Abstract
A theranostic nanoparticle was synthesized based on diethylstilbestrol conjugated with phosphate, copper, and indium (DESCIP) and labelled with 99mTc which can be used for SPECT imaging of ER-enriched cancers. In vitro biological activity of 99mTc-DESCIP was examined in breast adenocarcinoma cells (MCF-7), prostatic carcinoma cells (PC-3), and pulmonary epithelial cells (A-549). In vivo lymph node imaging was performed in normal and receptor blocked female New Zealand rabbits. Results demonstrated that 99mTc-DESCIP and DESCIP has potential for imaging ER-enriched tumors such as breast and prostate tumors, and their metastases in the lung, as well as improving management for their therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorescence-based detection is the most common method utilized in biosensing because of its high sensitivity, simplicity, and diversity. In the era of nanotechnology, nanomaterials are starting to replace traditional organic dyes as detection labels because they offer superior optical properties, such as brighter fluorescence, wider selections of excitation and emission wavelengths, higher photostability, etc. Their size- or shape-controllable optical characteristics also facilitate the selection of diverse probes for higher assay throughput. Furthermore, the nanostructure can provide a solid support for sensing assays with multiple probe molecules attached to each nanostructure, simplifying assay design and increasing the labelling ratio for higher sensitivity.
Cell imaging using fluorescence dye can supply detailed anatomic information of the cancerous cell or tissue. Consequently, it is used for both clinical and research purposes. Quantum dot nanoparticles present a promising alternative. They can be easily prepared using II-VI elements (such as CdS, CdSe, CdTe, ZnS, ZnSe, CuInP, InP, etc.) that are dissolved in various organic solvents or in aqueous media at appropriate conditions. They not only have a tendency to bioconjugate but also demonstrate fluorescence properties. Because of their semiconductor properties, they have multiple uses in other areas of technology besides medical imaging.
The major endocrine-related cancers in humans display gender-specific hormonal responsiveness. In women, estrogen plays an important role in breast and endometrial cancer, and in men, androgen plays an important role in prostate cancer [1,2,3,4,5,6]. Two types of estrogen receptors have been classified: ER-α and ER-ß. The treatment strategy depends on whether the receptors are positive or negative [7,8,9,10]. For example, estrogen receptor-blocking drugs such as tamoxifen have been used to treat ERα-positive tumors [4, 5].
Diethylstilbestrol (DES), a nonsteroidal estrogen, was first synthesized in 1938 [1]. It was the first synthetic estrogen to be made and was occasionally prescribed for the treatment of advanced breast and prostate cancer [2, 3]. A well-known teratogen and carcinogen, DES inhibits the hypothalamic-pituitary–gonadal axis, consequently blocking the testicular synthesis of testosterone, lowering plasma testosterone, and inducing a chemical castration [2, 3, 6]. Recently, we synthesized a phosphate derivative of diethylstilbestrol (DESP) and labelled it with 99mTc to study its imaging potential [11]. DESP is the diphosphate ester of a synthetic, nonsteroidal form of estrogen (Fosfestrol; Diethylstilbestrol diphosphate; Phosphestrol) [12].
The main objective of this work is to determine the potential of DES conjugated with phosphate-, copper-, and indium-nanoparticles (DESCIP) and labelled with 99mTc for targeting and detecting estrogen receptor-enriched cancer cells. By adding phosphorus-, copper- and indium-containing nanoparticles to the DES structure, we conferred multimodality imaging capability (single-photon emission computed tomography and fluorescence). In vitro cell labeling and in vivo lymph node imaging properties were determined. Figure 1 shows a schematic representation of DESCIP distribution in cancer cells.
Experimental
Equipment and sources of material
Na99mTcO4 was supplied by the Sifa University of Izmir/Turkey. DES was purchased from Sigma. All other chemicals were supplied from Merck Chemical Co., (Darmstadt Germany) and Aldrich Chemical Co., (Germany). Breast adenocarcinoma cells (MCF-7), prostate carcinoma cells (PC-3), and pulmonary epithelial cells (A-549) were supplied from the American Type Culture Collection (Manassas, VA USA).
The following equipment from Ege University Institute of Nuclear Sciences were used: Cd (Te) detector equipped with a thin layer radio chromatography (TLRC); high-performance liquid chromatography (Shimadzu Corporation, Somerset, NJ, USA) equipped with a LC-10ATvp quaternary pump, SIL- 20A HT auto sampler, and 7.0 µm reversed-phase-C-18 column 250 × 21 mm I.D. (Macherey–Nagel, Bethlehem, PA, USA); and Perkin Elmer Optima 2000 DV (to produce Inductively Coupled Plasma OES (ICP-OES) chromatograms). The Institute of Technology, Izmir provided X-ray powder diffraction (XRD) analyses using the Philips X’Pert Pro (Phillips, Amsterdam, Netherlands), imaging using the Philips XL-30 FEG Scanning Electron Microscope (SEM) (Phillips, Amsterdam, Netherlands), and size analyses in liquid ambience chromatograms using a Zetasizer. Fourier Transform Infrared Spectra (FTIR) spectra were obtained from the Celal Bayar University. Nuclear magnetic resonance (NMR) chromatograms were obtained from Ataturk University.
Synthesis of DESCIP
26.8 mg (0.1 mmol) of DES was dissolved in 4 mL pyridine using a magnetic stirrer. Then, 24.5 mg (0.2 mmol) of copper(I)acetate, 44.2 mg (0.2 mmol) of indium(III)chloride, and 100 µl of POCl3 were added drop-wise. The mixture was stirred for 24 h at room temperature. After the reaction completed and pyridine evaporated, the product was washed with 10 mL of dichloromethane and 10 mL of distilled water. The product was rewashed with 10 mL of dichloromethane and 10 mL of distilled water. It was centrifuged 10 min at 2500 rpm. Then, the upper (aqueous) phase was separated and the pH was adjusted to 9 using 3 M LiOH solution. The mixture was re-centrifuged for 10 min at 2500 RPM and the upper (aqueous) phase was eluted using with Dowex 50 anion exchange column. The eluate was stored at 4 °C.
Structural analysis of DESCIP
Inductively coupled plasma mass spectrometry (ICP-MS)
ICP analyses were conducted in order to understand the elemental composition of DESCIP. Standard solutions of copper, indium, and phosphorous were used to determine the elemental composition of DESCIP nanoparticles. 100 µL/µg of DESCIP solution was used for the analyses.
Fourier transform infrared spectra (FTIR) and nuclear magnetic resonance (NMR)
10 mg of lyophilized DESCIP were mixed thoroughly with 100 mg of anhydrous KBr in a mortar while grinding with the pestle; the pellet was prepared for the acquisition of FTIR spectra. 1H-NMR and 13C-NMR spectra were acquired to identify molecular structures (Fig. 2). CDCl3 was used as solvent. Theoretical (ACD/LogP Algorithm) spectra were used for comparison with experimental NMR spectra.
Characterization of DESCIP
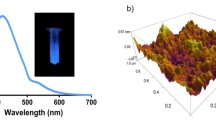
The mean particle size was determined by dynamic light scattering and the surface charge of the nanoparticles was investigated through zeta potential measurements after dilution in ethanol (Fig. 3). The particle size and morphology of DESCIP were assessed by SEM (Fig. 4).
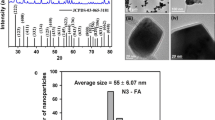
The nanoparticles were analyzed by XRD, Cu Kα (λ = 1.541 A°) radiation wavelength at 40 kV and 40 mA. The samples were scanned between 0° and 80 °C.
Fluorescence spectra of DESCIP
1 mg/mL of DESCIP was prepared in ethanol. The fluorescence spectra were acquired at 250 nm excitation and 450 nm emission wavelengths.
99mTc labeling of DESCIP
Stock solutions of DESCIP (1.19 mg/0.2 mL distilled water) and SnCl2 (1 mg/1 mL distilled water) were prepared. Then, 50 µL of DESCIP and 100 µL of SnCl2 were mixed in a tube at a pH of 7. 1 mCi (37 MBq) of 99mTcO4 was added to the mixture and the resulting product was incubated for 20 min at room temperature. Rf value and radiolabeling yield of 99mTc labeled DESCIP (99mTc-DESCIP) were determined by TLRC.
Quality control studies of 99mTc-DESCIP
Thin layer radio chromatography (TLRC)
The same procedure as in our previous reports was applied to obtain TLRC chromatograms (mobile phase: acid citrate dextrose and physiological serum (0.90% NaCl)). TLRC chromatograms were acquired using a Bioscan AR-2000 Scanner. Rf values of each component were determined. Table 1 presents Rf values of 99mTcO4 −, Reduced 99mTc and 99mTc-DESCIP.
Stability testing
To perform stability tests of the radiolabeling, 50 µg/50 µL aliquots of 99mTc-DESCIP were incubated in physiological serum at 37 °C in a humidified 5% CO2 incubator. Then, they were analyzed using RTLC at several time intervals to determine if there is any impurity up to 4 h during the observation period.
Lipophilicity studies
Lipophilicities were determined similar to our previous reports [13, 14]. Briefly, 100 μL aliquots of 99mTc-DESCIP were put into a test tube together with 300 μL of n-octanol and 300 μL of PBS volumes. Subsequently, the mixture was vortexed for 1 min at room temperature and centrifuged at 2500 rpm for 30 min. Then, the radioactivities of the top and bottom phases were counted. The partition coefficient (LogP) was determined by dividing the counts in the n-octanol layer with the counts in the aqueous layer.
In vitro Bioaffinities of 99mTC-DESCIP
In vitro bioaffinities of 99mTc-DESCIP were examined in breast adenocarcinoma (MCF-7), prostate carcinoma (PC-3), and pulmonary epithelial (A-549) cell lines. Cells were cultured in in DMEM, 10% FBS (Fetal Bovine Serum), 1%PSS, (100 units/ml penicillin and 100 μg/ml streptomycin), 1%l-Glutamine, 2% NaHCO3, 1%sodium pyruvate, 1% non-essential amino acid mixture in cell culture flasks. After cells have proliferated enough to cover 80% of the flasks, they were separated from the surface using 0.25% (w/v) trypsin–EDTA solution and distributed into 24-well plates (500 µl for each well). Time-dependent incorporation ratios were then examined in ER positive (+) and ER negative (−) cells.
Cell incorporation study with receptor unblocked ER negative (−) cells
10 µg (4 MBq/µg) of 99mTc-DESCIP was added to 5 mL of culture medium and the resulting solution was administered upon the cells in the 24-well plates. At 30, 60, and 120 min following administration, the radioactivity in the cells were counted with Cd(Te) semi-conductor detector (A0). Then, they were washed by RIPA buffer for three times and the counts were read for second (A1) time (Table 2) (Fig. 7).
Cell incorporation study with receptor blocked ER positive (±) cells
In order to determine receptor saturation, 1.2 mg 99mTc-DESCIP was dissolved in 20 µl ethanol, and then it was put into 5 ml culture medium and incubated with the cells for 2 h. Following that, the study continued similar to that for ER (−) cells (Table 3) (Fig. 7).
Fluorescence imaging studies of DESCIP
In order to obtain fluorescence images, cells (MCF-7, PC-3 and A-549) were cultured and prepared on chamber slide plates. Then, 200 µl of DESCIP was applied to cells and fluorescence imaging was obtained using the fluorescence microscope at 20X and 40X magnification over 4 h (Fig. 8).
For comparison, DESP labelled with FITC (fluorescein isothiocyanate) (FITC), a common fluorescence dye, was also prepared. For FITC labeling, 1 mg of DESP solution was prepared in 1 mL of 0.1 M sodium carbonate buffer (pH 9). Then, 1 mg of FITC was dissolved in 1 mL of anhydrous DMSO. 50 µL of the FITC solution was added to the DESP solution very slowly in 5 µL aliquots and continuously stirred. The obtained solution was incubated for 8 h at 4 °C in the dark. 50 mM of NH4Cl was added to the solution and it was incubated for 2 more hours at 4 °C. Finally, unbound FITC was separated from the conjugate by gel filtration using Sephadex G-25 column. MCF-7, PC-3 and A-549 cells cultured on chamber slides were washed with PBS and the medium with FITC-labeled DESP was added to the cells. After a 4 h incubation time, cells were washed with PBS(× 2) and imaging was acquired using fluorescence microscopy with a green filter (520 nm) (Fig. 9).
All fluorescence imaging studies were performed in dark conditions.
Cytotoxicities
The MTT assay procedure was applied similar to our previous reports [14, 15]. The cells (MCF-7, PC-3, and A-549) were sustained in minimal essential medium and were prepared from confluent monolayer cultures and plated at a density of 8 × 10E3 cells in a 96-well plate. The incubation conditions were 48 h at 37 °C. Stock solutions of DESCIP and DES (1, 2, 3, 4 ng/mL) were prepared by dissolving them in PBS. Appropriate dilutions of the samples were prepared in growth medium and added to the cells. The resulting mixture was incubated for 24, 48, and 72 h. After each incubation period, viable cells were determined using MTT.
The absorbance was measured on a Varioscan microplate reader (Thermo, USA) at 520 nm. The intensity of color is directly proportional to the percentage of viable cells. Tests were carried out in quadruplicate and each experiment was repeated six times.
Sentinel lymph node imaging studies in rabbits
Static imaging and dynamic imaging were performed using a gamma camera (Diacan Instruments) in Celal Bayar University, School of Medicine, Department of Nuclear Medicine. All procedures were approved by the Animal Ethics Committee at Ege University. Eight normal and eight receptor blocked female New Zealand white rabbits (weight 2.5–3.0 kg) were used in the study. A cocktail 3 mL of Ketamine (50 mg/kg)/xylazine (10 mg/kg) anesthetic was injected intramuscularly in the brachial muscle. The hair on the medial side of the distal hind limb was shaved. 99mTc-DESCIP (7.4 Mbq/10.8 mg/0.1 ml per rabbit) nanoparticles were injected intradermally into the distal hind limb. Receptor saturation was supplied as previously by using DES [11]. 1.2 mg of DES was dissolved in 20 µL of ethanol then diluted with 15 mL of physiological serum. 10 g of DES was taken and used for receptor saturation 15 min prior to 99mTc-DESCIP injection.
Dynamic (64 × 64 matrix, 1 zoom) and static (256 × 256 matrix, 1 zoom) lymphoscintigraphic images were taken up to 24 h. Fifteen dynamic images (each for 20 s) and 5 min static images (at 5th, 15th, 30th, 60th, 120th min and 24 h) were obtained in the supine position (Fig. 10).
Statistical analysis
One-way analysis of variance and non-linear regression was conducted using the GraphPad software (GraphPad Software, Inc, San Diego, CA). Statistical differences were considered significant at p < 0.05.
Results and discussion
Proposed structure of DESCIP
FTIR spectra of DESCIP showed OH stretch at 3434 nm; C=C stretch at 1641 nm; C–O stretch at 1118 nm; P–O stretch at 1034 nm; C–H stretch at 909 nm; and C–In stretch at 553 nm (Fig. 2).
1H-NMR and 13C-NMR spectra of DESCIP verified that the estrogen ring in the structure is protected; main peaks were 1 ppm (21 and 23 numbered aliphatic methyl groups); 2–3 ppm (20 and 22 numbered CH2 groups); 5 ppm (OH protons); and 7 ppm (aromatic ring protons).
13C-NMR spectra presented main peaks of 10–20 ppm for methyl carbons; 30 ppm for CH2 groups; 120, 150 ppm for aromatic ring carbons; and 136 ppm for methylene carbons (supplementary data).
Elemental ratios were found to be In2Cu5P7 according to ICP analyses
Lastly, fluorescence spectra showed green fluorescence (Fig. 6). The excitation wavelength was 250 nm and the emission wavelength was 450 nm. NMR, FTIR, and ICP analyses confirmed that the estrogen ring was existent in the structure and atomic ratios was 5/2/7 for copper, indium, and phosphorous.
Characterization of DESCIP
X-ray diffraction was used for characterization of nanomaterials. Although there were no data for CuInP nanoparticles, our results confirmed characteristic diffraction peaks for Cu, In, and P (Fig. 5). The characterization of the structures was verified by crystal characteristics of the face centered cubic structure.
SEM images determined that the size of DESCIP nanoparticles were about 10–20 nm (Fig. 4) while hydrodynamic diameters of DESCIP nanoparticles were about 50 nm (Fig. 3). Additionally, DESCIP nanoparticles were shown to have stable green fluorescence at 450 nm (350 nm excitation) (Fig. 6).
Quality control studies showed that DESCIP nanoparticles were successfully labelled with 99mTc, with a 99% labeling yield using SnCl2 as a reducing agent, and stability was established for at least 4 h at room temperature. Rf values of 99mTcO4 −, reduced 99mTc and 99mTc-DESCIP are presented in Table 1. Both DESP and DESCIP can be labeled with 99mTc by direct labeling using SnCl2 as reducing agent. 99mTc can be attached to DESCIP through P and O atoms similar mechanism with DESP [11] and methylenediphosphonate [16] which have been used as bone imaging agent .
The lipophilicities of 99mTc-DESP and 99mTc-DESCIP were found to be − 2.0 ± 0.01, and − 1,23 ± 0.20, respectively.
In vitro incorporation results
According to time-dependent in vitro incorporation results (Fig. 7, Tables 2, 3), 99mTc-DESCIP or 99mTc-DESP both showed higher uptake in every cell line than 99mTcO4 − as a control group (p < 0.05). The highest incorporation ratio was observed in the MCF-7 cell line and the lowest in the A-549 cell line. Estrogen plays an important role not only in the development of normal mammary glands, but also in the proliferation and progression of breast cancer. There are two types of estrogen receptors: ERα and ERβ which was first reported in 1996 [17, 18]. The MCF-7 cell line is one of the most frequently used cell lines for functional observation of estrogen response and represents an ERα-positive breast cancer cell line [19, 20]. On the other hand, the prostate is known as an androgen target; however, it is also targeted for estrogen. Recent studies have shown that estrogens, in combination with androgens, play critical roles in prostate carcinogenesis [21,22,23]. Moreover, ERα is also present in prostate stromal cells of normal, hyperplastic, and cancerous prostate tissue [23,24,25,26]. There are some reports indicating a possible role of ERα in promoting inflammation, proliferation, and metastasis in prostate cancer [27, 28] and thus ER receptor targeting can be considered as a potential target for prostate cancer prevention therapy [29, 30]. Lastly, although estrogen receptors are highly expressed in breast cancer tissue, there are also some reports indicating estrogen receptors (ERα and ERβ) expression in normal lung tissue and small cell lung cancer tissue [31,32,33]. Our results show that we achieved the highest incorporation ratios on ER (+) positive MCF breast cancer cells, followed by PC-3 prostate cancer cells, and then A-549 lung cancer cells. Therefore, we propose that incorporation ratios were consistent with the relative levels of ER expressions in the cells.
Fluorescence imaging of the cells
Stable fluorescence images were obtained of the DESCIP incorporated cells (MCF-7, PC-3, and A-549) using green fluorescence of the DESCIP nanoparticles. Figure 8 shows fluorescence images of the cells. DESCIP presented significant fluorescence intensity on all cell lines (Fig. 8). For comparison, FITC commercial green fluorescence dye labeled DESP were also incorporated into cells (Fig. 9). Fluorescein isothiocyanate (FITC) (3′,6′-dihydroxyspiro[isobenzofuran-1(3H),9′-[9H]xanthen]-3-one) is a commercial dye molecule for fluorescent labeling. It binds to biomolecules substituting (-N=C=S group with hydrogen. FITC conjugated with DESP for comparison DESCIP fluorescent within the cells.
Cytotoxicity results
Four different concentrations (1, 2, 3, 4 ng/ml) were tested at 0 min, 24 h, 48 h, and 72 h of exposure. The 0 min value was chosen to confirm the viability of the cells. Cell viability above 80% was detected at 24, 48 and 72 h for all cell lines (MCF-7, PC-3, and A-549) for DESP and DESCIP. The results are given in Tables 4 and 5 for DESP and DESCIP, respectively.
Previously, Sharp et al. reported that 10–5 M, DES blocked the formation of microtubules in vitro [34]. Additionally, DES-induced apoptosis in androgen independent prostate cancer cell lines has also been reported independent from estrogen receptors [35, 36]. Finally, both DES monophosphate and diphosphate were cytotoxic on different malignant and transformed cell lines [37, 38].
In vivo scintigraphic imaging studies with rabbits
To evaluate the in vivo localization of 99mTc-DESCIP, scintigraphic images were taken after intradermal injection. Figure 10 shows static scintigrams of normal and receptor blocked female rabbits at several time intervals. Significant lymph node uptake dissimilarities were seen between normal and receptor blocked rabbits (Fig. 10). The uptake ratios were increased 3–4 between 1 h and 24 h imaging.
The sentinel lymph node is the first node that serves a cancer. It is exponentially more likely to contain a metastasis than a downstream, non-sentinel node. If one fails to identify sentinel lymph nodes that contain metastases, a physician is likely to incorrectly stage a cancer and inaccurately inform a patient that they are metastasis free.
Multimodality imaging
There are many advantages to combining radionuclide imaging and FL imaging modalities into a multimodality imaging approach. Both PET/FL and SPECT/FL imaging involve the use of good, clinical contrast agents that lack toxicity and allow us to image disease at high spatial and longer temporal resolutions at trace quantities [39, 40]. The two modalities also complement each other in terms of spatial resolution at the histological and artificial level. Fluorescent probes are stable while the lifetime of radionuclide labeled probes depend on their half-life; however, radionuclide labeled probes are superior to FL for their efficacy in providing non-invasive, high resolution visualization of cell and tissue structure through deep tissue. Therefore, multimodality radionuclide/FL probes are superior to standalone SPECT/PET and FL contrast agents.
In many cancers, a primary tumor is easily resected and not a cause for concern. Cancer metastasis, however, can be lethal. For this reason, it is vitally important to accurately identify the presence of metastasis following diagnosis of cancer. Lymph node imaging is important to determine if metastasis exists or not. Metastasis is easily observed non-invasively in 18F-FDG-PET scans. Unfortunately, 18F-FDG-PET scans only indicate metabolism and do not provide biomarker or morphological information that an oncologist could use in determining appropriate drugs for treating a cancer. Because of this, it is vital to isolate and biopsy the lymph nodes that serve a cancer, even in patients who receive 18F-FDG-PET scans. This is especially true in breast, melanoma, and squamous cell carcinoma of the oral cavity.
The results showed that DESCIP nanoparticles can be radiolabeled with 99mTc in high yields. It may potentially be proposed as multifunctional probe both for SPECT and optical for estrogen receptor targeting.
Conclusion
DESCIP (DES containing nanoparticles) demonstrates both florescence capability and ER receptor targeting specificity. Nanostructured florescence property was successfully conferred, and desirable particle size and zeta potential values were obtained. DESCIP was also successfully radiolabeled with 99mTc. Biological behavior and ER targeting capability was determined by in vivo and in vitro studies. Results indicate that DESCIP may be beneficial for detecting estrogen-overexpressed tissues in animal models.
DESCIP improves the effects of DES and may be a novel and important means of increasing theranostic efficacy of cytotoxic chemotherapy and imaging agents.
References
Dodds EC, Goldberg L, Larson W, Robinson R (1938) Estrogenic activity of certain synthetic compounds. Nature 141:247–248
Turo R, Smolski M, Esler R, Kujawa ML, Bromage SJ, Oakley N, Adeyoju A, Brown SCW, Brough R, Sinclair A, Collins GN (2014) Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol 48:4–14
Benninka HJTC, Verhoevena C, Dutmana AE, Thijssenb J (2017) The use of high-dose estrogens for the treatment of breast cancer. Maturitas 95:11–23
de Vries Schultink AH, Alexi X, van Werkhoven E, Madlensky L, Natarajan L, Flatt SW, Zwart W, Linn SC, Parker BA, Wu AH, Pierce JP, Huitema AD, Beijnen JH (2017) An antiestrogenic activity score for tamoxifen and its metabolites is associated with breast cancer outcome. Breast Cancer Res Treat 161:567–574
Muftuler FZB, Unak P, Teksoz S, Acar C, Yolcular S, Yurekli Y (2008) I-131 labeling of tamoxifen and biodistribution studies in rats. Appl Radiat Isot 66:178–187
Palmieri C, Cheng GJ, Saji S, Zelada-Hedman M, Wärri A, Weihua Z, Van Noorden S, Wahlstrom T, Coombes RC, Warner M, Gustafsson JÅ (2002) Estrogen receptor beta in breast cancer. Endocr Relat Cancer 9(1):1–13
Gross JM, Yee D (2002) How does the estrogen receptor work? Breast Cancer Res 4:62–64
Strickland LA, Ponce YZ, Hunter DH, Zabel PL, Powe JE, Morrisey G, Driedger AA, Chamberlain MJ (1990) Amino and iodotamoxifens: synthesis estrogen receptor affinity and biodistribution. Drug Des Deliv 6:195–212
Skaddan MB, Wust FR, Jonson S, Syhre R, Welch JM, Spies H, Katzenellenbogen JA (2000) Radiochemical synthesis and tissue distribution of Tc-99m-Labeled 7α-substituted estradiol complexes. Nucl Med Biol 27:269–778
Van DeWiele C, De Vos F, Slegers G, Van Belle S, Dierckx RA (2000) Radiolabelled estradiol derivatives to predict response to hormonal treatment in breast cancer: a review. Eur J Nucl Med 27:1421–1433
Unak P, Muftuler FZB, Ichedef C, Medine IE, Ozmen K, Unak T, Yurt Kilcar A, Gumuser FG, Parlak Y, Bilgin ES (2012) Synthesis, radiolabeling and invivo biodistribution of diethylstilbestrol phosphate derivative (DES-P). J Radioanal Nucl Chem 293:57–66
Droz JP, Kattan J, Bonnay M, Chraibi Y, Bekradda M, Culine S (1993) High-Dose continuous-infusion fosfestrol in hormone-resistant prostate cancer. Cancer 71:1123–1130
Ertay T, Unak P, Ozdogan O, Biber FZ, Zihnioglu F, Medine EI, Durak H (2006) Tc-99 m-exorphin-glucuronide in tumor diagnosis: preparation and biodistribution studies in rats. J Radioanal Nucl Chem 269:21–28
Guldu OK, Unak P, Medine E, Barlas FB, Muftuler FZB, Timur S (2015) Radioiodinated magnetic nanoparticles conjugated with moxifloxacin: synthesis and in vitro biological affinities. Int J Polym Mater 64:253–259
Sanders JE, Chuang A, Swiec GD, Bisch FC, Herold RW, Buxton TB, McPherson JC (2011) The effects of enamel matrix derivative and cyclic mechanical strain on human gingival fibroblasts in an in vitro defect healing model. Int J Periodontics Restor Dent 31:671–678
Bordoloi JK, Berry D, Khan IU, Sunasseea K, de Rosales RTM, Shanahan C, Blower PJ (2015) Technetium-99 m and rhenium-188 complexes with one and two pendant bisphosphonate groups for imaging arterial calcification. Dalton Trans 44:4963–4975
Kuiper GGJM, Enmark E, PeltoHuikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930
Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M (1998) The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem Biophys Res Comm 243:122–126
Piccolella M, Crippa V, Messi E, Tetel MJ, Poletti A (2014) Modulators of estrogen receptor inhibit proliferation and migration of prostate cancer cells. Pharmacol Res 79:13–20
Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S (2003) Estrogen receptor (ER) β1 and ERbcx/β2 inhibit ERα function differently in breast cancer cell line MCF7. Oncogene 22:5011–5020
Leav I, Sm Ho, Ofner P, Fb Merk, Pwl Kwan, Damassa D (1988) Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Nat Cancer Inst 80:1045–1053
Bianco JJ, Handelsman DJ, Pedersen JS, Risbridger GP (2002) Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology 143:4922–4933
McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, Simpson ER, Risbridger GP (2001) Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology 142:2458–2467
Royuela M, de Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Arenas MI, Paniagua R (2001) Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol 168:447–454
Da J, Lu MJ, Wang Z (2015) Estrogen receptor alpha (erα)-associated fibroblasts promote cell growth in prostate cancer. Cell Biochem Biophys 73:793–798
Royuela M, de Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Arenas MI, Paniagua R (2001) Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol 168:447–454
Bonkhoff H, Fixemer T, Hunsicker I, Remberger K (1999) Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Int J Periodontics Restor Dent 155:641–647
Mobbs BG, Johnson IE, Liu Y (1990) Quantitation of cytosolic and nuclear estrogen and progesterone receptor in benign, untreated, and treated malignant human prostatic tissue by radioligand binding and enzyme-immunoassays. Prostate 16:235–244
Bonkhoff H, Berges R (2009) The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol 55:533–542
Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP (2008) Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB Journal 22:1512–1520
Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS (1997) Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138:4613–4621
Raso MG, Behrens C, Herynk MH, Liu SY, Prudkin L, Czburn NC, Woods DM, Tang XM, Mehran RJ, Moran C, Lee JJ, Wistuba II (2009) Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res 15:5359–5368
Abe K, Miki Y, Ono K, Mori M, Kakinuma H, Kou Y, Kudo N, Koguchi M, Niikawa H, Suzuki S, Evans DB, Sugawara S, Suzuki T, Sasano H (2010) Highly concordant coexpression of aromatase and estrogen receptor beta in non-small cell lung cancer. Hum Pathol 41:190–198
Sharp DC, Parry JM (1985) Diethylstilboestrol: the binding and effects of diethylstilboestrol upon the polymerisation and depolymer-isation of purified microtubule protein in vitro. Carcinogenesis 6:865–871
Robertson CN, Roberson KM, Padilla GM, O’Brien ET, Cook JM, Kim CS, Fine RL (1996) Induction of apoptosis by diethylstilbestrol inhormone-insensitive prostate cancer cells. J Natl Cancer Inst 88:908–917
Montgomery RB, Bonham M, Nelson PS, Grim J, Makary E, Vessella R, Stahl WL (2005) Estrogen effects on tubulin expression and taxane mediated cytotoxicity in prostate cancer cells. Prostate 65:141–150
Schulz P, Vetter I, Bauer HW, Fittler F (1988) Evaluation of the cytotoxic activity of diethylstilbestrol and its phosphorylated derivatives towards prostatic carcinoma, non prostatic neoplastic and non-transformed cells. Urol Itit 43(Suppl 1):5–9
Schneider MR, Schonenberger H (1988) Effects of diethylstilbestrol and its mono- and diphosphate on experimental mammary and prostatic tumors. Urol Itit 43(Suppl 1):l0–4
Tsien RY (2003) Imagining imaging’s future. Nat Rev Mol Cell Biol SS16–21
Nguyen QT, Tsien RY (2013) Fluorescence-guided surgery with live molecular navigation–a new cutting edge. Nat Rev Cancer 13:653–662
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moharrami, P., Unak, P., Guldu, O.K. et al. Multifunctional molecular imaging probes for estrogen receptors: 99mTc labeled diethylstilbestrol (DES) conjugated, cuinp quantum dot nanoparticles (DESCIP). J Radioanal Nucl Chem 314, 2609–2620 (2017). https://doi.org/10.1007/s10967-017-5630-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5630-2