Abstract
Tritium concentration was monitored in different water sources collected around Kaiga Nuclear Power plant, India. The concentration was in the ranges < 1.9–27.4 Bq L−1 (GM = 4.0 Bq L−1) for groundwater, < 1.9–42.1 Bq L−1 (GM = 3.5 Bq L−1) for surface water and in 12.4–42.0 Bq L−1 (GM = 24.07 Bq L−1) for reservoir water. The concentration values observed in this study are similar to those reported for other PHWR stations of the world. The radiation dose to the public due to ingestion of Tritium through groundwater was computed to be 0.08 μSvy−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tritium (3H) is a radioactive isotope of hydrogen and has a half-life of 12.3 years; it decays to 3He by emitting low energy beta radiation with an average energy of 5.7 keV (maximum energy = 18.6 keV). Tritium is produced continuously by nuclear reactions that occur naturally in the environment due to the interaction of high energy cosmic rays with oxygen and nitrogen atoms in the upper atmosphere [1]. These processes produce most of the world’s natural Tritium. In the upper atmosphere, Tritium converts into water and reaches the earth’s surface though rain and other processes of deposition. In addition to the natural sources, it can also be produced in nuclear power plants (NPPs) during the fission of heavy nuclei and by neutron interaction with coolants, moderators, and some light elements (such as lithium, beryllium, and boron), and can be released to the environment in small quantities during the normal operation of the power reactor [2].
Chemically, Tritium behaves similar to hydrogen. It can exist in a gaseous state or, more commonly, in the form of water. Tritium atoms have the tendency to replace one of the stable hydrogen atoms in water, thus becoming a part of the water molecule, and forming tritiated water with the chemical formula HTO or T2O [3]. This radionuclide is of ecological interest because it can be rapidly transported through environmental pathways and taken up by organisms [4]. During metabolic activities, a portion of the HTO incorporates into the organic molecules, including plant structural material or soil organic matter [5]. Hence, monitoring the environment around NPPs is important to ensure that the Tritium levels are well within the permissible limits (10,000 Bq L−1, WHO 2004).
In India, as part of the systematic waste management programme of the NPPs, all potential radioactive liquid wastes are collected and segregated, depending upon the activity levels. The low-level liquid waste is diluted and dispersed to the environment. Before release, the effluent activity levels are monitored to ensure that the concentration and total discharge are well within the limits specified by the Atomic Energy Regulatory Board (AERB). As a matter of practice, the releases from the plant are much below the permissible limit so that the impact to members of the public is minimal [6]. Although the Environmental Survey Laboratories of the NPPs conduct systematic environmental monitoring around a nuclear plant, it is important that external audit of the environmental impact assessment are performed by autonomous institutions from outside the atomic energy establishment through independent monitoring programmes. Such studies have greater acceptability among the general public.
The Centre for Advanced Research in Environmental Radioactivity, Mangalore University, has undertaken a systematic monitoring programme of Tritium concentration in the water bodies around the Kaiga NPP, India. The details of these studies are presented in this paper.
Experimental
About the study region
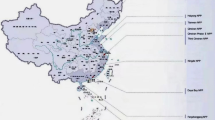
The Kaiga NPP (Latitude: 14° 52′18.5″N, Longitude: 74° 24′15.8″E) is situated on the banks of the river Kali, which emerges from the Western Ghats of India (Fig. 1a). It is surrounded by tropical forests and mountains with altitude ranging from 40 to 600 m from the mean sea level. Due to these features, it has a unique topography, and appears like a bowl with the Kaiga NPP, where 4 PHWR each 220 MW operating reactors are situated, located at the bottom of the bowl. There are several streams which flow through the mountains and join the river Kali and meanders through the valley of the Western Ghat and Kaiga region. The river Kali flows from the east to the west, and finally meets the Arabian Sea at Karwar. A dam is constructed on this river at Kadra (at about 5 km aerial distance in the downstream side of the NPP), which not only serves as a source and sink for the coolant water for the NPP, but also generates about 570 MU of hydroelectricity. The dense forest surrounding Kaiga manifests unique meteorological and ecological characteristics. The region receives an average rainfall of ~ 4000 mm y−1. This recharges the groundwater sources during the heavy southwest monsoon. The villages around Kaiga are moderately populated, and the people cultivate and consume rice as their staple food [7].
Sample collection and Tritium concentration measurement
The locations identified for collecting water samples are shown in Fig. 1a, and they are distributed in the radius zones of 2.3–5 km, 5–10 km, and 10–15 km of the Kaiga region, with the Kaiga nuclear power station at the centre of each zone. Sampling is performed only in places where there is human habitation. Since protected dense forest exists throughout the region, all the sectors around the NPP could not be covered under the study because of poor approachability.
A total of 120 groundwater (well) and 74 surface water samples (pond, stream, river, and reservoir) were collected from different sources in airtight polyethylene containers. The sampling was performed during the different months of the year from 2015 to 2018. The samples were then distilled after adding 100 mg of sodium thiosulphate and sodium carbonate to eliminate any volatile nuclides [8]. After distillation, the samples were taken in plastic liquid scintillation vials, and the Ultima Gold uLLT scintillator (Perkin Elmer, USA) was added in a sample to scintillator ratio of 10 mL: 10 mL, which is the optimized ratio as discussed in an earlier publication [9].
Quantulus-1220 (Perkin Elmer, USA) ultra low-level liquid scintillation spectrometer (LSS) was used for the determination of the Tritium concentration in the samples. The quench parameters were evaluated by preparing standards with different quench values, and these details were published elsewhere [9]. For a 10 mL: 10 mL sample to cocktail ratio, the detector efficiency was found to be 23% and the figure of merit (FoM) was 436 (for channels ranging between 40 and 150). The Tritium activity concentration of the water samples was computed as under:
where A is the Tritium activity concentration (Bq L−1), Cs is the counts obtained from the sample (CPM), Cb is the background counts (CPM), E is the counting efficiency (%) of the LSS, and V is the volume of the sample taken for counting (L).
The minimum detectable activity (MDA) at 2σ confidence level for a counting time of 6000 s and a sample (water) to scintillator combination of 10 mL + 10 mL was determined to be 1.9 Bq L−1. For each sample, triplicate analyses were performed to ensure reproducibility in the measurements.The mean value of triplicate measurements was considered as the representative value for Tritium activity concentration. The deviation among the results of triplicate measurements was < 3%.
Results and discussion
Tritium concentration in ground and surface water
Table 1 presents the Tritium activity concentration in the ground and surface water. The GM values presented in Table 1 are inclusive of those samples which exhibited concentration below MDA. The concentration in the groundwater varied in the range of < 1.9–27.4 Bq L−1 with GM value of 4.0 Bq L−1 and GSD of 2.0. Surface water exhibited similar activity concentration as that of the groundwater with values varying in the range of < 1.9–42.1 Bq L−1 (GM = 3.5 Bq L−1 and GSD = 1.9). The GM values indicate that the concentration is similar in the samples collected from the three zones covered in the present study. For comparison, samples were collected from a background region situated about 100 km away from the Kaiga NPP and analyzed in order to determine the increase in concentration in the vicinity of the NPP. All the samples collected from the background region exhibited activity concentration of < 1.9 Bq L−1. Several samples collected from 2.3 to 5 km zone have also exhibited values < 1.9 Bq L−1.
Figure 2a–c show the frequency distribution plots of the Tritium activity concentration in the groundwater sampled from the 2.3–5 km, 5–10 km, and 10–15 km zones, respectively. The Shapiro–Wilk test rejected the null hypothesis that the data sets come from normal distribution (with 95% confidence level) indicating the non-existence of normal distribution in all data sets. Skewness values showed positively skewed distribution and the kurtosis values suggested that the distribution was highly peaked. As evident from Fig. 2a and b, majority of the samples exhibited activity concentration values < 5 Bq L−1 in the 2.3–5 km and 5–10 km zones. Similar results were observed in the case of samples collected from the 10–15 km zone as well (Fig. 2c).
To distinguish causal variations from random fluctuations, trend analysis was carried out using the well-established Hurst exponent (H value) method (residual range normalized w.r.t. sigma, [10]). The time series trend of Tritium concentration in ground and surface water is depicted in Fig. 3a and b, respectively. The Hurst analysis also offers persistence of trends. For Tritium concentration values in ground and surface water H values were computed to be 0.77 (N = 120) and 0.65 (N = 69) respectively. H > 0.5 implies a certain degree of positive tendency of persistence and hence, the variations observed in the Tritium concentration are likely to be linked by causal relationships with underlying environmental factors like ambient temperature, humidity, rainfall, wind directions, etc.
Table 2 presents a comparison of the Tritium concentration observed in the Kaiga region with those reported for other NPPs. The details of different NPPs, for which the reported values for Tritium are available, are also presented in the same table. It is evident from the comparison that the results observed in the present study are similar to those reported for the environs of other NPPs across the globe.
Tritium concentration in reservoir water
Figure 4 presents the Tritium concentration in the water samples of the reservoir built on the Kali River, which is the source and sink for the coolant water to the condenser of the NPP. In total, 36 water samples were collected from different locations within the reservoir during the period 2015–2017. The concentration varied in the range of 12.4–42.0 Bq L−1 (GM = 24.07 Bq L−1 and GSD = 1.5). A comparison of the reservoir activity with the surface water and groundwater samples revealed that the concentration in the reservoir water was higher to that observed in other surface water and groundwater samples. However, the concentration reduced to background levels when the water from the dam was released at the downstream side of the reservoir and this is due dilution process.
Temporal and seasonal variation of concentration in ground and surface water
In order to study temporal and seasonal variations in the concentration, if any, samples were collected during different months from 3 locations of the 2.3–5 km zone, 2 locations of the 5–10 km zone, and 2 locations of the 10–15 km zone. Figure 5a, b present temporal variation of the Tritium concentration in different zones for the ground and surface water samples, respectively. Although the data presented in Fig. 5a for the ground water shows that the concentration is higher during May, statistical analysis (paired t test) performed between the mean values of concentration for this month against other months showed that the difference of their population means are not significantly different (at 95% significance level). Similarly, from Fig. 5b it can be seen that the concentration values in the surface water was higher in the months of December and January, but, paired t-test did not confirm this.
The samples collected in different seasons of all years were grouped according to the seasons, namely, summer, monsoon, and winter, and analyzed for seasonal variation. Figure 6 presents the variation of the concentration with respect to the different seasons. While plotting the data in Fig. 6, the data has been categorized with respect to the different zones (2.3–5 km, 5–10 km, and 10–15 km). This plot indicates that the concentration is similar in all the seasons, and the statistical tests confirmed the same. Some of the water sources were dried up during March–April (summer) and hence samples were not available during these months in all the sampling stations.
In Table 3 the concentration values observed in different sources of water, during different seasons, are categorized with respect to upstream and downstream direction of water flow. As outlined in Sect. 2, the zones covered under the study were divided into different sectors (each of 30°). To delineate the dependence of the concentration with the flow direction of the main water body, the river Kali, the data was pooled for all the downstream locations (situated in sectors 180°–360°) of the NPP together, and similarly, for the upstream locations (sectors 0°–180°), and the GM values for the pooled data was compared in Table 3. The dominant wind direction in the study area is easterly winds during winter and southwesterly during rainy and summer seasons (Fig. 1b), with respect to NPP. It is interesting to note from the GM values that irrespective of the wind direction and seasons, the concentration remained similar in the downstream and upstream locations. This was also confirmed through statistical analysis (paired t-test). This may be the reason for the observed trend, in which no seasonal variation in concentration is observed in the groundwater samples. Similar results are reported by other investigators as well [18, 19].
Dose calculation
The annual whole-body radiation dose to the public due to the ingestion of Tritium along with consumption of groundwater as drinking water was calculated as under [20]:
where Dwhole body is the annual whole-body dose (µSv y−1), Cw is the concentration of Tritium in water (Bq L−1), Uw is the water consumption rate (L y−1),and Dw is the dose coefficient (µSv Bq−1) = 1.8 × 10−5 µSv Bq−1 [20] for the whole body for an adult.
The data on daily water consumption rate by the population is an important parameter required for dose calculations. The daily consumption rate of water was considered as two liters per day as per World Health Organization (WHO) guidelines [21]. The dose due to the ingestion of Tritium along with drinking water, calculated using Eq. (2), was found to vary in the range of 0.03–0.55 µSv y−1 with a GM value of 0.1 µSv y−1 for the population residing in the 2.3–5 km zone, in the range of 0.03–0.4 µSv y−1 (GM = 0.1 µSv y−1) for the 5–10 km zone, and in the range of 0.03–0.13 µSv y−1 (GM = 0.05 µSv y−1) for the 10–15 km zone. The GM value for the entire region covered under the present study (2.3–15 km) was 0.08 µSv y−1. The concentration values of < 1.9 Bq L−1 (observed for several samples) were not considered for the dose calculations and hence dose values given above is considered as a higher estimate than the actual dose. The dose to the public resulting from the ingestion of Tritium through drinking water is ~ 0.01% of the regulatory dose limit of 1000 µSv y−1 prescribed by International Commission on Radiological Protection (ICRP) [22] and WHO [21] for the members of the public due to the operation of the NPP.
Conclusion
The Tritium concentration in the ground and surface water samples collected in the 2.3–15 km zones of the Kaiga NPP region was similar with overall GM values of 4.0 Bq L−1 and 3.5 Bq L−1 respectively. The concentration values were similar among the different months and seasons of the year. The statistical analysis of the data indicated that the concentration in the different water sources did not depend upon the predominant wind directions and flow direction of the major water body. The samples from the reservoir, which supplies coolant water to the NPP, showed a marginally higher concentration of Tritium when compared with the groundwater, but the concentration on the downstream side of the reservoir was observed to be similar to that of the groundwater samples. The observed concentration level was four orders of magnitude lower than the permissible value recommended by WHO for drinking water.
References
Catalano R, Imme G, Mangano G, Morelli D, Giammanco S (2014) Natural Tritium determination in groundwater on Mt. Etna (Sicily, Italy). J Radioannal Nucl Chem 299:861–866
Greg J (2008) Tritium issues in commercial pressurized water reactors. Fusion Sci Technol 54:329–332
Health Physics Society-Specialists in Radiation Safety Report. http://hps.org/documents/Tritium_fact_sheet.pdf. Accessed 22 Feb 2018
Nikolov J, Todorovic N, Jankovic M, Vostiner M, Bikit I, Veskovic M (2013) Different methods for Tritium determination in surface water by LSC. Appl RadiatIsot 71:51–56
Thompson PA, Kwamena NOA, Ilin M, Wilk M, Clark ID (2014) Levels of Tritium in soils and vegetation near Canadian nuclear facilities releasing Tritium to the atmosphere: implications for environmental models. J Environ Radioact 140:105–113
Atomic Energy Regulatory Board, Annual Reports (2017). https://aerb.gov.in/images/PDF/Annual_report/ar2017/annrpt2k17.pdf. Accessed 21 March 2019
Karunakara N, Somashekarappa HM, Narayana Y, Avadhani DN, Mahesh HM, Sidappa K (2003) 226Ra, 40K and 7Be activity concentration in plants in the environment of Kaiga, India. J Environ Radioact 65:255–266
Hegde AG, Varma PC, Rao DD (2008) Bhabha Atomic Research Center (BARC) Standard protocol for evaluation of environmental transfer factors around NPP sites. https://www.osti.gov/etdeweb/servlets/purl/21134202. Accessed 21 Nov 2014
Nayak RS, D’souza RS, Kamath SS, Mohan MP, Bharath S, Trilochana S, Sudeep KK, Narayana B, Dileep BN, Ravi PM, Karunakara N (2019) Organically bound Tritium: optimization of measurements in environmental matrices by combustion method and liquid scintillation spectrometry. J RadioannalNucl Chem. https://doi.org/10.1007/s10967-018-6395-y
Mohan MP, D’souza RS, Nayak RS, Kamath SS, Trilochana S, Sudeep KK, Yashodhara I, Mayya YS, Karunakara N (2018) A study of temporal variations of 7Be and 210Pb concentrations and their correlations with rainfall and other parameters in the South West Coast of India. J Environ Radioact 192:194–207
Chang KK, Byung HR, Kun JL (1998) Environmental Tritium in the areas adjacent to Wolsong nuclear power plant. J Environ Radioact 41:217–231
Baeza A, Garcia E, Parianez R (2005) Modelling the spatio-temporal evolution of Tritium in the waters of the River Tagus. J Environ Radioact 86:367–383
Paunescu N, CotarleaM GaleriuD, MargineanuR Mocanu N (1999) Evaluation of environmental Tritium level in preoperational period of Cernavoda CANDU Nuclear Power Plant. J RadioannalNucl Chem 239:465–470
Miljevich N, Sipka V, Zujic A, Golobocanin D (2008) Tritium around the Vinca Institute of Nuclear Sciences. J Environ Radioact 48:303–315
Results of environmental monitoring programs, Ontario power generation Inc, N-REP-03443-10013. http://www.opg.com/news-and-media/Reports/2013_EMP_Report.pdf. Accessed 22 Feb 2018
Bolsunovskii A, Bondareva LG (2005) Tritium in water bodies of the Yenisei basin in the impact zone of the mining-and-chemical plant of the ministry of the nuclear power industry of the Russian Federation. Russ J Ecol 36:52–56
Tonosaki K, Kudoh H, Kimura H (2000) Tritium concentrations of natural waters in Rokkasho-mura. J RadioannalNucl Chem 243:579–585
Valgma I, Torn H, Erg K (2006) The impact of infiltration dam on the groundwater regime in the kurtna landscape reserve area. Oil Shale 23:3–14
Dingwell S, Mills CE, Phan N, Taylor K, Boreham DR (2011) Human health and the biological effects of Tritium in drinking water: prudent policy through science—addressing the ODWAC new recommendation. International Dose Response Society 9:6–31
The International Commission on Radiological Protection (2007). Compendium of Dose coefficients based on ICRP Publication 60. Annals of ICRP. http://www.icrp.org/docs/P%20119%20JAICRP%2041(s)%20Compendium%20of%20Dose%20Coefficients%20based%20on%20ICRP%20Publication%2060.pdf. Accessed 31 Aug 2019
World Health Organization (2011) Guidelines for drinking-water quality, WHO Library Cataloguing-in-Publication Data NLM classification: WA 675. https://apublica.org/wp-content/uploads/2014/03/Guidelines-OMS-2011.pdf. Accessed 21 March 2019
The International Commission on Radiological Protection (2007) The 2007 Recommendations of the International Commission on Radiological Protection. Annals of the ICRP-103 http://www.icrp.org/docs/ICRP_Publication_103-Annals_of_the_ICRP_37(2-4)-Free_extract.pdf. Accessed 21 March 2019
Acknowledgements
The authors would like to thank the Board of Research in Nuclear Science (BRNS), Department of Atomic Energy, Government of India, for funding the research programme. The authors would also like to thank the Site Director and other officials of ESL, Kaiga Generating Station, Nuclear Power Corporation of India Ltd., for supporting this research programme. The help received from Mr. S. S. Managhanvi and his colleagues, Health Physics Unit, Kaiga Generating Station, Kaiga, during the sample collection is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest of the work reported in this article with any other agencies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamath, S.S., Narayana, B., D’Souza, R.S. et al. Tritium in water bodies around the Kaiga generating station. J Radioanal Nucl Chem 322, 389–397 (2019). https://doi.org/10.1007/s10967-019-06742-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06742-z