Abstract
Two diglycolamide homologs, N,N,N′,N′-tetraoctyl-3,6-dioxaoctane diamide (DOODA) and N,N,N′,N′-tetraoctyl-3,6,9-trioxaundecane diamide (TOUDA), were synthesized. Their extraction behaviors of Am3+ and Eu3+ were investigated and compared with those of N,N,N′,N′-tetraoctyl-3-oxapentane diamide (TODGA). The extraction ability toward Am3+ and Eu3+ decreased with increasing etheric oxygen number in the extractant structure. In contrast to TODGA, an very interesting inversion on the selectivity occurred for DOODA and TOUDA, which were more favor of extracting Am3+ than Eu3+. The maximum SF Am/Eu of 3.5 for DOODA can be achieved at 3.0 mol/L HNO3. The stoichiometries of extracted species and extraction thermodynamic parameters were also presented.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the fast growing energy demands, more and more countries consider the addition of their nuclear power projects to meet the demands for energy. The nuclear power stations are producing plenty of spent nuclear fuel while generating nuclear energy. Although most of uranium (U) and plutonium (Pu) in the spent fuel can be recycled by PUREX process, at the same time the PUREX process generated the high level liquid waste (HLLW), which contains long-lived radioactive minor actinides (MA), for instance, americium (Am) and curium (Cm) [1, 2]. The hazards of HLLW primarily originate from its long term radiotoxicity and the loading heat, of which MA are the main contributors. If MA can be removed from HLLW, a significant reduction of nuclear waste can be achieved. [3,4,5].
Diamides were found to be a very promising group of extractants for partitioning actinides (An) from HLLW [6, 7]. Diamides have high distribution ratios (D) for trivalent actinides in high HNO3 concentration solutions, and also have good chemical and irradiation stability [8, 9]. In addition, diamide includes only C, H, O, N four elements and supposed to be burned out thoroughly, which suggested a reduction of the secondary pollution significantly. Malonamides with alkyl bridges groups were the first diamide group that found to have good extraction power for acitinides and then were proposed for actinide partitioning in the DIAMEX processes. However, D value of malonamides for trivalent actinides is not high enough, which necessitates the use of high concentration of extractants in the process [10]. Thus, more efficient extractants need to be developed. In 1990s, Stephan et al. [11, 12] replaced the carbon of the diamide bridge of malonamides by etheric oxygen to synthesize diglycolamides (DGA). The change of malonamide from a bidentate chelating agent to a tridentate DGA ligand increased the extractability for trivalent actinides significantly. Among DGAs, N,N,N′,N′-tetraoctyl-3-oxapentane diamide (TODGA) was proved to be optimal in many aspects. For example, TODGA has strong extraction ability, good solubility in aliphatic diluents and favorable stability [7]. Nevertheless, for TODGA, third-phase could appear in the presence of high concentration of lanthanides (Ln). It must need 0.5 mol/L DHOA as phase modifiers for better operation on the bulk separation of metal ions [13]. Thus, the structural modification of TODGA was necessary.
It can be found that the diamide bridge in its molecular structure plays an important role in chelating with f-elements. Many attempts of structure modifications for DGA have been made to study the effect of diamide bridges on extraction ability. Ruhela and Sasaki [14, 15] replaced the central oxygen atom by sulfur atom and attained thiodiglycolamides. Thiodiglycolamides show great extraction ability and selectivity for palladium, but hardly extract actinides [14, 15]. Sasaki et al. also replaced the central oxygen atom by nitrogen atom. These extractants also have poor extraction ability for actinides [16]. These results indicated that the etheric oxygen in DGA is more effective to improve extraction power for actinides than sulfur and nitrogen. Recently, Sasaki et al. [16, 17] inserted one additional etheric oxygen group in TODGA and synthesized N,N,N′,N′-tetraoctyl-3,6-dioxaoctane diamide (DOODA). The addition of bridging oxygen leads to significant changes in extraction selectivity within the Ln. The exctraction capacity for TODGA increases, whilst that for DOODA varies to a much lesser extent from lanthanum to lutetium, suggesting that the number of etheric oxygen in diglycolamide could result in the difference on the extraction selectivity for Ln [13, 18]. Due to the very high similarity of An3+ to Ln3+, it can also be expected that the increase of ether group maybe have similar influences on the extraction of An3+ over Ln3+ too. Therefore, in this work, our aims are to separate Am3+ over Eu3+, unlike the co-extraction separation of Am3+ and Eu3+ vs other fission products reported by the previous literatures [13, 16, 19].
In the present paper, two homologs of TODGA, which are DOODA and N,N,N′,N′-tetraoctyl-3,6,9-trioxaundecane diamide (TOUDA) (Fig. 1), have been synthesized to explore the effect of different number of etheric oxygen group in diglycolamide structure on the extraction behaviors of Am3+ and Eu3+. Meanwhile, their coordination behaviors with Eu3+ were also examined by means of IR and MS.
Experimental
General experimental
The organic raw materials utilized in the synthesis experiments were obtained from Aladdin Chemical and Energy Chemical, China. The pure products were characterized by 1H NMR and MS. Nuclear magnetic resonance (NMR) spectra were tested on the Varian Inova NMR spectrometer operating at 400 MHz, using (CH3)4Si (TMS) as internal standard substance. MS were measured on a Bruker amazon SL spectrometer. Sulfonated kerosene was produced by referring to previous literature. Nitric acid (Energy Chemical, China) and europium nitrate (Aladdin Chemical, China) were of AR grade, which were used without further purification in the solvent extration. The nitrate solutions were diluted with deionized water to a constant volume. The tracer stock solutions of purified radionuclides 241Am3+ were supplied by China Institute of Atomic Energy.
Synthesis of ligands
TODGA and DOODA were prepared as the previous methods [16]. TODUA was synthesized according to Scheme 1.
N,N,N′,N′-tetraoctyl-3,6,9-trioxaundecane diamide (TOUDA)
3.07 g NaH (60% in oil) was added to 1.10 g diethylene glycol in 75 mL THF and refluxed for 1 h. Then 12.04 g 1-chloro-N,N-dioctylacetamide, which was dissolved in 50 mL THF, was dropped into the ethanediol solution via constant pressure funnel. Over a period of 24 h reflux, the solution was concentrated by reduced pressure distillation, affording yellow remnant. The remnant was dissolved in 100 mL EA, scrubbed with 0.5 mol/L HCl solution (3 × 100 mL), 10% NaOH solution (2 × 100 mL), and deionized water (2 × 100 mL) in turn. The EA phase was desiccated over anhydrous MgSO4 and concentrated, eventually at reduced pressure, giving the original product. The above raw product was purified using silica column as stationary phase and mixed eluant [EA/PE, 1:4 (v/v)] as flowing phase to afford TOUDA (6.03 g, yield: 90.1%), which was slightly yellow oil. 1H NMR (400 MHz, CDCl3) δ 4.21 (s, 4H, OCH2CO), 3.72 (s, 8H, OCH2), 3.29 (m, 4H, NCH2), 3.19 (m, 4H, NCH2), 1.54 (s, 8H, NCH2CH 2 ), 1.29 (s, 40H, (CH 2 )5CH3), 0.96–0.82 (m, 12H, CH3); MS: m/z 691.7 [M+Na]+, calculated: 691.6.
Solvent extraction
The extraction experiments of the metal ion were proceeded by different organic phases containing extractant and diluent, and various aqueous phase conditions, which includes 200 ppm Eu3+ mixed with tracer level 241Am3+ or 200 ppm Eu3+. Before extraction of the metal ion, the organic phases were mixed with equal volumes of appropriate concentration HNO3 solutions for the preequilibration. It was influential for ensuring the acidity of the aqueous did not change during the biphase equilibrium. 1.5 mL organic phase and the equal volume of aqueous phase were mixed into the 15 mL test tubes with stopper. The mixture was agitated by magnetic stirrer in water bath for 1 h to achieve extraction equilibrium. After separation by centrifugation, the amount of 241Am3+ in two phases were measured with NaI(Tl) scintillation counter. Eu3+ in the aqueous phase was measured by ICP-AES. The content of Eu3+ in the organic phase was calculated from mass balances by difference between the initial solution and the aqueous phase after extraction. The D values of metal nitrate are defined as the quotient of specific concentration of metal ion in organic phase to that in aqueous phase, D M = [M]tot, org./[M]tot, aq.. Here, the subscript aq. represented aqueous phase and subscript org. indicated organic phase, severally [19]. Separation factor (SF) of Am3+ over Eu3+ (SF Am/Eu) was the ratio of D Am to D Eu, SF Am/Eu = D Am/D Eu. The precision of the D values and SF Am/Eu values were expressed as the standard deviation (SD) of three-times measurements.
Results and discussion
Influence of diluent
The extraction behaviors can be influenced by the solvation between extractant and diluent. Thus, the extraction of tracer amount of Am3+ and Eu3+ using TODGA, DOODA and TOUDA as extractants were investigated in six different diluents: kerosene, toluene, n-dodecane, n-hexane, xylene and n-octanol according to previous works [14, 19, 20]. D values and SF Am/Eu values were shown in Table 1 and Fig. 2, respectively. It can be seen that relatively high D values and reliable SF Am/Eu values could be obtained when kerosene was used as diluent. No third phase was formed during the extraction. In this way, phases could be separated effectively for all extraction experiments even at the condition of high acidity. In addition to these, kerosene with low viscosity (2.190 mpa s at 25 °C) and high boiling point (170–270 °C) was already widely applied in the industrial process. Therefore, kerosene was chosen as the diluent for the following experiments.
Influence of HNO3 concentration
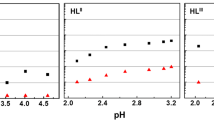
To compare the extraction behaviors of Am3+ and Eu3+ with TODGA, DOODA and TOUDA, D values in various HNO3 solutions were measured. The influence of HNO3 concentration on D Am and D Eu was shown in Fig. 3. An increase of D Am and D Eu of all the three extractants is observed as increasing the HNO3 concentration in the investigated acidity, which was due to the salting-out effect of HNO3. TODGA has the best extractability for both Am3+ and Eu3+. The order of distribution ratio is TODGA > DOODA > TOUDA, suggesting the extraction ability decrease with the increase of etheric oxygen group. In addition, it is worthy of note, for TODGA, D Eu is higher than D Am, but for DOODA and TOUDA, D Am are slightly higher than D Eu. It may be owing to the difference of ligand structures. As the increase of etheric oxygen, the cavity of diglycolamide is likely to be more suitable for Am3+ than Eu3+, leading to a reverse of the selectivity of Am3+ and Eu3+. The SF Am/Eu data at 3.0 mol/L HNO3 were summarized and listed in the Fig. 2. It can be found that the order of selectivity SF Am/Eu is DOODA > TOUDA > TODGA. The maximum SF Am/Eu of DOODA is 3.5.
Influence of extractant concentration
Since the three extractants all belong to neutral extractant. Thus, the extraction behavior for trivalent metal ions can be accounted for co-ordination mechanism, which was described in Eq. (1) within the scope of the HNO3 concentration investigated:
where M and L mean metal ions and extractants, severally.
The stoichiometries of the extracted complexes can be determined if the logD and log[L]org. are plotted with a good linear relationship at a constant acidity. The slope is roughly equivalent to the number of extractant molecules in the complexes.The extraction constant, K ex, is defined as:
The distribution ratio is refer to as Eq. (3):
Rearranging the mathematical formula after substituting the value of \(\left\{ {{{\left[ {{\text{M}}\left( {{\text{NO}}_{3}^{ - } } \right)_{n} \cdot m{\text{L}}} \right]_{{{\text{org}} .}} } \mathord{\left/ {\vphantom {{\left[ {{\text{M}}\left( {{\text{NO}}_{3}^{ - } } \right)_{n} \cdot m{\text{L}}} \right]_{{{\text{org}} .}} } {\left[ {{\text{M}}^{n + } } \right]_{{{\text{aq}}.}} }}} \right. \kern-0pt} {\left[ {{\text{M}}^{n + } } \right]_{{{\text{aq}}.}} }}} \right\}\)from Eq. (3) into Eq. (2), the K ex can be presented as follows:
The β n values for the NO3 − complexation of Eu3+ and Am3+ were estimated from the literature values as β 1 of 1.48, β 2 of 0.26 for Am3+ and β 1 of 1.66, β 2 of 0.26 for Eu3+ at ion strength of 3.0 [21]. For the reason that the concentration of extractant was roughly equal before and after equilibration, hence, the K ex values in Eq. (4) can be calculated.
The dependence of D Am and D Eu on the concentration of extractant is shown in Fig. 4. The logD values increased with the increase of log[L]org. in a linear relationship. This is due to the fact that more ligand molecules participate in the coordination. Table 2 listed the slope values and y-intercept values of three diglycolamide homologs. It can be easily observed that TODGA and DOODA extracted metal ions nearly at the mole ratio of 4:1 and 2:1, respectively. Nevertheless, for TOUDA, the slope values of Am3+ and Eu3+ are 1.52 and 1.59, severally, which are between 1 and 2. This result suggested the generating of both mono-solvated and di-solvated species and will be further discussed in the section of “mass spectrometry”.
From the above obtained results, the extraction mechanisms for both Am3+ and Eu3+ were concluded as the following:for TODGA:
for DOODA:
for TOUDA:
and
Influence of temperature
For the extraction equilibrium of Am3+ and Eu3+ at 3.0 mol/L HNO3, the extraction behaviors at different temperatures were performed to obtain the thermodynamic parameters. Equilibrium concentration constants in the range of 293–313 K calculated through Eq. (4) were given in Table 3. The dependence of the extraction of log K ex values on the inverse temperature ranging from 293 to 313 K offers a straight line in Fig. 5. In this way, the enthalpy (ΔH) and entropy (ΔS) in the process of biphase equilibrium can be computed through the Van’t Hoff equation:
where R is the gas constant.
The ΔH and ΔS were calculated on the basis of the slope values and intercept values. Furthermore, the gibbs free energy (ΔG) could be obtained from the Gibbs function:
The ΔG, ΔH and ΔS values of the extraction by TODGA, DOODA and TOUDA of this work were summarized and listed in Table 4. On the one hand, the negative ΔH value indicates that the exothermic enthalpic factor of the complexation with Am3+ and Eu3+ is the leading effect comparing with the other opposite enthalpic factors, such as dehydration of metal ions [22]. On the other hand, for the metal nitrate complex, the degree of freedom is reduced when the H2O molecules in the relatively complex primary coordination sphere is replaced by extractant. This can be reflected by the negative entropy value [23]. The negative value of ΔG suggests that the two phase equilibrium process is spontaneous at room temperature. It can be seen that the absolute ΔG values of three diglycolamide homologs were ranked in the sequence of TODGA > DOODA > TOUDA, which is same as the order of distribution values. In addition, the ΔG value of TODGA is much smaller than that of DOODA and TOUDA, implying that the reaction spontaneity of TODGA is stronger than that of DOODA and TOUDA obviously.
Stripping experiment
Efficient stripping of metal ions from loaded organic phase is an important feature for evaluating an extraction system. The stripping of Am3+ and Eu3+ was performed with various HNO3 solutions from the loaded 0.1 mol/L TODGA, DOODA or TOUDA in kerosene. As listed in Table 5, the higher stripping efficiency was achieved as the decrease of acidity. However, the stripping efficiency of three extractants were different. For TODGA, 1.0 mol/L HNO3 could barely strip Am3+ and Eu3+ from the loaded organic phase. More than 90% of metal ions could be stripped only if the concentration of HNO3 as low as 0.1 mol/L. In the case of DOODA and TOUDA, all the three HNO3 solution with concentrations of 0.1, 0.5 and 1.0 mol/L could effectively strip at least 97% Am3+ and Eu3+ into aqueous at one stage. Quantitative stripping of Am3+ and Eu3+ could be completed at two stages by all examined HNO3 solution for DOODA and TOUDA. Considering practicability and economy, 0.1 mol/L HNO3 is recommended as a stripping agent.
Spectrum analysis of Eu3+-complexes
In order to investigate the coordination relationship between metal ions and ligands deeply, the preparation and analysis of metal complexes were necessary. In this work, we produced three complexes: Eu-TODGA, Eu-DOODA and Eu-TOUDA, using IR spectroscopy and mass spectrometry for the further studies.
IR spectroscopy
The infrared spectra of three complexes were measured and shown in Fig. 6. The band associated with C=O stretching vibration shifted to lower wavenumber from 1650 to 1610 cm−1 after complexation with Eu. This result is similar to that reported by Sasaki et al. [20]. Wavenumbers of 1120 and 1040 cm−1 are attributed to the C–O–C group. The displacement of etheric oxygen group means that the ether O was also involved in the coordination to Eu. The C–O–C group of three complexes occured red shift approximately by 39 cm−1 for TODGA, 20 cm−1 for DOODA and 14 cm−1 for TOUDA, respectively. The degree of the shift was in the order of TODGA > DOODA > TOUDA, which was same as the descending sequence of distribution ratio and the absolute ΔG values in Table 4. This phenomenon can be explained as follows. The cavity of the structure was enlarged as the addition of C–O–C group. Meanwhile, the bond length of M–O (ether) was elongated as the increase of etheric oxygen number. The lengthening of M–O (ether) bond leads not only to the decrease of complex ability, but also to lower displacement of wavenumbers. Likewise, the reduction of thermodynamic spontaneity with the increase of ether O can be explained in the same way. Moreover, bands at 1384 cm−1 in the spectra of complexes demonstrate the existence of NO3 −, which can be accounted for the charge balancing effect of neutral extraction system.
Mass spectrometry
In order to identify the composition of complexes, ESI–MS analysis was performed for the determination of speciation during the extraction [24, 25]. After nebulisation of the samples, several ionic species M(L) x (NO3 −) y , with x = 1, 2 or 4 and y = 0 or 1 were observed for diglycolamide homologs, as presented in Figs. 7, 8, and 9. In fact, all of these peaks were isotopically resolved and agreed very well with their theoretical distribution. Meanwhile, the mass spectrograms with good purity made it apparently to identify the new species. The complexes of TODGA and DOODA shown in Figs. 7 and 8 indicated the formation of [Eu-4TODGA]3+ and [Eu-2DOODA-NO3 −]2+, severally. This result is in accordance with that of slope method research. Two molecular ion peaks observed in Fig. 9 meant that TOUDA coordinated with Eu at the mole ratio of 1:1 or 1:2, which was also in agreement with the conclusion obtained by slope analysis.uture.
Based on the results obtained from the spectra analysis, it can be seen that both ether O and carbonyl O were participated in the coordination with Eu3+. The combining ability of the three ligands was decreased with the increase of etheric oxygen. ESI–MS showed that a single kind of complex of TODGA and DOODA was formed during the extraction. However, two kinds of complexes, Eu-TOUDA and Eu-2TOUDA, were generated in the process of phases equilibrium, which was different from that of TODGA and DOODA.
Conclusions
Two TODGA homologs, DOODA and TOUDA were synthesized. The extraction behaviors of Am3+ and Eu3+ by three diglycolamides were investigated and compared. It has been shown that the D values of all the three extractants increased as the increase of HNO3 concentration. In the meantime, the extraction power of extractants for both Am3+ and Eu3+ decreased with the increase etheric oxygen number in the extractant structures. This result was in conformity with the thermodynamic parameters and IR spectrometry. Slope analysis and mass spectrometry indicated that TODGA and DOODA extracted metal ions at the mole ratio of 4:1 and 2:1, respectively. For TOUDA, slope values of metal ions were both close to 1.5, suggesting the formation of 1:1 and 2:1 ligand: metal complexes. An interesting inversion of the selectivity of Am3+ over Eu3+ was observed in the case of 2 or 3 etheric oxygen bridge and the maximum SF Am/Eu of DOODA can achieve 3.5 at 3.0 mol/L HNO3. Although the SF Am/Eu values are not high yet for TODGA, DOODA and TOUDA, the results of this paper would provide important guidance to design the new more efficient ligands without soft N- or S-donor for An3+/Ln3+ separation in the future.
References
Wang ZP, Ding SD, Hu XY, Li SM, Su DP, Zhang LR, Liu Y, Jin YD (2017) Selective extraction of americium(III) over europium(III) ions in nitric acid solution by NTAamide(C8) using a novel water-soluble bisdiglycolamide as a masking agent. Sep Purif Technol 181:148–158
Prathibha T, Selvan BR, Venkatesan KA, Rajeswari S, Antony MP (2017) Radiolytic stability of N,N-di-alkyl-2-hydroxyacetamides. J Radioanal Nucl Chem 311:1929–1935
Ning SY, Zou Q, Wang XP, Liu RQ, Wei YZ, Zhao YP, Ding YQ (2016) Evaluation study on silica/polymer-based CA-BTP adsorbent for the separation of minor actinides from simulated high-level liquid wastes. J Radioanal Nucl Chem 307:993–999
Xiao CL, Wang CZ, Mei L, Zhang XR, Wall N, Zhao YL, Chai ZF, Shi WQ (2015) Europium, uranyl, and thorium-phenanthroline amide complexes in acetonitrile solution: an ESI-MS and DFT combined investigation. Dalton Trans 44:14376–14387
Ravi J, Robert Selvan B, Venkatesan KA, Antony MP, Srinivasan TG, Vasudeva Rao PR (2014) Radiolytic stability of N,N-didodecyl-N′,N′-diethylhexyl diglycolamide. J Radioanal Nucl Chem 300:981–986
Manchanda VK, Pathak PN (2004) Amides and diamides as promising extractants in the back end of the nuclear fuel cycle: an overview. Sep Purif Technol 35:85–103
Ansari SA, Pathak P, Mohapatra PK, Manchanda VK (2012) Chemistry of diglycolamides: promising extractants for actinide partitioning. Chem Rev 112:1751–1772
Thiollet G, Musikas C (1989) Synthesis and uses of the amides extractants. Solvent Extr Ion Exch 7(5):813–827
Cuillerdier C, Musikas C, Hoel P, Nigond L, Vitart X (1991) Malonamides as new extractants for nuclear waste solutions. Sep Sci Technol 26(9):1229–1244
Ansari SA, Pathak P, Mohapatra PK, Manchanda VK (2011) Aqueous partitioning of minor actinides by different processes. Sep Purif Rev. 40:43–76
Stephan H, Gloe K, Beger J, Mühl P (1991) Liquid-liquid extraction of strontium with amido podands. Solvent Extr Ion Exch 9(3):435–458
Stephan H, Gloe K, Beger J, Mühl P (1991) Liquid-liquid extraction of metal ions with amido podands. Solvent Extr Ion Exch 9(3):459–469
Alyapyshev MY, Babain VA, Ustynyukc YA (2016) Recovery of minor actinides from high-level wastes: modern trends. Russ Chem Rev 85(9):943–961
Ruhela R, Sharma JN, Tomar BS, Panja S, Tripathi SC, Hubli RC, Suri AK (2010) N,N,N′,N′-tetra(2-ethylhexyl) thiodiglycolamide T(2EH)TDGA: a novel ligand for the extraction of palladium from high level liquid waste (HLLW). Radiochim Acta 98:209–214
Sasaki Y, Tachimori S (2002) Extraction of actinides (III), (IV), (V), (VI), and lanthanides (III) by structurally tailored diamides. Solvent Extr Ion Exch 20:21–34
Sasaki Y, Tsubata Y, Kitatsuji Y, Sugo Y, Shirasu N, Morita Y, Kimura T (2013) Extraction behavior of metal ions by TODGA, DOODA, MIDOA, and NTAamide extractants from HNO3 to n-dodecane. Solvent Extr Ion Exch 31:401–415
Usuda S, Yamanishi K, Mimura H, Sasaki Y, Kirishima A, Sato N, Niibori Y (2015) Separation of Am and Cm by using TODGA and DOODA(C8) adsorbents with hydrophilic ligand-nitric acid solution. J Radioanal Nucl Chem 303:1351–1355
Sasaki Y, Kitatsuji Y, Tsubata Y, Sugo Y, Morita Y (2011) Separation of Am, Cm and lanthanides by solvent extraction with hydrophilic and lipophilic organic ligands. Solv Extract Res Dev, Jpn 18:93–101
Su DP, Huang H, Huang S, Liu N, Ding SD (2015) Extraction of trivalent europium and americium from nitric acid solution with bisdiglycolamides. Sep Sci Technol 50:1384–1393
Sasaki Y, Rapold P, Arisaka M, Hirata M, Kimura T, Hill C, Cote G (2007) An additional insight into the correlation between the distribution ratios and the aqueous acidity of the TODGA system. Solvent Extr Ion Exch 25:187–204
Horwitz EP, Muscatello AC, Kalina DG, Kaplan L (1981) The extraction of selected transplutonium(III) and lanthanide(III) ions by dihexyl-N,N-diethylcarbamoylmethylphosphonate from aqueous nitrate media. Sep Sci Technol 16(4):417–437
Arisaka M, Kimura T (2011) Thermodynamic and spectroscopic studies on Am(III) and Eu(III) in the extraction system of N,N,N′,N′-tetraoctyl-3-oxapentane-1,5-diamide in n-dodecane/nitric acid. Solvent Extr Ion Exch 29:72–85
Ansari SA, Pathak PN, Husain M, Prasad AK, Parmar VS, Manchanda VK (2006) Extraction of actinides using N,N,N′,N’-tetraoctyl diglycolamide (TODGA): a thermodynamic study. Radiochim Acta 94:307–312
Gong Y, Hu HS, Tian GX, Rao LF, Li J, Gibson JK (2013) A tetrapositive metal ion in the gas phase: thorium(IV) coordinated by neutral tridentate ligands. Angew Chem Int Ed 52:6885–6888
Tian GX, Zhu YJ, Xu JM, Zhang P (2003) Investigation of the extraction complexes of light lanthanides(III) with bis(2,4,4-trimethylpentyl)dithiophosphinic acid by EXAFS, IR, and MS in comparison with the americium(III) complex. Inorg Chem 42:735–741
Acknowledgements
The authors are very grateful for the financial support by the National Science Foundation of China (Nos. 11675115, 91426302 and 91126016). Analytical & Testing Center of Key Laboratory of Green Chemistry & Technology (Sichuan University) of Education Ministry of China is acknowledged for NMR, MS and ICP-AES analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Z., Huang, H., Ding, S. et al. Extraction of trivalent americium and europium with TODGA homologs from HNO3 solution. J Radioanal Nucl Chem 313, 309–318 (2017). https://doi.org/10.1007/s10967-017-5317-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5317-8