Abstract
The variable values of 226Ra, 228Ra and 40K were identified in coal and coal combustion residuals (CCR) samples to redistribute radionuclides using 228Ra/226Ra activity ratios in CCRs and compared to their values in the corresponding feed coal. NORM concentrations in CCRs were found to be 6–12 times higher than the original coal. The effective dose rates in the original coal were calculated and ranged from 14.9 ± 0.9 to 370.3 ± 22.2 μSv year−1, whereas in CCRs ranged from 257.5 ± 20.6 to 1797.5 ± 143.8 μSv year−1. The average concentration of 40K (120 Bq kg−1 per 1% K2O in CCRs) was calculated. The chemical composition indicates that the majority of CCR samples are Type C, which has a high calcium oxide ratio, high melting points and low deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the increased thermal power from coal combustion, however, residues often contain high radioactive concentrations as natural occurring radioactive materials (NORM) and are used in the manufacture of certain building materials such as cement, concrete and bricks [1]. Since the 1960s, coal and CCR radioactivities have been identified [2, 3], due to the longevity of the common NORM (238U, 226Ra, 210Pb, 232Th, 228Ra, and 40K) [4,5,6]. Coal is categorized according to the concentrations of NORM, either high concentrations of NORM at low concentrations of sub-bituminous, brown and lignite, or low concentrations of NORM at high concentrations of bituminous [7, 8].
Some hazardous trace elements such as cadmium (Cd), arsenic (As), molybdenum (Mo), vanadium (V), mercury (Hg) and various acid gasses are detected during coal burning [9]. Several countries used the CCRs to manufacture Portland cement [10] and concrete [11] as allowed by the European Standard EN 197-1. There are 27 different common products of cement classified into five categories. Composite cement is the fifth category containing 18–50% of coal fly ash. A reference level (1 mSv year−1) [12] has been determined for external indoor exposure owing to gamma radiation emitted by building materials [13]. Many industrial residues are presently being studied as prospective construction materials [14].
On the other hand, European waste has studied and identified the chemical composition of coal [15, 16] and CCRs as non-hazardous waste [17, 18]. Coal contains predominantly carbon with variable quantities of other elements; primarily hydrogen, oxygen, sulfur and nitrogen [16]. The chemical composition of coal has been converted into CO2 and CH4 by breaking chemical bonds from hemicellulose, cellulose and most biomass and has lost up to 70% of the weight at 550 °C. Unburnt alkaline sulfate particles are formed at an elevated temperature and gain comparative weight. The elements of low melting point may agglomerate to form clusters of particles of high melting point [16]. Most of the carbon was eliminated at temperatures above 550 °C, increasing the proportions of elements with high melting points. Carbon values usually ranged 15–25%, whereas oxygen remains in the form of oxides, and slightly increased carbonates and sulfates [16]. The high peaks of silicon in coal (40%) compared to other elements. Ironically, K and Ca are the main elements contained in the slag as well as the first components of most coal samples exceeding 15%, while Mg values are nearly constant 3–5% values.
Electricity production in Kingdom of Saudi Arabia (KSA) depends on the main sources of energy are natural gas and petroleum. Domestic power generation demand is set to increase and looks forward to supplying it from the coal and renewable energy. Some countries of the Gulf Cooperation Council (GCC) used coal for coal-fired plants, but not so far in KSA. Several factors point to the possibility of using coal as a prospective source of electricity and cement production in KSA, such as lower operating costs and higher global reserves (990 billion tons of current consumption, sufficient for 150 years) [19]. Coal is expected to quickly play an important role in the electricity and cement sector in Saudi Arabia. Coal is currently used for narghile (hookah, shisha, goza) and grill of different types of meat, many Arab countries use coal as fuel for smoking tobacco. The present study is therefore a pre-emptive step for KSA to select high-quality coal by measuring NORM and chemical compositions of various kinds of coal on the local market and calculating adsorbed dose (nGy h−1) and effective dose (mSv year−1) before and after coal combustion.
Experimental
Samples preparation
Twenty-seven coal samples were purchased from the local market of the Kingdom of Saudi Arabia, and prepared for gamma analysis and elemental analysis. Eighteen samples were burned in order to analyze the CCRs (slag by-products and remaining solids). The samples were homogenized and packed with standard size cups (75 ml) and tightly sealed, then stored for more than a month to reach secular equilibrium of 226Ra and to prevent radon gas escape. In order to determine efficiency calibration, reference material (RGU-1) was also packed into the same standard size cups. Gamma-analysis was used to measure the samples and reference material.
Analytical techniques
Gamma-spectrometry analysis
A coaxial P-type (relative efficiency 50%) high purity germanium detector (HPGe) was used. A low level background due to the existence of a lead shield to protect the detector. The RGU-1 was used as a reference material for the HPGe detector efficiency calibration. It was verified that radium-226 is in equilibrium with uranium content. The certified activity of uranium is 400 ppm, which refers to 4960 Bq kg−1. To create the efficiency calibration curve, the 226Ra daughters (214Pb and 214Bi) were used. A polynomial fitting of the fourth degree was performed to obtain the best R2 value (≈ 0.997).
Radioisotopes 226Ra and 228Ra explained that concentrations of radioactivity ranged from below the detection limit to 13.8 and 17 Bq kg−1, respectively, while 40K ranged from 40.0 to 553 Bq kg−1 (53.0% potassium content).
Radium-226 was measured by achieving secular equilibrium with its daughter 214Pb (t1/2 = 26.9 min) at 295.2 keV (Iγ = 19.3%), 351.93 keV (Iγ = 37.6%), and 214Bi (t1/2 = 19.7 min) at 609.31 keV (Iγ = 46.1%), 1120.29 keV (Iγ = 15.1%), 1764.49 keV (Iγ = 15.4%). Radium-228 was determined at 911.2 keV (Iγ = 25.8%) by measuring the 228Ac (t1/2 = 6.15 h).
Potassium-40 was estimated at Eγ = 1490.83 keV, Iγ = 11%. The efficiency of each transition line was either determined by direct comparison of the energy lines in the reference material or obtained from the above-mentioned efficiency calibration curve.
The reference sample and the measured samples subtracted the background of each energy line. The detection limit based on the Currie detection limit method was calculated as follows:
The detection limit for each radionuclide was calculated individually. The calibration was tested against IAEA proficiency and no more than 5% bias was found.
XRF analysis
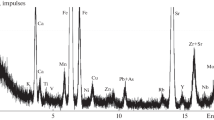
The JSX-3222 analyzer, Japan (type JEOL) is an energy-dispersive X-ray fluorescent spectrometer used to evaluate the constituent elements of coal consumed by KSA. Samples were assessed using loose powders were prepared for XRF that is one of the most important, steps in achieving accuracy. The range of elements measured ranges from sodium (Na) to uranium (U).
Results and discussion
Radioactive analysis
The classification of coal consumed in Saudi Arabia based on radioactivity concentrations is shown in Table 1. The 226Ra and 228Ra were indirectly determined by measuring 214Bi and 214Pb as daughters of 226Ra, 228Ac and 212Pb as daughters of 228Ra [20]. Table 1 shows total radioactivity of RaAC in CCRs 6–12 times higher than the total radioactivity of RaBC in feed coal, which may be attributed to the removal of approximately 10–15% of carbon during the coal burning [21]. The average values of the 226Ra, 228Ra and 40K before the burning of the coal are 6, 11 and 138 Bq kg−1, respectively, whereas the post-burning values are 30, 50 and 777 Bq kg−1. By comparing our data with the literature before coal burning, the average values of 226Ra are found to be relatively lower than the literature values (16.2 Bq kg−1) [9], but agree with the literature values of 228Ra and 40K (13.1 and 133.7 Bq kg−1, respectively) [9]. After the coal burning, the average value of 226Ra in our data is still lower than the literature value (55 Bq kg−1), our data are consistent with the literature data for 228Ra (50 Bq kg−1) and higher than the value of 40K (433 Bq kg−1) [9]. The low concentration of 226Ra may be due to uranium-238 (226Ra parent) found mainly in sedimentary rock organic layer materials, which can be easily removed during the combustion process, while 232Th (228Ra parent) occurs in inorganic layers [22]. On the other hand, burning organic matter leads to an increase in natural radionuclide concentrations in CCRs depending on the physical and chemical composition of coal as well as the circumstances of the combustion process and can be attributed to radionuclide accumulation in the form of oxides in CCRs [22].
Redistribution of radionuclides in coal and CCRs was investigated by calculating the proportions of 228Ra/226Ra, which are relatively higher in CCRs than the feed coal as shown in Table 2. The 228Ra/226Ra ratios in CCRs and feed coal are consistent with the ratios reported in literature [6, 21, 23]. The 238U and 232Th are found in radioactive equilibrium (secular equilibrium) with their decay products (their daughters) and expressed as the ratios 238U/226Ra and 232Th/228Ra equivalent to the unit in the closed system [21, 24]. The average ratio of 228Ra/226Ra in soil is supposed to be about 1.2 equal to the average Th/U activity ratio in continental crust. In CCRs, the average total radium radioactivity was estimated and found to be about 100 Bq kg−1, which is 1.5 times higher than in the soil (about 70 Bq kg−1). Most coal samples have a ratio of 228Ra/226Ra greater than one, meaning that the concentration of 226Ra is lower than concentration of 228Ra except for some samples such as Orinex, King Sford, Toppy and Earthquake. The 228Ra/226Ra ratios in CCR samples were slightly increased relative to their values in the feed coal indicating that the 226Ra removal due to the combustion process is lower than 228Ra.
Absorbed dose
According to the European Radiation Protection Guide 122 [25], for the materials to be used to make any use limitation decision, a dose assessment of the situation should be carried out [25]. A scenario for calculating the effective annual dose of coal radioactivity using the Markkanen model was proposed [26]. The conversion factor used to calculate the absorbed gamma dose rate D (nGy h−1) for 226Ra, 232Th and 40K corresponds to 0.92, 1.1 and 0.08 nGy h−1 per Bq kg−1, respectively [25,26,27] as in Eq. (1)
Table 3 indicates the absorbed dose rates in feed coal and CCRs range from 3 ± 0.2 to 75.6 ± 3.8 and from 40.7 ± 2.9 to 366.8 ± 25.7 nGy h−1 with the average of 19.5 and 165 nGy h−1, respectively, whereas the average worldwide value (55 nGy h−1) [25, 28]. The conversion coefficient (0.7 Sv Gy−1) was used to calculate the annual effective dose rates as the following equation:
The effective dose rates calculated in feed coal ranged from 14.9 ± 0.9 to 370.3 ± 22.2 μSv year−1, while from 257.5 ± 20.6 to 1797.5 ± 143.8 μSv year−1 ranged in CCRs that higher than those reported in the literature resulting of terrestrial radionuclides (460 μSv year−1) [29, 30].
Chemical composition
The proportions of the chemical constituents in the feed coal and CCRs were determined using the XRF technique as shown in Table 4. The variable element ratios depend on the physical and chemical properties of coal, which form metal oxides in CCRs after the feed coal burning in oxygen [16]. It is notable that the average concentration of silicon, potassium, calcium and iron in feed coal is 13, 11, 49 and 16%, respectively, as shown in Table 4. The low concentrations of elements Al, Ti, Mn, Zn, Rb, Sr and Zr ranged from less than 1 to 5% in the feed coal. Most CCR samples contain Si, K and Ca with an average concentration of 18, 9% and 49%, respectively. Zn, Rb, and Zr ratios are almost constant at around 1% for most samples, while Ti ratios range from 1 to 3%.
In this study, potassium is the only element could be measured by XRF and gamma analysis before and after the combustion to determine the volatilization ratio and the enrichment factor (Ef). The potassium enrichment factor was calculated by dividing 40K radioactivity in CCRs on its radioactivity in feed coal as in Eq. (3).
Where, the specific activities of 40K in CCRs and their feed coal represent \((A_{{40_{\text{K}} }} )_{\text{CCRs}}\) and \((A_{{40_{\text{K}} }} )_{\text{Coal}}\). The majority of coal has a higher potassium volatilization rate than its enrichment, as shown in Fig. 1 as in Bamboo charcoal, where the volatilization rate is 53% compared to the 6% enrichment factor. These results may be due to potassium has a low melting point (63 °C) as an alkali metal, making it more volatile and increasing the rate of its evaporation during the process of combustion. Table 5 indicates that the average concentration of 40K in CCRs was found to be 120 Bq kg−1 per 1% K2O, a half-value (252 Bq kg−1) reported by Mohanty in 2004 [31].
The base/acid ratio (Rb/a) calculated by Eq. (4) is shown in Table 6 (Electronic Supplementary Material):
Furthermore, the ratio of silica/alumina (S/A), iron/calcium (I/C), slagging index (Rs) and slag viscosity (SR) was determined using Eqs. (5–8).
As shown in Table 6 (Electronic Supplementary Material), it is generally expected that slag deposition (Rb/a) will be achieved. Figure 2 shows that most CCRs (ash samples) contain more than 50% CaO and less than 30% SiO2 with more than 15% K2O. The ternary diagram in Fig. 3 classified most CCR samples as low acidity and indicates type C, which has a high calcium oxide ratio and is anticipated to have high melting temperatures and small deposits due to high concentration of Ca [16]. S and K types describe Super Kima and King Sford, respectively, which have a high risk of deposition owing to the formation of silicates and presence of potassium [16].
Conclusion
The radioactivity of total Ra and 40K in CCRs is 6–12 times higher than in the feed coal due to the removal of 10–15% carbon. Before burning, most coal samples have the ratios of 226Ra/228Ra close to the soil ratio (1.2). Radionuclides were measured in CCR samples to study the redistribution of these radionuclides and found that the majority of the samples had a concentration of 226Ra less than 228Ra, resulting the ratios of 228Ra/226Ra are relatively higher in CCRs than feed coal except for a small number of these samples. The effective annual dose rates were calculated and found to be 370.3 and 1797.5 μSv year−1 for coal before and after burning, respectively. XRF was used to determine the proportions of Al, Si, K, Ca, Mn, Fe, Sr and Rb coal composition with variable concentrations obtained before and after the combustion process. The average concentration of 40K (120 Bq kg−1 per 1% K2O in CCRs) concentration was calculated and its lower value was observed by half as compared to the literature value. Most CCR samples are low acidity and represent type C, which has a high calcium oxide ratio and is anticipated to have a high melting temperature and low deposits owing to high concentrations of Ca.
References
Trevisi R, Risica S, D’Alessandro M, Paradiso D, Nuccetelli C (2012) Natural radioactivity in building materials in the European Union: a database and an estimate of radiological significance. J Environ Radioact 105:11–20
Tadmor J (1986) Radioactivity from coal-fired power plants: a review. J Environ Radioact 4(3):177–204
Eisenbud M, Petrow HG (1964) Radioactivity in the atmospheric effluents of power plants that use fossil fuels. Science 144(3616):288–289
Coles DG, Ragaini RC, Ondov JM (1978) Behavior of natural radionuclides in western coal-fired power plants. Environ Sci Technol 12(4):442–446
Völgyesi P, Kis Z, Zs Szabó, Cs Szabó (2014) Using the 186-keV peak for 226Ra activity concentration determination in Hungarian coal-slag samples by gamma-ray spectroscopy. J Radioanal Nucl Chem Lett 302:375–383
Beck HL, Gogolak C, Miller K, Lowder WM (1980) Perturbations on the natural radiation environment due to the utilization of coal as an energy source. U.S. Department of Energy, Washington
Zielinski RA, Budahn JR (1998) Radionuclides in fly ash and bottom ash: improved characterization based on radiography and low energy gamma-ray spectrometry. Fuel 77(4):259–267
Fardy J, McOrist G, Farrar Y (1989) Neutron activation analysis and radioactivity measurements of Australian coals and fly ashes. J Radioanal Nucl Chem Lett 133(2):217–226
Bem H, Wieczorkowski P, Budzanowski M (2002) Evaluation of technologically enhanced natural radiation near the coal-fired power plants in the lodz region of Poland. J Environ Radioact 61(2):191–201
Sanjuán MA, Argiz C (2012) The new European standard on common cements specifications EN 197-1:2011. Mater Constr 62:425–430
Argiz C, Menéndez E, Moragues A, Sanjuan MA (2015) Fly ash characteristics of Spanish coal-fired power plants. Afinidad 572:269–277
Council Directive 2013/59/Euratom of 5 Dec. (2013) Laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation and repealing directives 89/618/Euratom. 90/641/Euratom. 96/29/Euratom. 97/43/Euratom and 2003/122/Euratom. L13, vol 57. ISSN: 1977-0677
Schroeyers W, Puertas F, Alonso M, Torres Carrasco M, Rivilla P, Gasco C, Trinidad JA, Suarez JA, Navarro N, Yague L, Mora JC, Orellana JG, Masque P, Hierro A, Bolıvar JP, Vazquez M, Quintana B (2015) In: Verdu´ G (ed) Introduction of the COST Action: COST TU1301 ‘‘NORM4-building’’. 48 Joint Congress 20 SEFM/15 SEPR (Spanish Society for Radiological Protection. Ed.) Valencia. Spain
Puertas F, García-Díaz I, Palacios M, Gazulla MF, Gómez MP, Orduña M (2010) Clinkers and cements obtained from raw mix containing ceramic waste as a prime material. Characterization, hydration and leaching studies. Cem Concr Compos 32:175–186
Sanjuan MA, Quintana B, Argiz C (2019) Coal bottom ash natural radioactivity in building materials. J Radioanal Nucl Chem Lett 319:91–99
García R, Pizarro C, Álvarez A, Lavín AG, Bueno JL (2015) Study of biomass combustion wastes. Fuel 148:152–159
Carlson CL, Adriano DC (1993) Environmental impacts of coal combustion residues. J Environ Qual 22:227–247
Ural S (2005) Comparison of fly ash properties from Afsin-Elbistan coal basin, Turkey. J Hazard Mater B119:85–92
Bundesanstalt für Geowissenschaften und Rohstoffe – Federal Institute for Geosciences and Natural Resources (BGR) (2009) Reserves, resources and availability of energy resources—annual report 2009, BGR, Hannover, Germany. www.bgr.bund.de
Puertas F, Alonso MM, Torres-Carrasco M, Rivilla P, Gasco C, Yagüe L, Suárez JA, Navarro N (2015) Radiological characterization of anhydrous/hydrated cements and geopolymers. Constr Build Mater 101:1105–1112
Lauer NC, Hower JC, Hsu-Kim H, Taggart RK, Vengosh A (2015) Naturally occurring radioactive materials in coals and coal combustion residuals in the United States. Environ Sci Technol 49(18):11227
Pandit GG, Sahu SK, Puranik VD (2011) Natural radionuclides from coal fired thermal power plants—estimation of atmospheric release and inhalation risk. Radioprotection 46(6):S173–S179
Swanson VE (1976) Collection, chemical analysis, and evaluation of coal samples in 1975. U.S. Department of the Interior, Geological Survey, Washington
Allam KhA, Ahmed Z, El-Sharkawy S, Salman A (2017) Analysis and statistical treatment of 238U series isotopic ratios using gamma-ray spectrometry in phosphate samples Radiat. Prot Environ 40(3&4):110
European Commission (1999) Radiation protection 122-radiological protection principles concerning the natural radioactivity of building materials. Directorate General Environment. Nuclear Safety and Civil Protection. https://ec.europa.eu/energy/sites/ener/files/documents/112.pdf. Accessed 27 Jul 2018
Markkanen M (1995) Radiation dose assessments for materials with elevated natural radioactivity. Report STUK-B-STO 32. Radiation and Nuclear Safety Authority-STUK. Helsinki. Iceland
Stojanovska Z, Nedelkovski D, Ristova M (2010) Natural radioactivity and human exposure by raw materials and end product from cement industry used as building materials. Radiat Meas 45:969–972
UNSCEAR (1998) Sources, effects and risk of ionizing radiation. United Nations, New York
UNSCEAR (1993) Exposure from natural sources of radiation. United Nations, New York
Yang Y (2005) Radioactivity concentrations in soils of the Xiazhuang granite area, China. Appl Radiat Isot 63:255–259
Mohanty AK, Sengupta D, Das SK, Saha SK (2004) Natural radioactivity and radiation exposure in the high background area at Chatrapur beach placer deposit of Orissa, India. J Environ Radioact 75:15–33
Xiao R, Chen X, Wang F, Yu G (2011) The physicochemical properties of different biomass ashes at different ashing temperature. Renew Energy 36:244–249
Bridgeman TG, Darvell LI, Jones JM, Williams PT, Fahmi R, Bridgewater AV et al (2007) Influence of particle size on the analytical and chemical properties of two energy crops. Fuel 86:60–72
Fryda L, Sobrino C, Glazer M, Bertrand C, Cieplik M (2012) Study of ash deposition during coal combustion under oxyfuel conditions. Fuel 92:308
Vamvuka D, Pitharoulis M, Alevizos G, Repouskou E, Pentari D (2009) Effects during combustion of lignite/biomass blends in fluidized bed. Renew Energy 34:2662
Acknowledgements
This research was funded by Deanship of Scientific Research, Princess Nourah bent Adulrahman University through the Fast-track Research Funding Program. The authors would also like to thank editor-in-chief of Journal of Radioanalytical and Nuclear Chemistry and referees for their valuable comments and cooperation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aloraini, D.A., El-Azony, K.M. Measurement of natural radioactivity concentrations and chemical composition of coal and its post-combustion residues in KSA. J Radioanal Nucl Chem 323, 885–895 (2020). https://doi.org/10.1007/s10967-019-07001-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-07001-x