Abstract
Positron emission tomography (PET) had been applied in clinical early diagnosis of various tumors and other diseases. The methylated synthetic conditions of (-)-[11C]-(1R,2S)-meta-hydroxyephedrine ((-)-[11C]HED), considered as one of the most important radiopharmaceuticals for PET, were optimized through single factor and orthogonal design methods. Here, we reported an improved purification protocol. The radiochemical yields of the final product were over 45% (decay-corrected and based on [11C]methyl iodide) (n = 50). The radiochemical purities and chemical purities were over 99% (n = 50) and 97% (n = 50), respectively. The automatic radiosynthesis procedure of (-)-[11C]HED with relatively high radiochemical yield was convenient and reliable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Positron emission tomography (PET) and PET-CT have been used over decades worldwide. PET-CT has revolutionized medical diagnosis in many fields (oncology, surgical planning, radiation therapy and cancer staging), by adding precision of anatomic localization to functional imaging, which was previously lacking from pure PET imaging. According to the distinctive apoptosis way [1] and DNA synthetic methods [2] of tumor cells, various subtract mimics were recruited as radiopharmaceuticals for PET and PET-CT. Various high-selectivity inhibitors [3, 4] were considered as the candidates of potential radiopharmaceuticals. However, high production cost and extremely short half-life of radiolabeled compounds objectively limits its wide application. Mutual combination and mutual promotion of PET and its radiopharmaceuticals are developing in interaction and reciprocity. Carbon-11 labeled (-)-(1R, 2S)-meta-hydroxyephedrine, ((-)-[11C]HED) first reported by Rosenspire [5], was described as a key reagent to reflect the catecholamine transport, storage, and neuron recycling. (-)-[11C]HED is recruited as an in vivo marker of noradrenergic neurons. Biodistribution studies in experimental animals and human-being had shown its selective uptake in organs with rich sympathetic innervation, including the heart and adrenal medulla [5, 6]. PET studies with (-)-[11C]HED in the field of receptor imaging have permitted noninvasive assessment of the integrity of the human cardiac sympathetic nervous system in the normal and transplanted heart, and in disease states such as acute myocardial infarction, diabetic neuropathy and dilated cardiomyopathy [7,8,9,10,11,12]. (-)-[11C]HED PET is also used in the diagnosis of pheochromocytomas/paragangliomas and neuroblastomas [13,14,15,16,17,18,19,20,21,22]. Although the radiosynthesis protocols of (-)-[11C]HED have been described in several literatures, rarely research focus on its optimal methylation conditions [5, 23, 24]. In addition, the purification processes of the final product were complicated, time-consuming and needed for disposing of the hazardous solvents before released for using. According to the issues mentioned above, optimizations and improvements in synthesis and purification are continuously noteworthy for the further developments of radiopharmaceuticals. The objective of this study is to establish a high-efficient preparation strategy of (-)-[11C]HED utilizing [11C]CH3OTf with improved purification protocol. Furthermore, the optimal methylation conditions are also investigated through single factor and orthogonal design methods for exploring the reaction conditions for relatively high labeling yields and radiochemical reaction.
Materials and methods
Instruments
The MINItrace™ cyclotron (10 MeV) (General Electric Medical Systems, Uppsala, Sweden) and the TRACERlab FXc [11C]methylation module (GEMS, Münster, Germany) were used to gain the radiopharmaceutical. Semi-preparative radio-HPLC was performed using the original synthesizer chromatographic equipment. Quality controls with analytical HPLC were performed with a Varian system consisting of a ProStar 210 solvent delivery module (Varian, Washington, USA), a FC-1000 gamma detector (Bioscan, Washington DC, USA) and a Varian ProStar 325 UV–Vis detector (Varian, Mulgrave, Australia).
Materials
Chemicals and solvents were purchased from Fisher Scientific (Fair Lawn, NJ) and used without further purification. The precursors, metaraminol (free base) (ABX, Germany) were dissolved in acetonitrile for the radiochemical synthesis procedure.
The silver triflate was prepared based on the reported method [25].
Modification of the [11C]methylation module
The modifications of the commercial GE TRACERlab FXc were shown in Fig. 1. The normally opened position of valve V14 was connected to the normally closed position of valve V11 through a Luer lock adapter. This modification was designed to ensure that the final product, collecting (-)-[11C]HED peak of semi-preparative HPLC, was inflowing into the product bottle instead of into the round bottle flask.
Modified GE TRACERlab FXc for preparation of (-)-[11C]HED. The red arrow presented the site of modification (M), while the purple line presented the new connection inserting a pair of Luer lock adapters (L) Modified GE TRACERlab FXc for preparation of (-)-[11C]HED. The red arrow presented the site of modification (M), while the purple line presented the new connection inserting a pair of Luer lock adapters (L). (Color figure online)
Reagent preparation for synthesis of (-)-[11C]HED
Metaraminol (free base) was dissolved in acetontrile and then placed into the reactor. Sterile water was added into the reservoir above V2 which was prepared to quench the radiolabeled reaction and subsequently transferred to the injection loop of the HPLC-system passing the fluid detector. The semi-preparative HPLC column (NUCLESOIL 100-5C18Nautilus, 250 mm × 10 mm, 5 µm) was installed onto the synthesis module and mobile solution (saline/ethanol (95:5) (v/v %)) was placed into Eluent-1 bottle. The flow rate was 4.5 mL/min and wave length of UV detector was 280 nm.
Synthesis of (-)-[11C]HED
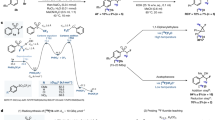
(-)-[11C]HED was synthesized by N-[11C]methylation of metaraminol with [11C]methyl triflate (Fig. 2). [11C]Methyl triflate was produced based on gas-phase synthesis of [11C]methyl iodide followed by on-column formation of [11C]methyl triflate [25], using the normal functions of the synthesizer. [11C]Methyl triflate from the triflate column was then bubbled into the reaction vessel containing the precursor solution at 0 °C until maximum radioactivity had been accumulated. The reactor was heated for several minutes to ensure that the radiolabeled reaction was well performed, and then sterile water was added into the reactor from the reservoir above V2 to quench the reaction. The mixture was then pushed through six-way valve into the semi-preparative HPLC for separation and purification of the product. The product peak (retention time ~ 8.5 min) was collected through L into the product vial. Final dose was transferred into a sterile vacuum vial through a 0.2 µm sterile filter.
Quality control
The radiochemical purity and chemical purity were determined by RP-HPLC with a Symmetry ™ C-18 column (150 mm × 4.6 mm, 5 µm) using the mobile phase whose ingredient was same to the mobile phase of semi-preparative HPLC separation, at a flow rate of 1.0 mL/min. The products were monitored by UV detector (wave length 280 nm) and a radio detector. The retention time was 5 min for (-)-[11C]HED.
Optimization of reaction conditions
Single-factor analysis and orthogonal experimental design were applied to investigate the optimized methylated synthesis conditions with L9 (34) orthogonal test.
Single-factor analysis
The effects of reaction temperature, reaction time and amount of precursor were examined as the relatively significant factors to optimize the [11C]methylation of metaraminol.
Effects of reaction temperature
While reaction time and amount of precursor were unchanged, the labeling yields of radiochemical reaction were measured under 0 °C, 25 °C, 50 °C, 60 °C and 70 °C to be reaction temperature, respectively.
Effects of reaction time
In this part of work, the labeling yields of radiochemical reaction were measured when several points of time had been chosen as reaction time, including 40 s, 1 min, 1.5 min, 2 min and 5 min. The reaction temperature and amount of precursor used during these experiments were 70 °C and 0.2 mg, respectively.
Effects of amount of precursor
In view of the optimal reaction temperature and time had been established, the labeling yields of radiochemical reaction were measured under 0.1 mg, 0.2 mg, 0.3 mg, 0.4 mg and 0.5 mg to be the amount of precursor, respectively. Higher amounts than 0.5 mg have not been investigated.
Orthogonal experimental design
The orthogonal array design was built in the above factors including reaction temperature, reaction time and amount of precursor with 3 relevant levels respectively, according to the results of single-factor analysis. The labeling yields were measured as the assessment criteria. The optimized methylated conditions were determined through analysis of variance. The orthogonal experiment factors and levels of radiosynthesis (-)-[11C]HED was listed in Table 2.
Results and discussion
Optimization of reaction conditions
Single-factor analysis
The effects of reaction temperatures, reaction times and different amounts of precursor are summarized in Table 1 and discussed below. As shown in Table 1, it is obviously that higher reaction temperature and amount of precursor resulted in higher labeling yields, meanwhile, prolongation of the reaction time gives significant lower yields. In order to get a decent yield, it is necessary to find a balance between reaction time and temperature, for that increased reaction temperature enhanced the activity of the reaction while the prolonged reaction time may lead to a radioactive decay.
Orthogonal test
The factors and levels of methylated synthesis conditions for orthogonal test and the analysis of L9 (34) test results are shown in Tables 2 and 3. The effect of methylated synthesis conditions on labeling yield is in the order A > C > B according to the R values. The reaction temperature is the main process factor, followed by the amount of precursor and the reaction time. The optimum condition was A3 B1 C3, which the reactor was heated to 70 °C to ensure 0.3 mg precursor methylated with [11C]-CH3OTf for 40 s.
Although higher temperature increases the labeling yield, a reaction temperature of 70 °C is preferred to avoid the evaporation of the solvent acetontrile. The amounts of precursor up to 0.5 mg show an almost linear positive correlation. Though, using of 0.2 mg of precursor is preferred when considering the final HPLC purification of the radioligands and the commercial availability and cost of precursors.
From the above, 70 °C, 40 s and 0.2 mg precursor have been chosen as optimal conditions for routine production of (-)-[11C]HED in our laboratory.
Production characteristics under the conditions chosen
(-)-[11C]HED was synthesized with the optimized methylated synthesis conditions using automated procedures. Typical irradiation was stopped as soon as the desired activity level was reached (~ 23 GBq). Typical beam currents were 30 µA and irradiation was stopped as soon as the desired activity level was reached ([11C]CO2, i.e. ~ 23 GBq). The total synthesis time was about 25 min from end-of-bombardment. The radiochemical yields were 62.00 ± 5.68% (50.34–71.91%) (n = 50) (decay corrected and based on [11C]CH3I). The radiochemical purities and chemical purities of final products were over 99% (n = 50) and 97% (n = 50), respectively. The specific activities of final products were 24.38 ± 2.17 (19.35–27.75) GBq/µmol (n = 50) at end-of-synthesis. The retention time of final product is 4.997 min while the precursor is 3.951, which are consist with the RT of references (Fig. 3).
In our study, the specific activities of final products were less than previous reports [5, 24]. In view of needed activities were bombed for radiosynthesis of (-)-[11C]HED, instead of standard bombardment, less radioactivities (the desired activity level was about 23 GBq) were achieved than reported previously. In case of more radioactivities are achieved for further radiosynthesis, the specific activities of our study will be increased.
The major highlights of our study are as following, (1) The short half-life (20.38 min) is one of the unique challenges for the art and science of radiopharmaceutical synthesis with carbon-11. The 25 min total synthesis time from end-of-bombardment in this research is relatively short, considering of using the HPLC purification method. (2) The research of effects of reaction conditions to labeling yield and the radiochemical yield of (-)-[11C]HED synthesis, using single-factor analysis and orthogonal experimental design in the study, is the first investigation as far as we know. The forthright benefit of this research is optimizing the radio synthesis reaction, for instance, the amount of precursor is only 0.2 mg. As is known to us, for better and faster chromatographic separation, a reduction in the amount of precursor is always desirable. (3) The HPLC purification methods of (-)-[11C]HED, previously reported in the literatures, are complicated, time-consuming or not suitable for intravenous administration except for further formulation. In this study, saline-ethanol eluent system is used in separating product from the impurities aiming to ensure that the product solution can be suitable for intravenous administration.
Conclusion
In this study, an optimized and improved processing technology for radiopharmaceuticals preparation had been descripted. Injectable (-)-[11C]HED was prepared with high radiochemical yield, radiochemical purity and chemical purity using [11C]methyl triflate method, by optimizing methylated conditions and improving purification protocol. The modification of the synthesis module can facilitate multiple syntheses of different 11C-labeled radiotracers in the same module and speed up radiosynthesis for growing clinical PET studies as well.
References
Wang B, Xie M, Li R, Owonikoko TK, Ramalingam SS, Khuri FR, Curran WJ, Wang Y, Deng X (2014) Role of Ku70 in deubiquitination of Mcl-1 and suppression of apoptosis. Cell Death Differ 21(7):1160–1169. https://doi.org/10.1038/cdd.2014.42
Xie MH, Wu QJ, Jiang YF, Bao P, Zhang Y (2009) A phage RNA-binding protein binds to a non-cognate structured RNA and stabilizes its core structure. Biochem Biophys Res Commun 378(2):168–173. https://doi.org/10.1016/j.bbrc.2008.10.160
Han B, Park D, Li R, Xie M, Owonikoko TK, Zhang G, Sica GL, Ding C, Zhou J, Magis AT, Chen ZG, Shin DM, Ramalingam SS, Khuri FR, Curran WJ, Deng X (2015) Small-molecule Bcl2 BH4 antagonist for lung cancer therapy. Cancer Cell 27(6):852–863. https://doi.org/10.1016/j.ccell.2015.04.010
You S, Li R, Park D, Xie M, Sica GL, Cao Y, Xiao ZQ, Deng X (2014) Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther 13(3):606–616. https://doi.org/10.1158/1535-7163.MCT-13-0608
Rosenspire KC, Haka MS, Van Dort ME, Jewett DM, Gildersleeve DL, Schwaiger M, Wieland DM (1990) Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: a false transmitter agent for heart neuronal imaging. J Nucl Med 31(8):1328–1334
Schwaiger M, Kalff V, Rosenspire K, Haka MS, Molina E, Hutchins GD, Deeb M, Wolfe E Jr, Wieland DM (1990) Noninvasive evaluation of sympathetic nervous system in human heart by positron emission tomography. Circulation 82(2):457–464
Allman KC, Stevens MJ, Wieland DM, Hutchins GD, Wolfe ER Jr, Greene DA, Schwaiger M (1993) Noninvasive assessment of cardiac diabetic neuropathy by carbon-11 hydroxyephedrine and positron emission tomography. J Am Coll Cardiol 22(5):1425–1432
Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M (2001) Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med 345(10):731–738. https://doi.org/10.1056/NEJMoa010519
Calkins H, Allman K, Bolling S, Kirsch M, Wieland D, Morady F, Schwaiger M (1993) Correlation between scintigraphic evidence of regional sympathetic neuronal dysfunction and ventricular refractoriness in the human heart. Circulation 88(1):172–179
Carrio I (2001) Cardiac neurotransmission imaging. J Nucl Med 42(7):1062–1076
Pietila M, Malminiemi K, Ukkonen H, Saraste M, Nagren K, Lehikoinen P, Voipio-Pulkki LM (2001) Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur J Nucl Med 28(3):373–376
Stevens MJ, Raffel DM, Allman KC, Dayanikli F, Ficaro E, Sandford T, Wieland DM, Pfeifer MA, Schwaiger M (1998) Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation 98(10):961–968
Franzius C, Hermann K, Weckesser M, Kopka K, Juergens KU, Vormoor J, Schober O (2006) Whole-body PET/CT with 11C-meta-hydroxyephedrine in tumors of the sympathetic nervous system: feasibility study and comparison with 123I-MIBG SPECT/CT. J Nucl Med 47(10):1635–1642
Hernandez FC, Sanchez M, Alvarez A, Diaz J, Pascual R, Perez M, Tovar I, Martinez P (2000) A five-year report on experience in the detection of pheochromocytoma. Clin Biochem 33(8):649–655
John H, Ziegler WH, Hauri D, Jaeger P (1999) Pheochromocytomas: can malignant potential be predicted? Urology 53(4):679–683
Lenders JW, Eisenhofer G, Mannelli M, Pacak K (2005) Phaeochromocytoma. Lancet 366(9486):665–675. https://doi.org/10.1016/S0140-6736(05)67139-5
Mann GN, Link JM, Pham P, Pickett CA, Byrd DR, Kinahan PE, Krohn KA, Mankoff DA (2006) [11C]metahydroxyephedrine and [18F]fluorodeoxyglucose positron emission tomography improve clinical decision making in suspected pheochromocytoma. Ann Surg Oncol 13(2):187–197. https://doi.org/10.1245/ASO.2006.04.022
Pacak K, Ilias I, Adams KT, Eisenhofer G (2005) Biochemical diagnosis, localization and management of pheochromocytoma: focus on multiple endocrine neoplasia type 2 in relation to other hereditary syndromes and sporadic forms of the tumour. J Intern Med 257(1):60–68. https://doi.org/10.1111/j.1365-2796.2004.01425.x
Shulkin BL, Wieland DM, Baro ME, Ungar DR, Mitchell DS, Dole MG, Rawwas JB, Castle VP, Sisson JC, Hutchinson RJ (1996) PET hydroxyephedrine imaging of neuroblastoma. J Nucl Med 37(1):16–21
Shulkin BL, Wieland DM, Schwaiger M, Thompson NW, Francis IR, Haka MS, Rosenspire KC, Shapiro B, Sisson JC, Kuhl DE (1992) PET scanning with hydroxyephedrine: an approach to the localization of pheochromocytoma. J Nucl Med 33(6):1125–1131
Trampal C, Engler H, Juhlin C, Bergstrom M, Langstrom B (2004) Pheochromocytomas: detection with 11C hydroxyephedrine PET. Radiology 230(2):423–428. https://doi.org/10.1148/radiol.2302021678
Yamamoto S, Hellman P, Wassberg C, Sundin A (2012) 11C-hydroxyephedrine positron emission tomography imaging of pheochromocytoma: a single center experience over 11 years. J Clin Endocrinol Metab 97(7):2423–2432. https://doi.org/10.1210/jc.2011-3342
Nagren K, Muller L, Halldin C, Swahn CG, Lehikoinen P (1995) Improved synthesis of some commonly used PET radioligands by the use of [11C]methyl triflate. Nucl Med Biol 22(2):235–239
Van Dort ME, Tluczek L (2000) Synthesis and carbon-11 labeling of the stereoisomers of meta-hydroxyephedrine (HED) and meta-hydroxypseudoephedrine (HPED). J Label CPD Radiopharm 43(6):603–612
Jewett DM (1992) A simple synthesis of [11C]methyl triflate. Int J Rad Appl Instrum A 43(11):1383–1385
Acknowledgements
This work was supported by the fund of National Key Clinical Specialty Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, L., Li, X., Dong, L. et al. A novel strategy for the preparation of the injectable PET/CT radiopharmaceutical (-)-[11C]-(1R,2S)-meta-hydroxyephedrine ((-)-[11C]HED. J Radioanal Nucl Chem 320, 543–549 (2019). https://doi.org/10.1007/s10967-019-06534-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06534-5