Abstract
Bifunctional resins having pyridinium and methyl ammonium-based dicationic groups, separated by a five carbon spacer, have been prepared by chemical modification of Reillex™ 402 poly(4-vinyl pyridine). Batch sorption studies carried out for the three resins (I, II, III), which differ by degree of functionalization and physical form, revealed very fast kinetics, with III having highest K D value (650 at 7.5 M). The capacity of III was 4.5 meq/g. The resin was selective for Pu sorption from nitric acid medium having assorted metal ions and the Pu content of resultant raffinate was below the disposal limit. Column experiments with III show fast loading and elution of Pu(IV).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various methods including precipitation, solvent extraction and ion exchange sequestration have been used for separation and purification of metal ions in the nuclear industry [1, 2]. While precipitation yields low decontamination, solvent extraction is advantageous in terms of fast kinetics, high capacities and selectivity for targeted metal ions. However, it has limited applications for lean aqueous streams. Ion exchange resins are employed for this purpose. Ion exchange has found wide scale applications in front as well as back end of nuclear fuel cycle beginning from uranium milling to tail end purification after reprocessing [3, 4]. For separation of plutonium, both cation and anion exchange resins have been employed. In general, cation exchange separation of Pu is characterized by high distribution ratio, fast kinetics but poor decontamination. Hence, it has been routinely used for concentration of Pu. Cation exchange resins generally contain sulphonic and carboxylic acid groups. While resins containing sulphonic acid lack selectivity, they have a faster kinetics of sorption. On the other hand, carboxylic acid containing resins are usable only at higher pH. Phosphorus-based bifunctional resins have been synthesized and evaluated for enhancing kinetics as well as selectivity of Pu sorption from acidic medium of cation exchange resins [5, 6].
Anion exchange separation, on the other hand, yields high decontamination factors. Pu(IV) forms a stable dianionic complex, \( {\text{Pu(NO}}_{3 } )_{6}^{2 - } \) in strong (7–9 M) nitric acid medium. Hence, anion exchangers can be used for the purification of Pu [7, 8]. Anion-exchange has been used routinely for the third cycle of plutonium purification following PUREX. Furthermore, Pu has been concentrated and purified effectively from irradiated fuel solutions. However, the major challenge with anion exchange separation of Pu was the diffusion controlled very slow exchange rate of the bulky dianion \( {\text{Pu}}({\text{NO}}_{3 } )_{6}^{2 - } \). New anion exchange resins, Tulsion A-PSL 4 and Tulsion A-PSL6, have also been evaluated in comparision to Dowex 1X4 by Kumaresan et al. [9]. Small scale batch sorption and radiolytic degradation studies revealed better performance of Tulsion A-PSL 6. However, selectivity studies in presence of other metal ions and column studies were not reported. Pu separation with Tulsion A-27 was investigated by Charyulu et al. [10].
In the quest for development of new Pu(IV) selective resins, resins based on solvation mechanism were also explored. Recently, diamide-based moiety was tagged to resin support for Pu(NO3)4 sorption from low nitric acid molarity [11].
Another aspect of anion exchange separation of Pu involves the fact that Pu loading at high nitric acid molarity may lead to radiolytic damage thus causing exothermic reaction between the resin material and acid medium [12, 13]. Hence, a fast loading and elution behavior warranting less residence of Pu in the resin column is desirable.
Relatively large volumes of Pu bearing solutions get routinely accumulated in our laboratory due to quality control operations of nuclear fuels and other research activities. The separation and purification of Pu from such solutions becomes inevitable before disposal. We have been using Dowex 1X4 traditionally for Pu recovery from such solutions. However, there exists a requirement for development of resins which yield selective and faster Pu separation while ensuring the safety aspects during large scale campaigns for Pu recovery.
A collaboration between Los Alamos National Laboratory and Reilly Industries, Inc. led to the development of Reillex™ HPQ, a new macroporous anion exchange poly(4-vinylpyridine) resin, that exhibited better kinetics as well as radiation stability [14]. Further, it was envisioned that presence of two cationic sites, separated by a given carbon spacer, may serve as a selective anchoring site for the dianionic Pu complex. It was found that such introduction of bifunctionality improved selectivity and exchange kinetics [15, 16].
The present study reports our attempts towards boosting Pu recovery efforts by the preparation of the pyridinium based dicationic resins (I, II and III) from the commercially available Reillex™ 402 and their characterization. Batch sorption studies were carried out to study the effect of nitric acid molarity and kinetics of sorption. Efficiencies of these resins were evaluated in terms of their applicability for selective sorption of Pu from nitric acid medium generated as a result of dissolution of irradiated fuel in nuclear reactor as well as analytical solution generated in the laboratory due to quality control of nuclear fuels. In order to evaluate the performance of the resin under actual process conditions, column studies were also carried out.
Experimental
The chemicals were procured from Sigma Aldrich and were used as received. Reillex™ 402 poly(4-vinylpyridine) was also procured from Sigma Aldrich. Other reagents were of AR grade. All solvents were freshly dried. All organic extracts were dried over anhydrous Na2SO4. IR spectra were recorded as films/KBr pellets with a JASCO model A-202 spectrophotometer. 1H and 13C NMR spectra were recorded in CDCl3/DMSO-d6 (as mentioned) with a 500 MHz Varian NMR spectrometer. Elemental analyses were recorded on an Elementar Vario microcube. Melting points were recorded on Buchi M 560 instrument.
Synthesis of Poly [N-(5-Trimethylammoniopentyl)-4-vinylpyridinium Dibromide] I/II/III
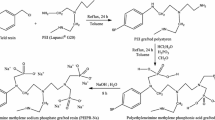
For the synthesis, tetrahydropyran (1) was converted into 1,5-dibromopentane (2) using 48% HBr and concentrated H2SO4 [17]. The dibromide was reacted with trimethylamine in THF at room temperature according to a methodology reported by Bartsch [18] et al. wherein, contrary to our expectation, (5-bromoalky1)trimethylammonium bromide (3) precipitated along with the corresponding bis-salt from the solution (mono-salt:bis-salt 70:30 as revealed by 1H NMR). Fortuitously, the bis-salt could be easily removed by shaking the mixture with CHCl3. The pure mono-salt was then refluxed with Reillex™ 402, a poly(4-vinylpyridine) resin, in anhydrous ethanol with/without magnetic stirring for 2–4 days. The conditions were optimised to get I, II and III with 82, 97 and 94% coupling respectively as is evident from the elemental analyses data. Of these, the bead-like morphology of Reillex™ 402 was best preserved in III due to less mechanical disintegration in absence of magnetic stirring. However, a marginal reduction in coupling is observed in III which can be attributed to the mass-diffusion limitation resulting from the mechanical integrity of the resin. Gratifyingly, the granular form of III is more suited to its use in the column mode for uptake of Pu(IV) as against powder formulation which results in compaction (Scheme 1).
(i)48% HBr, H2SO4/reflux/15 h; (ii) Me3N/THF/RT/21 h; (iii) Method A: PVP Resin Reillex™ 402/EtOH/reflux/2 days with stirring (for I); Method B: PVP Resin Reillex™ 402/EtOH/reflux/4 days with stirring (for II); Method C: PVP Resin Reillex™ 402/EtOH/reflux/4 days without stirring (for III). # I, II, III differ in the extent of coupling of 3 with Reillex™ 402 and their morphology
Synthesis of 1,5-dibromopentane 2
To an ice-cooled round-bottomed flask containing 48% HBr (250 g, 170 mL), concentrated H2SO4 (75 g, 41 mL) was added dropwise and allowed to stir for 1 h. Distilled THP (1) (21.5 g, 0.25 mol) was added to it and the mixture was allowed to come to room temperature. The mixture was refluxed gently at 80 °C for 14 h. It was brought to room temperature and the contents were transferred to a separating funnel. The upper layer containing the excess acid was removed and the bottom layer containing bromide was diluted with H2O (150 mL) and extracted using CHCl3 (4 × 75 mL). The organic extract was washed with H2O (4 × 50 mL) till it was neutral, brine (1 × 25 mL) and dried over Na2SO4. Solvent removal in vacuo and purification by column chromatography over SiO2 gel (0–5% EtOAc/hexane) furnished pure 2 as a pale yellow liquid.
-
Yield: 44.8 g (78%);
-
IR: 1257, 1437, 732, 643 cm−1.
-
1H NMR (500 MHz) (CDCl3): δ 1.58–1.64 (m, 2H), 1.87–1.93 (m, 4H), 3.42 (t, J = 7.0 Hz, 4H).
-
13C NMR (125 MHz) (CDCl3): 26.8, 31.9, 33.1.
Synthesis of (5-Bromopentyl)trimethylammonium Bromide 3
In a 250 mL two necked round-bottomed flask containing KOH beads (50 g) was added 30% aqueous Me3N solution (51.3 mL, 260.94 mmol) dropwise. Through a vacuum bend attached to the other neck of the flask, the evolved trimethylamine gas was passed through two KOH traps (to check moisture) and finally introduced gradually into a two necked round-bottomed flask containing a solution of 2 (20 g, 86.98 mmol) in dry THF (120 mL) at room temperature. A KOH drying tube was attached to the other neck of the reaction flask. After complete addition, the tubes were replaced with stoppers and the reaction mixture stirred for 24 h. The precipitate was filtered and the filtrate was returned to the flask. The trimethylamine was added as before. Stirring for 24 h yielded more precipitate. This cycle was repeated again on the filtrate obtained. The combined precipitates over three cycles gave a highly hygroscopic white solid which was a mixture of 3 and its corresponding bis-salt in the ratio 70:30 (based on IH NMR in DMSO d6). The solid mixture was dissolved in CHCl3 and gratifyingly, 3 dissolved into it while the bis-salt remained insoluble (cf. 1H NMR in CDCl3). The mixture was filtered and the filtrate was concentrated under vacuum to get 3 as a white solid.
-
Yield of 3: 17.2 g (68%);
-
m.p. 140 °C [10]
-
IR (thin film, CHCl3): 1482 (C–H bending in –CH2–N+) cm−1
-
1H NMR (500 MHz) (DMSO d6) δ 1.25–1.31 (m, 1H), 1.36–1.42 (m, 2H), 1.67–1.77 (m, 4H), 1.83–1.88 (m, 2H), 3.04 (s, 9H mono-salt), 3.07 (s, 18H bis-salt), 3.28–3.31 (m, 2H), 3.56–3.59 (m, 2H).
-
Mono-salt: Bis-salt = 70:30
-
1H NMR (500 MHz) (CDCl3): δ 1.52–1.58 (m, 2H), 1.79–1.86 (m, 2H), 1.91–1.96 (m, 2H), 3.43 (t, J = 5.0 Hz, 2H), 3.45 (s, 9H), 3.65–3.69 (m, 2H).
-
13C NMR (125 MHz) (CDCl3): 22.4, 24.8, 31.8, 33.3, 53.5, 66.5.
Synthesis of Poly [N-(5-trimethylammoniopentyl)-4-vinylpyridinium dibromide] I/II/III
Method A
Reillex™ 402 poly(4-vinyl pyridine) (2.00 g, 19.02 mmol) was added to a round bottomed flask containing a solution of 3 (5.50 g, 19.02 mmol) in absolute ethanol (30 mL). The reaction mixture was refluxed under nitrogen and stirred magnetically for 2 days. After cooling to room temperature, it was filtered and the solid resin was washed with ethanol (3 × 10 mL) and water (3 × 15 mL). The solid was dried with benzene in a Dean Stark apparatus to give I as a white powder.
-
Yield: 5.3 g (82%)
-
m.p. > 300 °C
-
IR: (KBr pellet):1640 (C=N), 1474 (C–H bending in –CH2–N+) cm−1
-
Anal. Calcd. for I (C15H26N2Br2)0.82(C7H7N)0.18: C, 47.58; H, 6.60; N, 7.45; Br, 38.36%; Found: C, 47.39; H, 6.31; N, 7.69; Br, 38.61%.
Method B
Same as method A except that the reaction mixture was refluxed under nitrogen and stirred magnetically for 4 days and worked-up as above to afford II as an off-white powder. Yield: 6.4 g (87%).
-
m.p. > 300 °C
-
IR: (KBr pellet):1644 (C=N), 1472 (C–H bending in –CH2–N+) cm−1
-
Anal. Calcd. for II (C15H26N2Br2)0.97(C7H7N)0.03: C, 45.96; H, 6.60; N, 7.16; Br, 40.28%; Found: C, 46.02; H, 6.36; N, 7.17; Br, 40.45%.
Method C
Same as method A except that the reaction mixture was refluxed under nitrogen for 4 days without stirring followed by usual work-up to give III as a granular off-white solid.
-
Yield: 6.1 g (85%).
-
m.p. > 300 °C
-
IR (KBr pellet):1644 (C=N), 1471(C–H bending in –CH2–N+) cm−1
-
Anal. Calcd. for III (C15H26N2Br2)0.94(C7H7N)0.06: C,46.25; H, 6.60; N, 7.21; Br, 39.93%; Found: C, 46.01; H, 6.36; N, 7.53; Br, 40.10%.
Batch sorption experiments
Stock solution of Pu(IV) was prepared by TTA extraction and stripping the extracted Pu(IV) into 7.5 M HNO3, NaNO2 was added as a holding oxidant for Pu(IV). For batch sorption studies, accurate amount of the resins (~250 mg, converted to the nitrate form) were equilibrated with the Pu(IV) solutions (~10−4 M, 1 mL). For determination of the capacity, known amount of the resin was washed several times with deionized water. It was then equilibrated with 1 M HNO3 till the effluent was free from bromide ions. Capacity was determined by the titration of the supernatant with a standard AgNO3 solution. High Level Waste solution in nitric acid containing tracers of 103Ru, 137Cs, 95Zr, 95Nb, 140Ba, 141Ce, 140La was obtained from Radioanalytical Chemistry Division (RACD). 241Am and 233U tracers were obtained from RACD.
Column studies
For the column studies, the nitrate form of the resin III was transferred into a glass column (1 cm diameter, 10 cm height, 2 ml bed volume) and conditioned with 7.5 M HNO3. The Pu(IV) stock solution in 7.5 M HNO3 was loaded onto the column that was washed with four bed volumes of 7.5 M HNO3. Pu(IV) was then eluted with 9 bed volumes of 0.5 M HNO3. The effluents, collected for each bed volume were estimated for their Pu(IV) contents by a liquid scintillation counter.
Assay techniques
Pu and U alpha activity was determined by liquid scintillation counting using dioxane-based scintillator cocktail. Am present in the analytical solution was determined using NaI(Tl) scintillation detector coupled to 2 K Multichannel analyser. Fission products were assayed by gamma spectrometry with a shielded 30% HPGe coupled with 4096 channel analyzer in standard geometry. 152Eu source (5 mL) was used for calibration of the system efficiency.
Results and discussion
One of the most established methods of Pu separation and purification happens to be that employing anion exchange. Pu(IV) forms a stable dianionic complex, \( {\text{Pu}}({\text{NO}}_{3 } )_{6}^{2 - } \) at ~7 M HNO3 whereas most of the other metal ions remain in neutral or cationic form. Under this condition, few metal ions also form anionic complexes which have a low stability constant.
In the present study, the synthesized resin consists of cationic exchange site, one pyridinium and the other N-methyl ammonium, separated by a five carbon chain.
The distribution coefficient (K D) was calculated with the following formulae:
where C 0 and C e are the metal ion concentration at t = 0 and at equilibrium (mg/L) in the solution, V is the total volume of solution (mL); and m is the mass of adsorbent (g).
Effect of variation of nitric acid molarity
Molarity of acid dictates the nature of Pu(IV) species present in nitric acid medium. Pu(IV) exists as a hexanitrato anionic complex, \( {\text{Pu}}({\text{NO}}_{3 } )_{6}^{2 - } , \) in strong nitric acid solutions which undergoes sorption as per the following equation:
The sorption of \( {\text{Pu}}({\text{NO}}_{3 } )_{6}^{2 - } \) on the resin leads to pushing the equilibrium towards its further formation. Hence, there is an observation of enhancement in K D with nitric acid molarity. However, at high nitric acid molarity, plutonium forms acid species as given below:
These acid species are not sorbed as strongly as the \( {\text{Pu}}({\text{NO}}_{3 } )_{6}^{2 - } \) species. Also, under these conditions there exists a competition between nitrate ions and Pu complexes for the resin exchange sites. These two effects lead to a decrease in the Pu distribution coefficient at nitric acid molarities beyond a certain limit as observed in Fig. 1. The trend in variation of K D with nitric acid molarity is similar to that reported in literature [14, 15]. The results (Fig. 1) of variation of K D with HNO3 molarity revealed highest sorption at 7.5 M and negligible sorption at 0.5 M. Thus, loading of Pu(IV) and washing of other metal ions could be conveniently carried out with 7.5 M. Subsequent elution of Pu(IV) could be carried out with 0.5 M HNO3 when the dianionic Pu complex morphs to cationic form. It is to be noted here that the distribution coefficient values obtained for I, II, III under our experimental conditions were much lower than reported [14]. Nevertheless, this was sufficient to remove Pu from our laboratory solutions so that the resultant raffinate contained Pu concentrations that were in the order of ~1 mg/L.
Kinetics of sorption
In order to find the time required by the dianionic \( {\text{Pu}}({\text{NO}}_{3 } )_{6}^{2 - } \) to reach equilibrium between resin and nitric acid medium, the Pu concentration was determined after different time intervals. The batch sorption studies, carried out by equilibrating a known amount of resin with Pu(IV) solution, at 7.5 M nitric acid, for different contact times (0.5, 1, 2, 3 and 24 h) revealed (Fig. 2) that 0.5 h was sufficient to achieve equilibrium. However, all further experiments were carried out after 1 h of equilibration to ensure completion of the ion exchange. This was a remarkable enhancement in kinetics of sorption for anion exchange resins in comparison to widely used commercial resins like AG 1X4, Dowex 1X4 and developed ones like Tulsion resins [9, 10] requiring a equilibrium time ≥4 h. A direct implication of the fast kinetics is the less contact time of resin with Pu(IV) present in high nitric acid molarity and improved stability of resin. The sorption kinetics was also better than the Reillex™ HPQ resin [15] and comparable to that of bifunctional resins as reported by Marsh et al. [15].
The pseudo-second-order model can be given by following equation
The sorption kinetics data was fitted into pseudo-second-order kinetic models [19] (Fig. 3). Here, t, q t, k s and q e refer to time, sorption capacity at time t, pseudo-second order rate constant, sorption capacity at equilibrium respectively. Since the correlation coefficient is determined to be closer to unity for plot of ‘t/q t’ against ‘t’, it can be inferred that the sorption kinetics of \( {\text{Pu}}({\text{NO}}_{3 } )_{6}^{2 - } \) on III could be explained well in terms of pseudo-second-order kinetic model for the polymeric adsorbents. This suggests that the dianionic Pu species, \( {\text{Pu}}({\text{NO}}_{3 } )_{6}^{2 - } \), gets chemically sorbed onto the dicationic resin site and this involves the rate determining step.
Since III was found to have higher K D value in 7.5 M HNO3 and the resin was of bead form, all further experiments were carried out with III. For III, K D of 650 ± 10 was obtained at 7.5 M nitric acid molarity and for a equilibration time of 1 h. Its capacity was found to be 4.5 meq/g of dry resin.
Selectivity
High Level Waste solution obtained after removal of U, Pu by solvent extraction (PUREX) from dissolver solution contains residual Pu along with high yield fission products. Due to quality control operations on U–Pu based fuels, analytical waste solution gets generated in the laboratory that contains actinides (Pu, U) along with Am from 241Pu decay. Separation and purification of Pu from such acidic medium containing various metal ions is imperative. The selectivity of Pu(IV) over other metal ions is based on the fact that the stability constants of nitrato complexes of the tetravalent actinides are very much greater than the hexavalent, trivalent and pentavalent actinides. Fission products are not known to form anionic complexes in nitric acid medium. The K D values of Pu(IV) along with U(VI), Am(III) and some of the high yield fission products were determined and are shown in Table 1 at the two acid molarities. The selectivity of Pu(IV) over other metal ions is evident from the results. U, Nb, Ba, La, Ru show K D <10 at 7.5 M nitic acid and can come out as a part of the loading effluent. Zr, Am and Ce, having K D >10 can be washed with a few columns of 7.5 M nitric acid. Hence, Pu can be separated from other metal ions under suitable conditions.
Column studies
Column experiments were carried out by packing the nitrate form of III in a glass column, conditioning with 7.5 M nitric acid and ensuring the absence of formation of any void. Pu(IV) stock, in 7.5 M nitric acid, was loaded in two bed volumes followed by washing with four bed volumes of 7.5 M nitric acid which was followed by elution of Pu with 0.5 M nitric acid for 9 bed volumes. Assay of the effluents collected separately for each bed volume was carried out by liquid scintillation counting. Column experiments (Fig. 4) showed a sharp profile. This shows that the resin has fast kinetics viz. fast loading and fast elution leading to concentrated and pure Pu(IV) product in 0.5 M HNO3. It, therefore, ensures less residence of Pu in the resin column leading to better safety during recovery operations.
Conclusion
Bifunctional anion exchange resins (I, II, III) based on pyridinium and methyl ammonium groups were synthesized and characterized. III was found to have highest distribution ratio (650 ± 10). Best resin performance was obtained at 7.5 M nitric acid medium with an equilibration time of 1 h. Its capacity was found to be 4.5 meq/g of dry resin. The resin was selective towards Pu(IV) over U(VI), Am(III) and high yield fission products. Hence, Pu could be successfully separated from nitric acid medium containing different metal ions. III was in bead form and hence suitable for column operation. Column studies revealed fast loading and elution behavior.
References
Navratil JD (1989) Ion exchange technology in spent fuel reprocessing. J Nucl Sci Technol 26(8):735–743
Jenkins IL (1984) Ion Exchange in the atomic energy industry with particular reference to actinide and fission product separation: a review. Solvent Extr Ion Exch 2(1):1–27
Ion Exchange Technology in the Nuclear Fuel Cycle. IAEA-TECDOC-365, 1986
Jenkins IL (1984) Ion exchange in atomic energy industry with particular reference to actinide and fission product separation. Solvent Extr Ion Exch 2(1):1–27
Alexandratos SD, Quillen DR, Mcdowell WJ (1987) Bifunctional phosphinic acid resins for the complexation of lanthanides and actinides. Sep Sci Technol 22(2&3):983–995
Mohandasa J, Kumara T, Rajan SK, Velmurugan S, Narasimhan SV (2008) Introduction of bifunctionality into the phosphinic acid ion-exchange resin for enhancing metal ion complexation. Desalination 232:3–10
Ryan JL, Wheelwright EJ (1959) Recovery and purification of plutonium by anion exchange. Ind Eng Chem Res 51:60–65
Ryan JL, Wheelwright EJ (1959) The recovery, purification, and concentration of plutonium by anion exchange in nitric acid HW-55893
Kumaresan R, Sabharwal KN, Srinivasan TG, Vasudeva Rao PR, Dhekane G (2006) Evaluation of new anion exchange resins for plutonium processing. Solvent Extr Ion Exchange 24:589–602
Charyulu MM, Pawar SM, Ray M, Nagi S, Sivaramakrishna CK (1986) The plutonium loading and elution behaviour of the resin Tulsion A-27 (MP). In: Radiochemistry and Radiation Chemistry Symposium held at Tirupati, December 13–17, pp. 347–349
Ruhela R, Panja S, Singha AK, Dhami PS, Gandhi PM (2016) Benzododa grafted polymeric resin- plutonium selective solid sorbent. J Hazard Mater 318:186–193
Marsh SF (1990) The effects of ionizing radiation on Reillex TM HPQ, a new macroporous polyvinylpyridine resin, and on four conventional polystyrene anion exchange resins LA–11912
Bartenev SA, Zachinyaev GM, Nazin ER, Lazarev LN, Kalashnikov VM, Romanovskii VN, Strelkov SA, Firsin NG, Egorov GF, Hyder ML (2002) Radiation-chemical resistance of anion exchangers safety of sorption processes in nitric acid solutions: III. radiation-chemical resistance of VP-1AP anion exchanger. Radiochemistry 44(2):157–165
Fredric S (1989) Marsh, Macro porous polyvinyl pyridine resin for separating plutonium using nitrate anion exchange. Solvent Extr Ion Exch 7(5):889–908
Marsh SF, Jarvinen GD, Kim JS, Nam J (1997) New bifunctional anion-exchange resins for nuclear waste treatment. React Funct Polym 35:75–80
Marsh SF, Veirs DK, Gordan DJ, Barr ME, Moody EW (2000) Molecularly engineered reins for Pu recovery. Los Alamos Sci 26:463
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (2008) Vogel’s textbook of practical organic chemistry, 1,5-dibromopentane from tetrahydropyran. Pearson, London, p 563
Bartsch RA, Zhao W, Zhang Z-Y (1999) Facile synthesis of (ω-bromoalkyltrimethyl ammonium bromides. Synth Commun 29(14):2393–2398
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Acknowledgements
Authors gratefully acknowledge the support from Dr. S. Chattopadhyay, Head, Bio-Organic Division, BARC during this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S.S., Chatterjee, S., Rao, A. et al. Evaluation of pyridinium-based bifunctional resins for the separation of Pu(IV) from acidic solutions. J Radioanal Nucl Chem 314, 1031–1037 (2017). https://doi.org/10.1007/s10967-017-5470-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5470-0