. The linear plot of Ce/qe versus Ce shown in (Fig. 6) and (Table 6) clarifies that the adsorption process obeys Langmuir model. The correlation coefficient for the linear regression R2 = 0.9995 for AL and 0.995 for QSLC. The slope and intercept of the straight line were used to determine qo and b as (69.44 mg/g and 0.645 g/mg) for AL and (217.39 mg/g and 0.0327 g/mg) for QSLC, respectively. Langmuir isotherm can be expressed in term of a dimensionless constant (separation factor), RL; which is defined by the equation:
where b is the Langmuir constant and Co is the initial uranium concentration. If the values of RL < 1, this indicates a favorable adsorption process, (Table 7).
The Freundlich isotherm model was applied to the adsorption as a means of data interpretation [36]. The Freundlich isotherm model is represented by the equation:
where Ce is the concentration at equilibrium, qe is the amount of uranium adsorbed at equilibrium; Kf and n are Freundlich constants corresponding to the adsorption uptake capacity and adsorption intensity, respectively. A plot of Log qe versus Log Ce is shown in (Fig. 7).
The constants Kf and n were found to be (33.24 mg/g, 8.32 for AL), and (29.55 mg/g, 2.45 for QSLC), respectively. The correlation coefficient R2 = 0.477 for AL and 0.7476 for QSLC, indicating that experimental data are better represented by Langmuir model rather than by Freundlich model (Table 6).
Effect of adsorbent dose
The effect of adsorbent dose in the range of 0.01–0.1 g was studied. Results presented on Fig. 8 show that uranium uptake increases with increasing adsorbent dose till 0.05 g then starts to decrease for both AL and QSLC. The most convenient dose was chosen as 0.05 g. At low adsorbent doses, all the active sites are entirely exposed and adsorption is encouraged till all the surface of adsorbent becomes saturated. At higher adsorbent doses, the available uranium species in bulk are not sufficient enough to fulfill the entire adsorbent empty sites [37,38,39]. The maximum uptake capacities were 69 mg/g for AL and 207 mg/g for QSLC.
Furthermore, at high adsorbent doses, the available uranium ions in solution are not
Effect of temperature
The effect of temperature on uranium uptake was studied using 0.05 g of each of the 2 adsorbents contacted with 25 mL of uranium solution of conc. 150 mg/L for AL and 450 mg/L for QSLC at pH 2.5 for 30 min. at temperature range 25–70 °C. Results shown in (Fig. 9) illustrate that uranium uptake decreased linearly from 69 to 37.5 mg/g for AL and from 207 to 90 mg/g for QSLC with increasing temperature from 25 to 70 °C. Such behavior is due to the exothermic nature of uranium uptake process. The most suitable temperature that corresponds to the most efficient uranium adsorption was considered as the room temperature.
Thermodynamic studies of uranium adsorption
Thermodynamic parameters are calculated from the following equations [40]:
where ∆G is the Gibbs free energy, ∆H is the enthalpy change and ∆S is the change in entropy. R is the universal gas constant (8.314 mol−1 k−1) and T is temp. in °K. The values of (∆H, KJ/mol) and (∆S, KJ/mol K−1) were calculated from the slope and intercept of the plot of Log Kd versus 1/T, giving a slope of 2.3677 and intercept − 7.2229 for AL and a slope of 2.7606 and intercept of − 8.5368 for QSLC, (Fig. 10).
The results mentioned in (Table 8) indicate positive value of ∆H, confirm that uranyl ions adsorption onto AL or QSLC is an exothermic process. The negative value of ∆S, indicate decrease in the randomness of the adsorption process in the investigated system with change in the hydration of the adsorbed uranyl ions. The increase values of ∆G, showed that the adsorption process is spontaneous. The increase in ∆G values from 25 to 70 °C, with increasing temperature showed that the adsorption is unfavorable at high temperatures.
Effect of interfering ions
The tested of interfering elements were chosen as they persist within the matrix of the studied leach liquor. The effect of interfering elements were separately studied by introducing each one in 25 mL of uranium solution containing 150 mg/L or 450 mg/L mixing with 0.05 g of each adsorbent AL or QSLC under optimum adsorption conditions. Obtained results shown in (Table 9).
The interfering elements can cause a decline in uranium adsorption that does not exceed 2%. The coexistence of such elements does not interfere with the adsorption process and it proves it possible to be applied successfully to extract uranium from the studied geologic sample.
Uranium (VI) elution
Desorption is an economically important parameter in adsorption processes [41]. Three mineral acids (H2SO4, HCl and HNO3) with different concentrations 0.1–1 M were tested. A volume of 25 mL of acid solution was allowed to elute uranium from 0.05 g loaded adsorbent for 20 min. Results in (Table 10) show that uranium elution efficiency increased by increasing acid concentration. 0.25 M H2SO4 solution was chosen to be the most convenient eluting agent due to its cheapness.
Characterization of AL and QSLC adsorbents
Fourier transform infrared spectrometer characterization
FTIR spectra are useful in identifying molecular functional groups [42]. Thermo Scientific Nicolet IS10 FTIR instrument was used in a range 400–4000 cm−1. The FTIR spectra of AL and QSLC before and after uranium adsorption are given in (Fig. 11).
The bands appeared at 3780.04 and 3699.33 cm−1 are attributed to OH stretching vibration of water adsorbed. Band found at 3400.21 cm−1 is related to OH stretching in carboxylic [43, 44]. The peak at 3023 cm−1 is related to Ar–H stretching. The C–H stretching in alkanes is obtained at 2925.02 cm−1. One band at 1724.98 cm−1 is related to monomer C=O in carboxylic. The peak at 1620.12 cm−1 is related to NH2 in plane bending (–NH3+). The bands at 1481.92 and 1418.64 cm−1 are attributed to Aromatic C=C in ring stretching [45, 46]. The peak at 1278.16 cm−1 is related to Ar –N stretching.
The C–N stretching in amine is obtained at 1198.15 cm−1. The peak at 979.27 cm−1 is related to N–O.The band at 888.28 cm−1 is related to=C–H alkenes out of plane. While, 825 91 cm−1 is attributed to aromatic C–H out of plane. On the other hands, two bands at 762.88 and 705.50 cm−1 are obtained due to N–H wag amines (broad) bands. While, 631.92 and 499.36 cm−1 are related to N–H oscillation (–NH3+), (Fig. 11a). On the other hand, the major contributions of AL groups have been shifted or absence due to the adsorption of uranium (Fig. 11b).
The band at 3405.03 cm−1 is related to monomer OH carboxylic. The peak at 3026.59 cm−1 is related to broad (–NH3+). Stretching band of alkane CH is related to 2924.78 cm−1. The peak observed at 1727.53 cm−1 correspond to monomer C=O in carboxylic. The peak for C=N ring stretching in quinoline is observed at 1644.18 cm−1. Two peaks are observed at 1607.96 and 1563.39 cm−1 for NH in plane bending (–NH3+). The band of Ar C–C in ring stretching is obtained at 1483.72 cm−1. The peaks at 1321.32 and 1382.08 cm−1 are related to S=O sulfate. While, 1190.72 cm−1is related to S=O sulphonyl chloride stretching band. One peak at 1045.10 cm−1 is attributed to Si–OR (broad) band. On the other hand, the band of CH out of plane is obtained at 823.44 cm−1. The peak at 823.72 cm−1 is attributed to aromatic C–H out of plane. One peak at 771.35 cm−1 is related to alkyl halides stretching. While the band at 707.54 cm−1 is related to S–OR. Two peaks are observed at 584.13 and 467.93 cm−1 for N–H oscillation (–NH3+), (Fig. 11c). On the other hand, the major contributions of QSLC groups have been shifted or absence due to the adsorption of uranium (Fig. 11d).
Scanning electron microscope (SEM)
Activated Lewatit, AL before and after uranium adsorption were tested by SEM. It’s obvious that AL with high effective surface area and high sorption capacity for uranium, as shown in (Fig. 12a, b). Quinoline Silicate Lewatit composite QSLC and after uranium adsorption were shown in (Fig. 12c, d).
Energy-dispersive X-ray spectroscopy (EDX)
EDX was used to determine the elements present in 4 different samples mentioned in Fig. 13b.
Elemental analysis (CHNS)
The CHNS elemental analysis of AL and QSLC were obtained (Table 11). The elemental analysis of QSLC showed an elevated C, H, and N content than the activated Lewatit. This confirms the successful incorporation of quinoline. The presence of S element (3.15%) in QSLC is due to the sulfuric acid washing step during the synthesis of the composite.
X-ray Fluorescence (XRF)
The two adsorbents were identified by XRF after adsorption of uranium. Figure 14 involves the appearance of silica and sodium in the composite, supporting the fact that sodium metasilicate was successfully integrated into the surface of Lewatit. The composite shows much more affinity to uranium than AL.
Uranium (VI) recovery from a geologic sample
The applied experiments were carried out under optimum conditions by mixing 3 L of leach liquor solution of concentration 443 mg/L of uranium with 2 g of QSLC and stirring 9L assaying 147 mg/L of uranium with 2 g of AL at pH 2.5 for 30 min. The obtained results revealed that uranium adsorption efficiency was 92%. The uranium (VI) loaded on AL or QSLC was eluted by 500 mL of 0.25 M H2SO4 solution. Eluted uranium was precipitated using 40% NaOH at pH 7 as sodium diuranate (Na2U2O7). The uranium concentrate was characterized by ICP-OES, XRF and EDX analysis techniques. Results are shown in (Table 12) and (Figs. 15, 16). Uranium content in the concentrate produced by AL & QSLC was 70% attaining a purity of 93.33% with a small amount of impurities.
A comparative study for uptake capacity (mg/g) of different adsorbents for uranium is shown in (Table 13).
Conclusion
Both adsorbents were comparatively used for uranium (VI) adsorption from sulfate solution. The optimum conditions of batch technique: 3 Liters solution volume assaying 443 mg/L U(VI) with 2 g of QSLC or 9 Liters assaying 147 mg/L U (VI) with 2 g of AL at pH 2.5 for 30 min. at room temperature. Under these conditions, the obtained maximum uptake capacities for each adsorbent AL and QSLC were 69.44 mg/g and 217.39 mg/g. The studied thermodynamic parameters resulted in more negative values for ∆H and ∆S for QSLC indicating more exothermic process with a decrease in randomness of the system. Values of ∆G indicate a spontaneous adsorption process. The obtained kinetic data fitted well with pseudo-second order kinetic model. Langmuir adsorption isotherm model was found more suitable for explaining the adsorption process. Uranium elution can be easily performed using 0.25 M H2SO4 acid. The eluted uranium was precipitated using 40% NaOH as sodium diuranate (Na2U2O7). The final uranium concentrate from studied sample had a uranium content of 70% with a purity of 93.33% and acceptable level of impurities.
References
Dong W, Brooks SC (2008) Formation of aqueous MgUO2(CO3)2−3 complex and uranium anion exchange mechanism onto an exchange resin. Environ Sci Technol 42:1979–1983. https://doi.org/10.1021/es0711563
Shavinskii BM (2003) Anion-exchange recovery of thorium from uranium: analytical and preparation aspects. Radiochemistry 45:146–148. https://doi.org/10.1023/A:1023877008197
Wen Z, Huang K, Niu Y et al (2020) Kinetic study of ultrasonic-assisted uranium adsorption by anion exchange resin. Colloids Surf A 585:124021. https://doi.org/10.1016/j.colsurfa.2019.124021
Wódkiewicz L, Dybczyński R (1972) Effect of resin cross-linking on the anion-exchange separation of rare earth complexes with DCTA. J Chromatogr A 68:131–141. https://doi.org/10.1016/S0021-9673(00)88770-1
Rychkov VN, Smirnov AL, Gortsunova KR (2014) Sorption of uranium from underground leaching solutions with highly basic anion exchangers. Radiochemistry 56:38–42. https://doi.org/10.1134/S1066362214010081
Stoliker DL, Kaviani N, Kent DB, Davis JA (2013) Evaluating ion exchange resin efficiency and oxidative capacity for the separation of uranium(IV) and uranium(VI). Geochem Trans 14:1. https://doi.org/10.1186/1467-4866-14-1
Riegel M, Tokmachev M, Hoell WH (2008) Kinetics of uranium sorption onto weakly basic anion exchangers. React Funct Polym 68:1072–1080. https://doi.org/10.1016/j.reactfunctpolym.2008.02.009
Kowalczyk M, Hubicki Z, Kołodyńska D (2013) Removal of heavy metal ions in the presence of the biodegradable complexing agent of EDDS from waters. Chem Eng J 221:512–521. https://doi.org/10.1016/j.cej.2013.02.010
Riegel M, Schlitt V (2017) Sorption dynamics of uranium onto anion exchangers. Water 9:268. https://doi.org/10.3390/w9040268
Koodynska D, Hubicki Z (2012) Investigation of sorption and separation of lanthanides on the ion exchangers of various types. In: Kilislioglu A (ed) Ion exchange technologies. InTech
Pehlivan E, Cetin S (2009) Sorption of Cr(VI) ions on two Lewatit-anion exchange resins and their quantitative determination using UV–visible spectrophotometer. J Hazard Mater 163:448–453. https://doi.org/10.1016/j.jhazmat.2008.06.115
Galán B, Calzada M, Ortiz I (2006) Separation and Concentration of Cr(VI) from ground waters by anion exchange using lewatit MP-64: mathematical modelling at acidic pH. Solv Extr Ion Exchange 24:621–637. https://doi.org/10.1080/07366290600762413
Purkayastha D, Mishra U, Biswas S (2014) A comprehensive review on Cd(II) removal from aqueous solution. J Water Process Eng 2:105–128. https://doi.org/10.1016/j.jwpe.2014.05.009
Khawassek Y (2017) Anion exchange of uranium from sulfuric acid solution: adsorption and kinetics characteristics. In: ALTA 2017 URANIUM-REE SESSIONS. https://www.researchgate.net/publication/328062508_ANION_EXCHANGE_OF_URANIUM_FROM_SULFURIC_ACID_SOLUTION_ADSORPTION_AND_KINETICS_CHARACTERISTICS. Accessed 23 Dec 2019
Orabi AH, Rabia K, Elshereafy E, Salem A (2017) Application of commercial adsorbent for rare earth elements—uranium mutual separation and purification. MediterrJChem 6:238. https://doi.org/10.13171/mjc66/01712211014-orabi
Afifi SY, Elashry SM, Abo-Aly MM (2017) Alkaline leaching for recovery of uranium and copper from calcareous shale, um bogma formation, g. allouga, southwestern sinai. Egypt Arab J Nucl Sci Appl 50:213–228
Kołodyńska D, Hubicki Z, Skiba A (2009) Heavy metal ions removal in the presence of 1-hydroxyethane-1,1-diphosphonic acid from aqueous solutions on polystyrene anion exchangers. Ind Eng Chem Res 48:10584–10593. https://doi.org/10.1021/ie901195j
Kadous A, Didi MA, Villemin D (2011) Removal of uranium(VI) from acetate medium using Lewatit TP 260 resin. J Radioanal Nucl Chem 288:553–561. https://doi.org/10.1007/s10967-010-0970-1
Wołowicz A, Hubicki Z (2012) The use of the chelating resin of a new generation Lewatit MonoPlus TP-220 with the bis-picolylamine functional groups in the removal of selected metal ions from acidic solutions. Chem Eng J 197:493–508. https://doi.org/10.1016/j.cej.2012.05.047
Liu Y, Cao X, Le Z et al (2010) Pre-concentration and determination of trace uranium (VI) in environments using ion-imprinted chitosan resin via solid phase extraction. J Braz Chem Soc 21:533–540. https://doi.org/10.1590/S0103-50532010000300020
Praveen R, Metilda P, Daniel S, Rao T (2005) Solid phase extractive preconcentration of uranium(VI) using quinoline-8-ol anchored chloromethylated polymeric resin beads. Talanta 67:960–967. https://doi.org/10.1016/j.talanta.2005.04.019
Kouraim MN, Hagag MS, Ali AH (2019) Sorption of uranium from radioactive wastes by silicate-neutralised polyacrylic. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2019.1641600
Sartore L, Dey K (2019) Preparation and heavy metal ions chelating properties of multifunctional polymer-grafted silica hybrid materials. Adv Mater Sci Eng 2019:1–11. https://doi.org/10.1155/2019/7260851
Massey MS, Lezama-Pacheco JS, Nelson JM et al (2014) Uranium incorporation into amorphous silica. Environ Sci Technol 48:8636–8644. https://doi.org/10.1021/es501064m
Zhang S, Li J, Wen T et al (2013) Magnetic Fe3O4@NiO hierarchical structures: preparation and their excellent As(v) and Cr(vi) removal capabilities. RSC Adv 3:2754. https://doi.org/10.1039/c2ra22495j
Shapiro L, Brannock WW (1962) Rapid analysis of silicate, carbonate, and phosphate rocks. US Geological Survey Bulletin 114A (Revised Edition): https://doi.org/10.3133/b1144A
Kuppusami Govindaraju, Guy Mevelle, Charles Chouard (1976) Automated optical emission spectrochemical bulk analysis of silicate rocks with microwave plasma excitation. Anal Chem 48:1325–1331. https://doi.org/10.1021/ac50003a018
Marczenko Z, Balcerzak M (2000) Principles of Spectrophotometry. In: Analytical spectroscopy library. Elsevier, pp 26–38
Davies W (1964) A rapid and specific titrimetric method for the precise determination of uranium using iron(II) sulphate as reductant. Talanta 11:1203–1211. https://doi.org/10.1016/0039-9140(64)80171-5
Mathew KJ, Bürger S, Vogt S et al (2009) Uranium assay determination using Davies and Gray titration: an overview and implementation of GUM for uncertainty evaluation. J Radioanal Nucl Chem 282:939–944. https://doi.org/10.1007/s10967-009-0186-4
Cheira MF, Atia BM, Kouraim MN (2017) Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO4 resin: kinetic, equilibrium and thermodynamic studies. J Radiat Res Appl Sci 10:307–319. https://doi.org/10.1016/j.jrras.2017.07.005
Cheira MF, Orabi AS, Atia BM, Hassan SM (2018) Solvent extraction and separation of thorium(iv) from chloride media by a schiff base. J Solution Chem 47:611–633. https://doi.org/10.1007/s10953-018-0740-1
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18:1501–1507. https://doi.org/10.1016/0043-1354(84)90124-6
Weber J, Morris JC, Weber W et al (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div A Soc Civil Eng 89:31–60
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Freundlich H (1907) Adsorption in solution. Zeitschrift für Physikalische Chemie. https://doi.org/10.1515/zpch-1907-5723
Das DP, Das J, Parida K (2003) Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTlc) towards removal of fluoride from aqueous solution. J Colloid Interface Sci 261:213–220. https://doi.org/10.1016/S0021-9797(03)00082-1
Liao X, Shi B (2005) Adsorption of Fluoride on Zirconium(IV)-Impregnated Collagen Fiber. Environ Sci Technol 39:4628–4632. https://doi.org/10.1021/es0479944
Chaudhary N, Balomajumder C (2014) Optimization study of adsorption parameters for removal of phenol on aluminum impregnated fly ash using response surface methodology. J Taiwan Inst Chem Eng 45:852–859. https://doi.org/10.1016/j.jtice.2013.08.016
Atia BM, Gado MA, Abd El-Magied MO, Elshehy EA (2019) Highly efficient extraction of uranyl ions from aqueous solutions using multi-chelators functionalized graphene oxide. Sep Sci Technol. https://doi.org/10.1080/01496395.2019.1650769
Kannamba B, Reddy KL, AppaRao BV (2010) Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J Hazard Mater 175:939–948. https://doi.org/10.1016/j.jhazmat.2009.10.098
Dong J, Ozaki Y (1997) FTIR and FT-raman studies of partially miscible poly(methyl methacrylate)/poly(4-vinylphenol) blends in solid states. Macromolecules 30:286–292. https://doi.org/10.1021/ma9607168
Khalifa ME (1998) Selective separation of uranium using alizarin red S (ARS)-modified anion-exchange resin or by flotation of U-ARS chelate. Sep Sci Technol 33:2123–2141. https://doi.org/10.1080/01496399808545719
Sid Kalal H, Panahi HA, Hoveidi H et al (2012) Synthesis and application of Amberlite xad-4 functionalized with alizarin red-s for preconcentration and adsorption of rhodium (III). J Environ Health Sci Engineer 9:7. https://doi.org/10.1186/1735-2746-9-7
Anirudhan TS, Rijith S (2012) Synthesis and characterization of carboxyl terminated poly(methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium(VI) from aqueous media. J Environ Radioact 106:8–19. https://doi.org/10.1016/j.jenvrad.2011.10.013
Atrees MS, Metwally E, Demerdash M, Salem H (2016) Sorption behavior of Pr and Nd upon chitosan benzoyl thiourea derivatives. J Radiat Res Appl Sci 9:207–216. https://doi.org/10.1016/j.jrras.2015.02.004
Solgy M, Taghizadeh M, Ghoddocynejad D (2015) Adsorption of uranium(VI) from sulphate solutions using Amberlite IRA-402 resin: equilibrium, kinetics and thermodynamics study. Ann Nucl Energy 75:132–138. https://doi.org/10.1016/j.anucene.2014.08.009
Massoud A, Masoud AM, Youssef WM (2019) Sorption characteristics of uranium from sulfate leach liquor by commercial strong base anion exchange resins. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06770-9
Semnani F, Asadi Z, Samadfam M, Sepehrian H (2012) Uranium(VI) sorption behavior onto amberlite CG-400 anion exchange resin: effects of pH, contact time, temperature and presence of phosphate. Ann Nucl Energy 48:21–24. https://doi.org/10.1016/j.anucene.2012.05.010
Ladeira ACQ, Gonçalves CR (2007) Influence of anionic species on uranium separation from acid mine water using strong base resins. J Hazard Mater 148:499–504. https://doi.org/10.1016/j.jhazmat.2007.03.003
Mattigod SV, Golovich EC, Wellman DM, et al (2010) Uranium Adsorption on Ion-Exchange Resins—Batch Testing
Kosari M, Sepehrian H (2016) Uranium Removal from Aqueous Solution Using Ion-Exchange Resin DOWEX® 2x8 in the Presence of Sulfate Anions. Int J Eng 29:1677–1683
Cheira FM, El-Didamony AM, Mahmoud FK, Atia BM (2014) Equilibrium and kinetic characteristics of uranium recovery by the strong base ambersep 920U Cl resin. IOSRJAC 7:32–40. https://doi.org/10.9790/5736-07533240
Balanovsky NV, Koshcheeva AM, Cherednichenko AG (2016) Synthesis and properties of strongly basic acrylate polyfunctional anion-exchange resin for uranium extraction. Moscow Univ Chem Bull 71:336–340. https://doi.org/10.3103/S0027131416050023
Khawassek YM, Eliwa AA, Haggag EA et al (2017) Equilibrium, kinetic and thermodynamics of uranium adsorption by ambersep 400 SO4 resin. Arab J Nucl Sci Appl 50:100–112
Venkatesan KA, Shyamala KV, Antony MP et al (2008) Batch and dynamic extraction of uranium(VI) from nitric acid medium by commercial phosphinic acid resin, Tulsion CH-96. J Radioanal Nucl Chem 275:563–570. https://doi.org/10.1007/s10967-007-6888-6
Acnowledgements
This work was funded by the Nuclear Materials Authority as a part of its research activities. This article was reviewed and approved for publishing by the Nuclear Materials Authority with no obligation on the authors’ part to revise the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, A.A. Kinetics of uranium adsorption from sulfate medium by a commercial anion exchanger modified with quinoline and silicate. J Radioanal Nucl Chem 324, 1387–1403 (2020). https://doi.org/10.1007/s10967-020-07169-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07169-7