Abstract
A ligand of N-2-(furylmethyl iminodiacetic acid) (FMIDA) has been easily labeled by a tetradentate chelating agent of [99mTc]. Factors like a stannous chloride solution as a reducing agent (100 μg), substrate amount (100 μg), pH (7), in vitro stability (8 h) and temperature (37 °C) have been systematically studied to optimize high radiochemical yield (98.0%). The radiochemical conversion was calculated on thin-layer chromatography, paper electrophoresis, and high performance liquid chromatography. Biodistribution study showed that this complex was removed from the kidneys and bladder path way during 1 h post injection. Therefore, [99mTc]FMIDA may be used as renal function radiotracer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many radiotracers have been evaluated [1–11] as drugs for renal imaging (radioisotope renography). The drug of diethylenetriaminepentaacetic acid (DTPA) has high stability and polarity because of the presence of five (COOH) groups and three (NH2) groups. Therefore, [99mTc]DTPA appears to be a single radiochemically pure species. In addition, Glomerular filtration rate (GFR) agents have been developed like [99mTc]DTPA and [169Yb]DTPA [12–17]. [99mTc]mercaptoacetyltriglycine ([99mTc]MAG3) is considered as a better diagnostic agent than [99mTc]DTPA, specially, in case of neonates including impaired function and patients including suspected obstruction [17]. However, p-aminohippurate (PAH) was considered the gold standard for the determination of effective renal plasma flow (ERPF) but its clearance required a constant plasma infusion and long time for analysis [18, 19]. Therefore, [131I]ortho-iodohippurate was considered a standard radiotracer used as imaging agent and to measure ERPF [20]. Additionally, [99mTc]MAG3 had become commercially available kit, and is the most widely used as an independent measure of renal function, because of the effective renal plasma flow (ERPF) [17, 21, 22]. But its clearance was limited than [131I]OIH. To overcome the defects result from this radiotracer, a new radiotracer ([99mTc]FMIDA, Fig. 1b) has been systematically prepared in this study. Due to its concentration in the target organ (kidneys) considers high compared to other radiotracers used, and using 99mTc (γ-emitter, [99mTc]FMIDA) instead of 131I (β-emitter, [131I]OIH) is more favorable in nuclear medicine field. Biodistribution study has been achieved using Normal Swiss Albino mice to show the concentration of this complex in target organ (kidneys).
Experimental
Materials and methods
All chemicals were purchased from British drug houses, Aldrich chemical company, and Merck Co. and they were analytical grade reagents. The water used was purged deoxygenated bidistilled water. 99mTc was eluted as 99mTcO4 − from 99Mo/99mTc generator, Gentech, Turkey. Ethanol and methanol were purchased from Sigma-Aldrich. Thin layer chromatography (TLC) aluminum sheets (20 × 25 cm) SG-60 F254 were supplied by Merck. Whatman paper number (PC) 1, Whatman International Ltd, Maidstone, Kent, UK. All chemicals were of analytical or clinical grade and were used directly without further purification unless otherwise stated.
Apparatus
Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer at 70 eV. The 1HNMR and 13CNMR spectra were run in CDCl3 or DMSO-d6 and recorded on a Varian Mercury VXR-400 MHz spectrometer and the chemical shifts were measured as δ (ppm) down field from tetramethylsilane (TMS) as an internal standard. A well-type NaI scintillation γ-Counter model Scalar Ratemeter SR7 (Nuclear Enterprises Ltd., USA) was used for radioactive measurement. Paper electrophoresis (PE) apparatus was form E.C. Corporation (Albany, OR, USA). The IR spectra of samples (discs pressed with potassium bromide) were recorded on a Pye Unicam SP 3300 and a Shimadzu FT IR 8101 PC infrared spectrophotometers. High performance liquid chromatography analysis (HPLC) is combination with a Shimadzu model detector SpD-6A, model which consists of pumps LC-9A, Rheodyne injector and UV spectrophotometer detector at 260 nm wavelength, the mobile phase, acetonitrile: methanol: H2O (3:3:5 v/v/v), and the column Li Chrosorb RP-C18-250 mm × 4.6 mm, 5 μm). Fractions of a volume 1.0 mL were collected separately up to a volume of 15 mL and counted with a γ-ray scintillation counter. Gamma camera imaging, the body distribution profile in mice was recorded in the gamma camera with a 5-mm pinhole collimator, window setting of 190 keV, and 20% width for gamma-imaging studies. After intravenous administration of radiolabeled [99mTc]FMIDA (injected dose: 0.1 mg FMIDA in 0.5 mL phosphate buffer (pH 7), activity: 0.2 mCi), the animal was anesthetized by intramuscular injection of xylazine (10.0 mg/kg) and 0.5 mL of ketamine hydrochloride (100.0 mg/kg) for 5 min before imaging. The doses were given according to Laboratory Animal Sciences Program in NCI Fredric Center for Cancer Research, and this anesthetic agent is believed to have a negligible effect on both blood pressure and the biodistribution of the radiolabeled samples. The animals where fixed promptly on a board in the posterior anterior position, and imaging was performed at different time intervals using a gamma camera. The images were taken in the two positions anterior and posterior. Gamma camera images were acquired at 5, 10, 15, 30 and 60 min after injection. The static images were stored in a 512 × 512 matrix size and acquisition times were 300 s. Scintigraphy was the diagnostic Nuclear Medicine test used in this study, where radiolabeled [99mTc]FMIDA was administered intravenously and the emitted gamma radiation captured by the gamma camera (Philips axis gamma 2) to form two-dimensional images.
Synthesis of N-2-(furylmethyliminodiacetic acid)
Furan-2-ylmethanamine (0.19 g, 0.002 mol) was dissolved in methanol (30 mL) and added to a mixture of bromoacetic acid (0.572 g, 0.004 mol, two equivalents) with 0.028 g of crushed NaOH. The reaction mixture was stirred at room temperature for 5 days, then the solvent was removed using a rotary evaporator. The solid formed was collected, thoroughly washed with diethyl ether, recrystallized, and purified by column chromatography (silica hexane: ethyl acetate 2:1 v/v) giving brown crystals (45%) [23].
Radiolabeling procedure
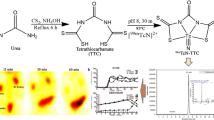
The reaction mixture volume was fixed to ~ 2000 μL. Accurately weighed 100 μg FMIDA dissolved in water (1 mg:1 mL) was added to the reaction flask, followed by SnCl2·2H2O (100 μg). A volume of 1000 μL (99mTcO4 −, ~ 500 MBq) reaction mixture was transferred to the above reaction vial. The pH of the reaction mixture was settled to 7 using phosphate buffer, the volume adjusted with DI-H2O and purged with N2. The [99mTc]FMIDA complex can be formed according to the literature [23, 24] presented as (Fig. 1b). That can be explained with bidentate N,O coordination of two ligands (FMIDA) to the metal oxo cation, with tridentate O,N,O coordination of one ligand to the metal oxo cation as a ration of 2:1 (bidentate chelation) and occupation of the remaining with the formation of an octahedral complex. The [99mTc]FMIDA complex was purified by HPLC.
Radiochemical analysis of [99mTc]FMIDA complex
The radiochemical conversion to [99mTc]FMIDA was determined using aluminum-backed silica gel GF254 plates. A volume of 2 μL (1.80 MBq, 99mTcO4 −) reaction mixture was placed above the lower edge, which was allowed to evaporate. The plate was developed in acetone and a mixture of ethanol:water:ammonium hydroxide (2:5:1 v/v/v) as a developing solvents. The strips were removed, dried and cut into 1 cm segments and assayed for radioactivity using SR.7 gamma counter. The percent ratio of free [99mTcO4 −] at Rf (0.7–1.0) to colloid and [99mTc]FMIDA at Rf (0.0–0.6) in case of acetone while the percent ratio of colloid at Rf (0.0–0.1) to [99mTcO4 −] and [99mTc]FMIDA at Rf (0.2–1.0) in case of mixtures [25, 26]. On completion of development, the paper was removed, dried, cut into 1 cm wide strips, and the strip counted in a γ-counter. High performance liquid chromatography analysis (HPLC) analysis gave a purity for [99mTc]FMIDA to give < 99% by direct injection of 20 μL of the reaction mixture (see method above). The Rt values of free 99mTcO4 −, [99mTc]FMIDA and FMIDA were 4, 9 and 8.1 min, respectively (Fig. 2).
Biodistribution and animal studies
Animal experiments were approved by Ethical Committee of the Labeled Compounds Department. Mice as Swiss Albino mice (30–40 g). Five groups of normal mice (5 mice for each group to give 25 mice in total) intravenously injected with 0.2 mL (3.6 MBq) of [99mTc]FMIDA adjusted to physiological pH via the tail vein were used for quantitative determination of organ distribution (per time point) and sacrificed at various times post-injection at (5, 10, 15, 30 min, 1 h) [27–31]. Corrections were made for background radiation and physical decay during the experiments. Differences in the data were evaluated with the Student t test was applied. Results of P value are reported using the 2-tailed test. The level of significance was set at P < 0.05.
Determination of the partition coefficient for the [99mTc]FMIDA complex
The octanol/water partition coefficient of [99mTc]FMIDA ropinirole was determined at pH value of 7.4 by measuring its distribution in n-octanol and phosphate buffered saline (PBS), respectively. A sample of 200 μL was added to an immiscible liquid containing PBS (800 μL; pH 7.4) and n-octanol (1 mL), then after 5 min vigorous vortex, the mixture was incubated for 30 min at room temperature. Centrifugation at 5000 rpm for 5 min ensured complete separation of the organic and the aqueous layers. An aliquot (100 μL) from each layer was measured with a γ counter. The partition coefficient value can be expressed as log P o/w values and repeated for five times.
Plasma protein binding
About 0.2 mL of [99mTc]FMIDA radiotracer incubated with 1 mL of human plasma at ambient temperature for 1 h. Then, the plasma protein is precipitated by adding 0.20 mL of 50% trichloroacetic acid (TCA) to 1 mL of plasma. The supernatant and the precipitate are separated by centrifugation at 5000 rpm for 10 min. To calculate the activities present in two phases that are measured separately in a well-type NaI (Tl) scintillation detector (γ-counter).
Results and discussion
Characterization of the synthesized of FMIDA
The structure of the new product was confirmed by elemental and spectral analyses. IR υ (cm-1): 3620-2780 (COOH, H-bonding); 3090 (C–H aromatic); 2930 (C–H aliphatic); 1736 (C=O of COOH); 1630 (C=C); 1238 (C–O–C). MS (ESI): mass calculated for C9H11NO5, 213; observed, 81 (M-132). 1H-NMR. 3.85 (s, 4 H, 2(-CH2); 3.99 (s, 2 H, N-CH2); 6.39, 6.48, 7.61 (3H, ArH), 8.50 (s, 2H, COOH). 13C-NMR (DMSO-d6), d ppm 35.50 (CH 2 –N), 59.75(CH 2 –COOH), 111.14, 111.48, 144.20, 147.53 (4 C, furan), 174.98 (COOH).
Optimization of reaction
Figure 3a indicates the effect of changing of substrate amounts (FMIDA) to give high radiochemical yield. The optimum radiochemical conversion to [99mTc]FMIDA is 98% at 100 μg of substrate and free 99mTcO4 − as (7.5 MBq). Figure 3b indicates that the conversion to [99mTc]FMIDA increased up to a ceiling of 100 μg of stannous chloride dihydrate as a reducing agent giving an optimum radiochemical conversion of 98.0%, the other reaction parameters were kept constant. Excess stannous chloride dihydrate lead to decrease the radiochemical conversion to give 66% at 5 mg [24,25,26,27]. In addition, pH is a critical factor in the labeling process (see Fig. 3c) which needs to be controlled, pH 7 proving optimal which may reflect in part the stability of [99mTc]FMIDA. The effect of reaction time was also studied giving a maximum conversion at 15 min (Fig. 3d). The in vitro stability of [99mTc]FMIDA was confirmed in two different media. It was obtained that [99mTc]FMIDA was stable in saline for up to 48 h. In contrast, in serum after 24 h, the purity dropped to 90.0% and then decreased to 82% at 48 h. The effect of temperature was finally studied to give a maximum conversion at 40 °C. Further increasing in temperature more than 40 °C up to 100 °C decrease the radiochemical conversion to [99mTc]FMIDA to give 80% may be due to the thermal decomposition of the [99mTc]FMIDA complex [28,29,30,31,32,33,34].
a Effect of FMIDA concentration on the labeling yield of 99mTc-FMIDA complex. Conditions: 0.25–10 mg of FMIDA, 100 μg Sn (II), pH 7 and 15 min reaction time, n = 3. b Effect of Sn (II) content on the labeling yield of 99mTc-FMIDA, complex. Conditions: 0.10 mg FMIDA, 1–10 mg Sn (II), pH 7 and 15 min reaction time, n = 3. c Effect of pH on the labeling yield of 99mTc-FMIDA complex. Conditions: 0.10 mg FMIDA, 0.10 mg Sn (II), pH 2–12 and 15 min reaction time, n = 3. d Effect of reaction time on the labeling yield of 99mTc-FMIDA complex. Conditions: 0.10 mg FMIDA, 0.10 mg Sn (II), pH 7, the 1–60 min reaction time, n = 3
Gamma scintigraphy imaging results
Figure 4 indicates [99mTc]FMIDA complex that concentrated into its target, the kidneys of mice with a maximum in 5 min post injection, then gradually decreases with time till 1 h. In addition, Fig. 4a indicates the amount of activity that concentrated in organ (Kidneys) by pixels unit, as gamma counting, during 1 h.
The effect of partition coefficient factor
The logarithm of the partition coefficient (log P) value of [99mTc]FMIDA is −3.5 ± 0.02. Therefore, [99mTc]FMIDA is a hydrophilic complex [8].
Plasma protein binding (PPB)
The PPB method for [99mTc]FMIDA complex has been evaluated to give 41% protein bound that less than [131I]OIH which have protein binding of 44% [8].
Biodistribution study
Table 1 indicates the biodistribution of [99mTc]FMIDA complex in different body organs and fluids. All radioactivity levels are expressed as average percent-injected dose per organ tissue (% ID/organ ± SD). The uptake within the kidneys increased up to 8.5% at 5 min p.i. and decreased to 1.1% at 1 h p.i. Therefore, [99mTc]FMIDA complex considered a highly radiotracer accumulates with localizing selectively in target organ with fast clearance during 1 h. This indicated that the tracer is excreted through urinary pathways. That was confirmed by gamma camera analysis (see Fig. 4). The uptake within the blood is more clearance through 1 h post injection to give 10.6, 8.11, 3.9, 1.2% and 0.51 at 5, 10, 15, 30 and 60 min post injection respectively. Therefore, [99mTc]FMIDA complex considered less than [99mTc]PMIDA and [99mTc]DTPA that gave 6.05 and 6.03% respectively at 15 min post injection. The published % ID/organ ± SD for a number of common radiotracers accumulated in kidneys as a target organ e.g., [99mTc]PMIDA [8], [131I](ortho-iodohippurate,OIH) [8], [99mTc]DTPA [8], [99mTc](CO)3(ASMA), Al[18F]NODA-butyric acid [6], [99mTc(CO)3(CMT-IDA)]−2 [5] and [99mTc(CO)3(NTA)]−2 [27] which gave maximum up take in 6.84, 7.25, 7.35, 6.7, 4.6, 0.8 and 1.8% ID/organ respectively. Our results indicate that [99mTc] FMIDA complex has a higher % ID/organ ± SD value than these materials.
Conclusion
An optimized protocol for the synthesis of [99mTc]FMIDA complex in the optimum radiochemical conversion (98%) has been achieved by optimum conditions. Biodistribution studies indicated that the [99mTc]FMIDA complex has a high kidneys uptake of 8.5 ± 0.13% ID/organ at 5 min post injection, confirmed by gamma camera. The radiotracer, [99mTc]FMIDA complex considers more better than recently discovered agents such as [99mTc]PMIDA, [99mTc](CO)3(ASMA), Al[18F]NODA-butyricacid, [99mTc(CO)3(CMT-IDA)]−2, [99mTc(CO)3(NTA)]−2 and [99mTc]DTPA.
References
Hosain F, Reba RC, Wagner HN Jr (1969) Int J Appl Radiat Isot 20:517–521
Bianchi C (1972) Prog Nucl Med 2:21–53
Skov PE (1970) Acta Med Scand 187:419–428
Richards P, Atkins HL (1976) J Nucl Med 7:165–170
Mohini B, Drishty S, Sweety S, Haladhar DS, Meera V, Sharmila B (2012) Curr Radiopharm 5:65–70
Malgorzata L, Jeffrey K, Dinesh S, Jonathon AN, Hyunsuk S, Andrew TT (2014) Nucl Med Biol 41:248–253
Malgorzata L, Jeffrey K, Luigi G, Marzilli A, Taylor T (2012) J Nucl Med 53(8):1277–1283
Yoshiharu K, Koji I, Jiro T (1999) Ann Nucl Med 13(2):127–132
Andrew TT, Malgorzata L, Luigi GM (2010) J Nucl Med 51(3):391–396
Jeffrey K, Malgorzata L, Andrew TT, Luigi GM (2012) Eur J Inorg Chem. https://doi.org/10.1002/ejic.201200599
Klingensmith WC, Fritzberg AR, Spitzer VM et al (1984) J Nucl Med 25:42–48
Taylor A, Eshima D, Alazraki N (1987) Eur J Nucl Med 12(10):510–514
González A, Jover L, Mairal LI, Martin-Comin J, Puchal R (1994) Nuklearmedizin 33(6):244–247
Kramer W, Baum RP, Scheuermann E, Hör G, Jonas D (1993) Urol A (in German) 32(2):115–120
Li Y, Russell CD, Palmer-Lawrence J, Dubovsky EV (1994) J Nucl Med 35(5):846–850
Al-Nahhas AA, Jafri RA, Britton KE et al (1988) Eur J Nucl Med 14(9–10):453–462
O’Malley JP, Ziessman HA, Chantarapitak N (1993) Clin Nucl Med 18:22–29
Shattuck LA, Eshima D, Taylor A et al (1994) J Nucl Med 35:349–355
Brandoni A, Anzai N, Kanai Y, Endou H, Torres AM (2006) Biochim Biophys Acta 1762(7):673–682
Flannery A, Veber J (1980) A literature review. Med Phys 7:249–250
Eshima D, Taylor A (1992) Sem Nucl Med 22:61–73
Esteves FP, Taylor A, Manatunga A, Folks R, Krishnan M, Garcia EV (2006) Am J Roentgenol 187:W610–W617
Alberto R, Abram U (2011) Handbook of nuclear chemistry. Springer, New York, pp 2073–2120
Motaleb MA, El-Said H, Abdallah M, Atef M (2012) Radiochemistry 54(5):501–505
Chen X, Guo Y, Zhang Q, Hao G, Jia H, Liu B (2008) Organomet. Chem. 693:1822–1828
Zhuang ZP, Kung MP, Hou C, Plössl K, Kung HF (2005) Nucl Med Bio 32:171–184
Malgorzata L, Luigi GM, Andrew TT (2009) J Nucl Med 50(3):454–460
Drishty S, Ketaki B, Archana M, Sharmila B, Kanchan K, Meera V (2006) Appl Radiat Isot 64:888–892
Sanad MH, Salama DH, Marzook FA (2017) Radiochim Acta 105(5):389–398
Sanad MH, Gehan MS, Marzook FA (2017) J Label Compd Radiopharm. https://doi.org/10.1002/jlcr.3541
Rhodes BA (1974) Semin Nucl Med 4:281–293
Sanad MH (2013) Radiochemistry 55(5):539–544
Sanad MH, Challan SB (2017) Radiochemistry 59(3):307–312
Sanad MH, Talaat HM (2017) Radiochemistry 59(4):396–401
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanad, M.H., Ibrahim, A.A. & Talaat, H.M. Synthesis, bioevaluation and gamma scintigraphy of 99mTc-N-2-(furylmethyl iminodiacetic acid) complex as a new renal radiopharmaceutical. J Radioanal Nucl Chem 315, 57–63 (2018). https://doi.org/10.1007/s10967-017-5617-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5617-z