Abstract
A rapid method for the separation and qualitative analysis of several neutron activation products (198Au, 192Ir, 72Ga, 51Cr, 191/195m/197Pt, 54Mn, 57Co, and 59Fe) from fission products and soil matrixes has been developed. Analytes were isolated within 20 h using ion exchange chromatography. After separation, the activation products were characterized by γ-spectroscopy and inductively coupled plasma-optical emission spectroscopy. Validation experiments demonstrated versatility of the method, showing that the activation products could be isolated from fresh fission products and other contaminants associated with complex soil matrixes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advancing environmental fate-and-transport models for non-actinide activation products—those generated from reactions like (n, γ), (n,×n), (n, p), etc.—released from nuclear testing, radiochemical processing, the storage of nuclear material, and from nuclear power facilities is of widespread interest for accurately evaluating health risks from nuclear contamination sites [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Although substantial efforts have been invested historically to characterize impacts of long-lived activation products (t½ > 5 y; 60Co, 94Nb, 106mAg, 63Ni, 99Tc, 93Mo, 152/154Eu, etc.) [17], relatively little information is available for activation products that have rapid decay rates (t½ < 5 y; 51Cr, 54Mn, 57Co, 59Fe, 72Ga, 192/194Ir, 191/195m/197Pt, 198Au, among others) [18,19,20,21,22,23,24,25,26].
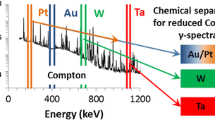
The information reported on short-lived activation products is scarce in comparison to data available for fission products and actinides [27,28,29,30,31,32,33,34,35,36,37,38,39]. This lack in information has created a clear knowledge gap, and nuclear signatures based on inter-element ratios for non-actinide activation products have yet to be established. This issue can be largely attributed to limited accessibility. For instance, production of many short-lived activation products requires high-energy neutrons (14 MeV), which limits access and impedes efforts to develop separation processes and spectroscopic methods for isotope quantification. The short half-lives also present technical barriers, in that information for short-lived activation product isotopes is often lost for fresh samples—those close in time to a fission event—because fission product and actinide isotopic quantification is prioritized. As demonstrated graphically in Fig. 1, some of these shorter-lived activation product isotopes decay away and are unavailable for detection after a timescale of tens of days or less. Overcoming these obstacles has potential for dramatic impact. For example, making non-actinide activation product quantification more accessible could transition this area of research from an academic curiosity into an analytical tool used broadly to inform policies in global health and national security.
Herein, we describe the first in a series of studies focused on overcoming non-actinide activation product analyses obstacles. We contribute a screening method that removes activation products from fission products and actinides. The procedure avoids complicated and lengthy chromatographic separation schemes [40] and was validated against sample matrixes that contained microscopic quantities of activation products (10−3–10−6 atoms per 235U fission), soil matrixes (90 mg: Standard Reference Material (SRM) 2702), and large quantities of fissioned 235U (up to 80 mg). We observed that precious metals (198Au, 192Ir, 191/195m/197Pt), main group elements (72Ga), and first row transition metals (51Cr, 54Mn, 57Co, and 59Fe) could be separated from fission products and uranium rapidly for qualitative analysis using gamma spectroscopy (within 20 h). The results are discussed in terms of potential application and future utility for the rapid and qualitative separation method.
Experimental
General procedure for separation of activation product elements from fission product elements, uranium, and soil matrix
Two stable carrier solutions are prepared, one containing activation product elements (Au, Co, Cr, Cu, Fe, Ga, Ir, Mn, Ni, Pt, Sc, and Zn at final concentrations of ~ 0.2 mg mL−1 each, and Pb at ~ 1 mg mL−1), and the other containing fission product elements and uranium (Ba, Ce, La, Mo, Nd, Sr, Te, Y, Zr, and U at final concentrations of ~ 0.1 mg mL−1 each). Each solution is generated by combining individual inductively coupled plasma (ICP) standard solutions (SPEX CertiPrep), followed by a matrix transposition to 4 M HNO3 (Fisher Scientific, Trace Metal Grade). For this study, a National Institute of Standards and Technologies (NIST) SRM 2702 was utilized as a soil standard as it is certified for several activation product analytes employed in this study. As such, the values can be used to make inductively coupled plasma optical emission spectroscopy (ICP-OES) chemical yielding corrections. Tracer solutions of 54Mn, 57Co, and 59Fe were purchased from Eckert and Ziegler and used as received. Optima-grade (Fisher Scientific) acids (HCl, HNO3, HClO4, and HF) were used to dissolve 9.99 g of the soil standard following a previously reported standard protocol [41] to generate a 4 M HNO3 solution with a concentration of ~ 8.9 mg soil g−1 solution.
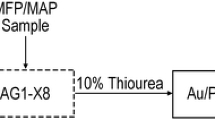
The sample matrix solution is prepared by combining 5 mL of the activation product solution, 2 mL of the fission product/U solution, and 10 mL of soil solution. Precipitation of tungstic acid [42] is completed by the addition of 1 mL of W carrier solution (SPEX CertiPrep, 10,000 μg mL−1 in H2O). Following centrifugation, the supernate is removed to yield a W pellet. To the supernate, 0.5 mL of Pb carrier solution is added (SPEX CertiPrep, 10,000 μg mL−1 in 5% HNO3), followed by 30 mL of fuming HNO3, then the solution cooled in an ice bath for 1 h to yield lead nitrate [43]. The mixture is centrifuged and the supernate removed to yield a Pb pellet, then the solution matrix is converted to 6 M HCl (10 mL). Using a corrosion-resistant Eldex Model 1SIP High Pressure Liquid Metering Pump (flow rate = 5 mL min−1), the solution is loaded onto a pre-prepared AGMP-1M column (25 mL of resin, 50–100 mesh, in a 40 cm × 6.6 mm Omnifit column). Thirty-seven individual fractions (10 mL each) are collected using a flow rate of 5 mL min−1. Elution conditions are as follows: 6 M HCl (9 × 10 mL) to elute Mn/Cr/Sc/Ni, Pb/Co (Cut I),Footnote 1 and Ir/Cu (Cut II); 4 M HCl (7 × 10 mL); 2 M HCl (8 × 10 mL) to elute Ga/Fe (Cut III); H2O (4 × 10 mL) to remove Zn (Cut IV); 1% (w/w) thiourea (3 × 10 mL) for the elution of Au (Cut V), and 7% (w/w) thiourea (6 × 10 mL) to recover Pt (Cut VI). For each fraction collected, ICP-OES analysis is performed to determine chemical yields.
Guided by the ICP-OES results, the first three fractions are combined and reduced in volume (to 5 mL) to generate the Mn/Ni/Cr/Sc cut, which undergoes further separation on DGA resin (Eichrom; 3 mL of resin in a 20 mL Bio-Rad Econo-Pac column) in order to further remove lanthanide and Groups 2/3/4 fission product elements. The DGA-based separation includes an initial load of 5 mL in 6 M HCl, followed by 6 M HCl (1 × 5 mL) to yield Cr/Ni (Cut VII); 6 M HCl (1 × 5 mL) followed by 4 M HCl (2 × 5 mL) to elute Mn (Cut VIII); and 2 M HCl (2 × 5 mL) followed by H2O (2 × 5 mL) to recover Sc (Cut IX). Pressure was added to the column using the in-house air line, with a flow rate of approximately 1 drop per second. The resultant Cr/Ni, Mn, and Sc fractions are analyzed by ICP-OES to determine chemical yields.
Target irradiations and sample preparation
To generate fresh activation products and fission products, two concurrent irradiations were conducted. Irradiation targets were prepared from 235U metal and several stable materials (0.12% Au–Al wire, a K2IrCl6/KCl pellet, Ga2Cl4, CrCl3·6H2O, and H2PtCl6·xH2O). A thermal irradiation of highly enriched (93.13% 235U) uranium (HEU) metal, sealed in a quartz ampule, was conducted at the Massachusetts Institute of Technology (MIT) Nuclear Reactor Laboratory for 200 min at 90 kW under a flux of 7.2 × 1010 n/cm2/s to generate fission products. The five solid neutron activation targets sealed in LDPE vials were irradiated at the United States Geological Survey (USGS) TRIGA Reactor for 300 min in the Lazy Susan position at 1000 kW under a flux of 3.7 × 1012 n/cm2/s. All targets were allowed to cool for approximately 48 h, then shipped to Los Alamos National Laboratory (LANL) for radiochemical analysis.
Each target was dissolved separately in individual beakers, then used to make solutions implemented in the validation experiments. All dissolutions were conducted using Fisher Scientific Optima Grade acids and 18 MΩ·cm water. The HEU foil was dissolved while heating using a mixture of 8 M HNO3, 6 M HCl, and dilute HF [44]. Once dissolved, the solution was transposed to a 4 M HNO3 matrix, then diluted to achieve a final concentration of 0.866 mg HEU g−1 solution. Each activation product target was dissolved in a separate Pyrex beaker. The Au–Al wire was dissolved in aqua regia at room temperature. To the beaker containing the K2IrCl6/KCl powder, 0.5 M HCl was added at room temperature until the powder fully dissolved. The CrCl3·6H2O powder and the H2PtCl6·xH2O powder were each dissolved using H2O at room temperature. The Ga2Cl4 was dissolved in 8 M HNO3 at room temperature. The resultant HEU/fission product solution and each activation product solution were characterized individually via γ-spectroscopy to determine the activity of the fission products and activated isotopes, respectively. Each solution was also analyzed using ICP-OES to determine the elemental concentrations in each solution.
Following characterization of the HEU/fission product solution and each activation product solution, the sample used for validation experiments was prepared by combining aliquots of each the HEU/fission product, 198Au, 192/194Ir, 72Ga, 51Cr, and 191/195m/197Pt solutions in an Erlenmeyer flask. Tracer solutions of 54Mn, 57Co, and 59Fe, as well as an aliquot of SRM 2702 solution (10 mL, ~ 8.9 mg g−1 solution), were also added. The activation product and fission product stable carrier solutions were then added in 5 mL and 2 mL portions, respectively. At this stage, the activation product, fission product, and SRM mixture was converted to a 4 M HNO3 matrix with a final volume of 10 mL in preparation for separations.
Instrumentation and sample handling
Chemical yielding was conducted by ICP-OES using a ThermoFisher Scientific ICAP 6500 (Waltham, MA, USA) instrument fitted with an Elemental Scientific Inc. (ESI, Omaha, NE, USA) auto-sampler. The ICP-OES is fitted to a radiological fume hood to assist in preventing personnel contamination. An axial view of the ICP was employed for photon collection to achieve optimal sensitivity. An ESI Perfluoroalkoxy (PFA) low-flow nebulizer housed within a Teflon® cyclonic spray chamber was utilized. This instrument configuration for the analysis of radiological materials has been described previously [45, 46]. Calibration curves were developed for each element prior to each analysis using custom-made, matrix-matched, certified standard solutions from High-Purity Standards. All samples analyzed by ICP-OES were diluted as needed to fall within the concentration range of the calibration curves. The instrument was evaluated for reproducibility, stability, and instrument drift over a test in-run analysis (6 h length) as well as day-to-day measurements (over 60 d). No significant variations were observed in the timeframes evaluated, indicating that the impact of instrument drift is minimal even when long run times are required for sample analysis. Uncertainties in the ICP-OES measurement were evaluated and determined using the data collected during this study.
Calibrated high-purity Ge (HPGe) detectors with either an aluminum or beryllium window were used for γ-spectroscopy measurements. Certified multi-line standards containing emissions from 59 keV (241Am) to 1836 keV (88Y) were used to determine the efficiency of each detector and position for the specified sample geometry. All analytical samples were measured using the calibrated sample geometry.
Results and discussion
The primary goal of this effort was to develop a method for rapidly isolating and qualitatively analyzing microscopic quantities (10−3–10−6 atoms per 235U fission) of several activation products (198Au, 192/194Ir, 72Ga, 51Cr, 191/195m/197Pt, 54Mn, 57Co, and 59Fe) from soil matrixes contaminated with fresh fission products. Following the method development using stable carriers, the process was validated by conducting several experiments with fresh activation products and fission products. Representative details of the experiments are described herein. A general discussion of the activation product separation procedure follows.
Method validation: separation of activation products from fission products, uranium, and soil matrix
Following the development of the separations protocol using stable carriers, several validation experiments were carried out using fresh activation products and fission products to test the effectiveness of the procedure for use in activation product qualitative radiometric analyses. Fresh fission products and short-lived neutron activation products (51Cr, 72Ga, 192/194Ir, 191/195m/197Pt, and 198Au) were produced, returned to LANL, dissolved, and characterized. Tracer solutions of 54Mn, 57Co, and 59Fe were also characterized prior to use (Table 1).
The individual solutions were subsequently combined to prepare the experimental sample solution employed in the experiments used for separations validation. This involved combining aliquots of each the HEU/fission product, 198Au, 192/194Ir, 72Ga, 51Cr, 191/195m/197Pt, 54Mn, 57Co, and 59Fe solutions to achieve activation product quantities that ranged from 10−3 to 10−6 atoms per 235U fission (Table 2). To simulate environmental samples—those that contain soil matrix and chemical interferences from the environment—the fission product and activation product cocktail was combined with an aliquot of SRM 2702 solution (containing ~ 89 mg of soil). Our activation product processing method relies on the addition of macroscopic amounts of stable elements, on the order of 0.2 mg for the stable fission product elements (Ba, Ce, La, Mo, Nd, Sr, Te, Y, and Zr) and 1 mg for the stable activation product elements (Au, Co, Cr, Cu, Fe, Ga, Ir, Mn, Ni, Pb (5 mg), Pb, Pt, Sc, and Zn) product elements. Having analytes present in these large quantities ensures that the isotopes adopt predictable behavior associated with a bulk sample. In addition, it provides potential opportunity to determine separation yields gravimetrically.
After adding the stable carriers to the sample (the solution containing soil matrix, fission products, uranium, and the desired activation products), the aqueous matrix was converted to HNO3. The solution was then processed as summarized in Fig. 2. The first step in the procedure was developed to isolate a tungsten fraction (W) via precipitation of tungstic acid [42, 47,48,49,50]. Quantitative removal of W from the stock solution made it impossible to determine a decontamination factor (defined hereafter as the amount of ingoing analyte divided by the amount of analyte remaining in the process stream, Table 1). Subsequently, Pb was removed by a similar precipitation of lead nitrate using Pb carrier [43]. Typically, Pb recoveries for this separation step were near quantitative, with the Pb recoveries often near 100%. However, this step is not robust and at times Pb yields can be as low as 5%. The variability in yield is likely a result of the short ice bath cooling time (1 h) used for the Pb solution following addition of the fuming HNO3. As this separations approach was centered on achieving short timelines, a longer cooling period was not attempted. When Pb yields are low from the precipitation step, screening can still occur using the AGMP-1M chromatographic step described below. Although it is tempting to eliminate the Pb precipitation, we recommend retaining this separation step. It is valuable because several fission products (Ce, ~ 80%; Mo, ~ 20%; Te, ~ 10%; and U, ~ 30%) co-precipitate with the W and Pb carriers, which facilitates the subsequent screening effort [48].
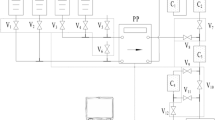
After removing W and Pb, the sample was converted to 6 M HCl matrix and subjected to ion exchange chromatography. A moderately sized column was used to accommodate the large variability in analyte distribution constants. To ensure rapid separation for this step (within 90 min), sample processing was integrated with high performance liquid chromatographic (HPLC) methodology. Given the high corrosivity of the eluents, special consideration was made to employ a pump with acid-resistant components. After loading the sample onto the column in HCl (6 M), a step-wise gradient elution was conducted from HCl (6 M) to H2O, and then to thiourea (7%, w/w). The described approach results in six main cuts that were collected in thirty-seven individual fractions. A representative separation profile showing the percent recovery of the activation products and cumulative analyte recovery vs fraction is presented in Fig. 3. The first cut (Cut I) contained Pb and Co, with smaller quantities of Sc, Ir, Ni, Cu, Cr, Ga, and Mn. Cut II contains primarily Cu and a sufficient amount of Ir for qualitative evaluation by gamma spectroscopy. Cut III contained appreciable amounts of Au and Ga, with small quantities of Pt and Ir also present. Iron was also able to be detected by γ-spectroscopy, despite the poor chemical recovery in the Ga/Fe cut (Cut III), as indicated in Table 3.Footnote 2 Changing the mobile phase to water (H2O), Cut IV containing only Zn eluted. To elute Au and Pt, the mobile phase was changed to thiourea. In dilute thiourea (1%) Au and some Pt eluted in Cut V. In more concentrated thiourea (7%), Pt is fully removed in Cut VI, which also contained small amounts of Au and Ir. Typical decontamination factors for each element are shown in Table 1.
Percent recovery of activation products from AGMP-1M separation, with the chromatographic fraction vs the cumulative analyte recovery shown on the top. Determined by ICP-OES. Along the bottom, from left to right, are the individual cuts for Pb/Co (I), Ir/Cu (II), Ga/Fe (III), Zn (IV), Au (V), and Pt (VI). Illustrated for each cut are the activation products that also co-elute
Following the chromatography step with AGMP-1M, separated fractions were characterized by ICP-OES and γ-spectroscopy. In general, fission products were removed to the point that they did not interfere with the activation product analyses. Exceptions to this generality were associated with the 194Ir, 54Mn, and 51Cr analytes. The gamma spectrum from the Ir/Cu cut had significant contributions from 140La (328.76 keV), which interfered with 194Ir detection. Fission products that also eluted in the 54Mn/51Cr/Sc/Ni fraction contributed to a high background, preventing these two activation products from being accurately detected. An additional challenge was encountered with the Au and Pt recoveries, which were significantly greater than 100%. This discrepancy likely resulted from precipitation of Au and Pt from the ingoing stock solution of stable activation product carriers, which hindered an accurate determination of starting Au and Pt concentrations needed for yield quantification [51, 52].
Following γ-spectroscopy measurements and chemical yielding determination for the first three fractions from the AGMP-1M separation, the fractions were combined and subjected to additional chromatography to further resolve 54Mn and 51Cr from the fission product interferences. The combined fractions underwent an additional chromatography step using DGA resin. This added step was attractive because it enabled pure fractions of 51Cr/Ni (Cut VII), 54Mn (Cut VIII), and Sc (Cut IX) to be isolated from main group, lanthanide, and transition metal fission product interferences (e.g. Ba, La, Ce, Sr, Nd, Y, Zr). Moreover, it could be completed rapidly (only adding 1 h to the entire process). As represented in Fig. 4, the sample was loaded in HCl (6 M) onto the column and subjected to a four-step gradient elution profile ending with H2O. Chromium and Ni eluted in 6 M HCl (Cut VII). With continued use of 6 M HCl followed by 4 M HCl, Mn eluted to yield Cut VIII. The mobile phase was changed to 2 M followed by H2O to recover Sc in Cut IX. To determine decontamination factors and chemical yields, the resultant Cr/Ni, Mn, and Sc fractions were analyzed by ICP-OES. Typical results achieved from the DGA separation step are illustrated in Fig. 4.
The results from these experiments validated that sufficient amounts of interferants (fission products and soil matrix) can be removed to screen for the presence of a series of activation product analytes. Further, the method can be completed rapidly (within 20 h) to provide qualitative results, despite low chemical yields in some cases.
Outlook and conclusion
Contributed here is an HPLC-based processing method that rapidly removes activation products from fission products, uranium, and contaminants associated with soil matrixes. Interferences are sufficiently removed such that activation product identities can be qualitatively characterized using γ-spectroscopy and elemental abundances quantified by ICP-OES. Although additional research is needed to demonstrate generality, the hallmark of this study is proof-of-principle. As a testament, the procedure was validated for 198Au, 192Ir, 72Ga, 51Cr, 191/195m/197Pt, 54Mn, 57Co, and 59Fe.
The simplicity of the procedure is attractive; the process can be carried out by researchers with a wide range of technical expertise. Broad operator access should facilitate implementation into a wide range of scientific laboratories and provide potential support for numerous scientific campaigns with diverse mission scope. Consider the importance of characterizing a nuclear fission event by identifying the presence (or absence) of a given activation product. This qualitative screen could serve as a diagnostic to guide subsequent experimental efforts. In addition, the procedure could be used as a crude separation step that precedes further sample processing and quantification efforts. We hope the process will find applicability in characterizing the impact of activation products emitted from nuclear contamination sites and inspire researchers to identify nuclear signatures and define inter-element ratios for non-actinide activation products.
Notes
If Pb does not completely precipitate during the Pb precipitation step, it co-elutes with Co.
In the presence of soil matrix, Fe recoveries are not reproducible. In the absence of soil matrix, Fe recoveries were determined by ICP-OES to be near quantitative.
References
Horan JR (1963) The health physics aspects of the SL-1 accident. Health Phys 9:177–186
Mitsugashira T, Hara M, Nakanishi T et al (2000) Passive gamma-ray spectrometry for the determination of total fission events in the JCO criticality accident’99 in Tokai. J Environ Radioact 50:21–26. https://doi.org/10.1016/S0265-931X(00)00056-4
Imanaka T (2000) Neutron dose and power released by the JCO criticality accident in Tokai-mura. J Environ Radioact 50:15–20. https://doi.org/10.1016/S0265-931X(00)00055-2
Furuta K, Sasou K, Kubota R et al (2000) Human factor analysis of JCO criticality accident. Cogn Technol Work 2:182–203. https://doi.org/10.1007/PL00011501
Komura K, Yamamoto M, Muroyama T et al (2000) The JCO criticality accident at Tokai-mura, Japan: an overview of the sampling campaign and preliminary results. J Environ Radioact 50:3–14. https://doi.org/10.1016/S0265-931X(00)00054-0
Komura K, Yousef AM, Murata Y et al (2000) Activation of gold by the neutrons from the JCO accident. J Environ Radioact 50:77–82. https://doi.org/10.1016/S0265-931X(00)00064-3
Takada J, Hoshi M (2000) External doses to 350 m zone residents around the Tokai-mura criticality accident site. J Environ Radioact 50:43–48. https://doi.org/10.1016/S0265-931X(00)00059-X
Imanaka T (2001) Transport calculation of neutrons leaked to the surroundings of the facilities by the JCO criticality accident in Tokai-mura. J Radiat Res 42:S31–S44. https://doi.org/10.1269/jrr.42.S31
Tanaka S-I (2001) Summary of the JCO criticality accident in Tokai-mura and a dose assessment. J Radiat Res 42:S1–S9. https://doi.org/10.1269/jrr.42.S1
Selby JM, Moeller DW, Vallario EJ, Stephan JG (1986) Use of radiological accident experience in establishing appropriate perspectives in emergency planning. In: ANS topical meeting on radiological accidents—perspectives and emergency planning. Bethesda
McLaughlin TP, Monahan SP, Pruvost NL et al (2000) A review of criticality accidents. Los Alamos Lab, Los Alamos
Inaba J (2000) Radiological and environmental aspects of the criticality accident in Tokai-mura. Radiat Prot Dosimetry 92:239–246. https://doi.org/10.1093/oxfordjournals.rpd.a033277
Napier BA, Schmieman EA, Voitsekovitch O (2007) Radioactive waste management and environmental contamination issues at the Chernobyl site. Health Phys 93:441–451. https://doi.org/10.1097/01.HP.0000279602.34009.e3
Podlazov LN, Trekhov VE, Cherkashov YM et al (1994) Computational modeling of the accident in the fourth power-generating unit of the chernobyl nuclear power plant. At Energy 77:580–587. https://doi.org/10.1007/BF02407430
Kofuji H, Komura K, Yamada Y, Yamamoto M (2000) An estimation of fast neutron flux by reaction. J Environ Radioact 50:49–54. https://doi.org/10.1016/S0265-931X(00)00060-6
Muroyama T, Murata Y, Kofuji H et al (2000) Neutron activation of chemical reagents exposed to the neutrons released by the JCO criticality accident. J Environ Radioact 50:55–59. https://doi.org/10.1016/S0265-931X(00)00061-8
Evans JC, Lepel EL, Sanders RW et al (1984) Long-lived activation products in reactor materials. Pacific Northwest Lab, Richland
Buesseler K, Aoyama M, Fukasawa M (2011) Impacts of the Fukushima nuclear power plants on marine radioactivity. Environ Sci Technol 45:9931–9935. https://doi.org/10.1021/es202816c
Shozugawa K, Nogawa N, Matsuo M (2012) Deposition of fission and activation products after the Fukushima Dai-ichi nuclear power plant accident. Environ Pollut 163:243–247. https://doi.org/10.1016/j.envpol.2012.01.001
Schwantes JM, Orton CR, Clark RA (2012) Analysis of a nuclear accident: fission and activation product releases from the Fukushima Daiichi nuclear facility as remote indicators of source identification, extent of release, and state of damaged spent nuclear fuel. Environ Sci Technol 46:8621–8627. https://doi.org/10.1021/es300556m
Mattsson S, Finck R, Nilsson M (1980) Distribution of activation products from barsebäck nuclear power plant (Sweden) in the marine environment. Temporal and spatial variations as established by seaweed. Environ Pollut Ser B Chem Phys 1:105–115. https://doi.org/10.1016/0143-148X(80)90031-2
Le Petit G, Douysset G, Ducros G et al (2014) Analysis of radionuclide releases from the Fukushima Dai-Ichi nuclear power plant accident part I. Pure appl Geophys 171:629–644. https://doi.org/10.1007/s00024-012-0581-6
Achim P, Monfort M, Le Petit G et al (2014) Analysis of radionuclide releases from the Fukushima Dai-ichi nuclear power plant accident part II. Pure appl Geophys 171:645–667. https://doi.org/10.1007/s00024-012-0578-1
Donaldson LR, Seymour AH, Nevissi AE (1997) University of Washingtonʼs radioecological studies in the Marshall Islands, 1946–1977. Health Phys 73:214–222. https://doi.org/10.1097/00004032-199707000-00018
Oughton DH, Day JP (1993) Determination of cesium, rubidium and scandium in biological and environmental materials by neutron activation analysis. J Radioanal Nucl Chem Artic 174:177–185. https://doi.org/10.1007/BF02040345
Inaba J (2015) Environmental transfer parameters of radionuclides. Radioisotopes 64:335–349. https://doi.org/10.3769/radioisotopes.64.335
Warren GA, Runkle RC (2013) New concepts for radiometric measurements of environmental samples. J Radioanal Nucl Chem 296:829–833. https://doi.org/10.1007/s10967-012-2133-z
Winkler SR, Steier P, Carilli J (2012) Bomb fall-out 236U as a global oceanic tracer using an annually resolved coral core. Earth Planet Sci Lett 359–360:124–130. https://doi.org/10.1016/j.epsl.2012.10.004
Biegalski SR, Bowyer TW, Eslinger PW et al (2012) Analysis of data from sensitive U.S. monitoring stations for the Fukushima Dai-ichi nuclear reactor accident. J Environ Radioact 114:15–21. https://doi.org/10.1016/j.jenvrad.2011.11.007
Thakur P, Ballard S, Nelson R (2013) An overview of Fukushima radionuclides measured in the northern hemisphere. Sci Total Environ 458–460:577–613. https://doi.org/10.1016/j.scitotenv.2013.03.105
Quinto F, Golser R, Lagos M et al (2015) Accelerator mass spectrometry of actinides in ground- and seawater: an innovative method allowing for the simultaneous analysis of U, Np, Pu, Am, and Cm Isotopes below ppq levels. Anal Chem 87:5766–5773. https://doi.org/10.1021/acs.analchem.5b00980
Froehlich MB, Tims SG, Fallon SJ et al (2017) Nuclear weapons produced 236 U, 239 Pu and 240 Pu archived in a Porites Lutea coral from Enewetak Atoll. J Environ Radioact 178–179:349–353. https://doi.org/10.1016/j.jenvrad.2017.05.009
De Cesare M, De Cesare N, D’Onofrio A et al (2015) Mass and abundance 236 U sensitivities at CIRCE. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 361:483–487. https://doi.org/10.1016/j.nimb.2015.05.029
De Cesare M, Gialanella L, Rogalla D et al (2010) Actinides AMS at CIRCE in Caserta (Italy). Nucl Instrum Methods Phys Res Sect B Beam Interact with Mater Atoms 268:779–783. https://doi.org/10.1016/j.nimb.2009.10.029
Mahara Y, Kudo A (1995) Plutonium released by the Nagasaki A-bomb: mobility in the environment. Appl Radiat Isot 46:1191–1201. https://doi.org/10.1016/0969-8043(95)00161-6
Yuanzhong L, Jianzhu C (2002) Fission product release and its environment impact for normal reactor operations and for relevant accidents. Nucl Eng Des 218:81–90. https://doi.org/10.1016/S0029-5493(02)00200-5
Tadmor J (1976) Determination of the type and amount of fission products released in a nuclear reactor accident. Health Phys 30:95–112. https://doi.org/10.1097/00004032-197601000-00011
Hanson SK, Pollington AD, Waidmann CR et al (2016) Measurements of extinct fission products in nuclear bomb debris: determination of the yield of the Trinity nuclear test 70 y later. Proc Natl Acad Sci 113:8104–8108. https://doi.org/10.1073/pnas.1602792113
Goldstein SJ, Hinrichs KA, Nunn AJ et al (2018) Sequential chemical separations and multiple ion counting ICP-MS for 241Pu–241Am–237Np dating of environmental collections on a single aliquot. J Radioanal Nucl Chem 318:695–701. https://doi.org/10.1073/pnas.1602792113
Morrison SS (2015) Activation product analysis in the presence of fission products. Washington State University, Pullman
Kleinberg J (1990) Collected radiochemical and geochemical procedures. Los Alamos National Lab, Los Alamos
Dams R, Hoste J (1961) Gravimetric determination of tungsten by homogeneous precipitation. Talanta 8:664–672. https://doi.org/10.1016/0039-9140(61)80163-X
Willard HH, Goodspeed EW (1936) Separation of strontium, Barium, and lead from calcium and other metals—by precipitation as nitrates. Ind Eng Chem Anal Ed 8:414–418. https://doi.org/10.1021/ac50104a003
Laue CA, Gates-Anderson D, Fitch TE (2004) Dissolution of metallic uranium and its alloys. J Radioanal Nucl Chem 261:709–717. https://doi.org/10.1023/B:JRNC.0000037117.01721.f1
Gao J, Manard BT, Castro A et al (2017) Solid-phase extraction microfluidic devices for matrix removal in trace element assay of actinide materials. Talanta 167:8–13. https://doi.org/10.1016/j.talanta.2017.01.080
Montoya DP, Manard BT, Xu N (2016) Novel sample introduction system to reduce ICP-OES sample size for plutonium metal trace impurity determination. J Radioanal Nucl Chem 307:2009–2014. https://doi.org/10.1007/s10967-015-4648-6
Korob RO, Cohen IM, Agatiello OE (1976) Tungsten and molybdenum co-precipitation by α-benzoinoxime for activation analysis of tungsten. J Radioanal Chem 34:329–333. https://doi.org/10.1007/BF02519582
Knowles HB (1932) The use of alpha-benzoinoxime in the determination of molybdenum. Bur Stand J Res 9:1–8. https://doi.org/10.1016/S0016-0032(13)90386-0
Yagoda H, Fales HA (1938) Studies on the analytical chemistry of Tungsten and Molybdenum. II. J Am Chem Soc 60:640–643. https://doi.org/10.1021/ja01270a041
Taylor-Austin E (1937) The determination of molybdenum in cast iron. Analyst 62:107–117
Borgarello E, Serpone N, Emo G et al (1986) Light-induced reduction of rhodium(III) and palladium(II) on titanium dioxide dispersions and the selective photochemical separation and recovery of gold(III), platinum(IV), and rhodium(III) in chloride media. Inorg Chem 25:4499–4503. https://doi.org/10.1021/ic00245a010
Torigoe K, Esumi K (1992) Preparation of colloidal gold by photoreduction of tetracyanoaurate(1-)-cationic surfactant complexes. Langmuir 8:59–63. https://doi.org/10.1021/la00037a013
Acknowledgements
The authors are grateful for support from the Defense Threat Reduction Agency.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bennett, K.T., Kozimor, S.A., Manard, B.T. et al. Rapid activation product separations from fission products and soil matrixes. J Radioanal Nucl Chem 322, 281–289 (2019). https://doi.org/10.1007/s10967-019-06678-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06678-4