Abstract
89Sr and 90Sr are important fission products. Their measurement is of interest for a number of reasons, including nuclear forensic scenarios. A rapid method has been developed for the analysis of 89Sr and 90Sr by a combination of Cerenkov and liquid scintillation counting; results can be available with 3–4 days. There is no strict time requirement for the Cerenkov counting and no additional step to separate 90Y after ingrowth. Counting efficiencies are determined using certified 89Sr and 90Sr (in equilibrium with 90Y) solutions without the need of 90Sr/90Y separation. The method has been validated using inter-laboratory comparison samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
AWE provides radiochemical analytical support to UK Nuclear Forensics Programme. Fission products in a nuclear forensics sample are required to be separated, purified and quantified. The analytical results should be provided promptly during a nuclear event. Strontium measurement is of interest for a number of reasons, including analysis in nuclear forensics scenarios. 89Sr and 90Sr are important fission products with high cumulative fission yields of 4.7 and 5.8% from the irradiation of 235U by thermal neutrons, and 1.7 and 2.0% from the irradiation of 239Pu by fission spectrum neutrons [1]. Both radiostrontium isotopes are beta emitters, whose nuclear data are shown in Table 1. 89Sr decays to stable 89Y with a half-life of 50.57(3) days. 90Sr decays to 90Y with a half-life of 28.80(7) years. 90Y also beta decays with a half-life of 2.6684(13) days to the stable nuclide 90Zr [2].
The measurement of 89Sr and 90Sr is complicated because they cannot be chemically separated. If the presence of both isotopes are significant in a sample, their activities need to be corrected from each other for the measurement. Also, 90Y ingrows immediately after the separation of strontium and yttrium as shown in Eq. (1)
where A is the activity at time t, λ is the decay constant, and t is the decay time. As the half-life of 90Y is much less than that of 90Sr (λ 90Sr << λ 90Y), this equation can be simplified to Eq. (2).
The preparation of a pure strontium source is not achievable due to the quick ingrowth of 90Y. All three radionuclides, with overlapping beta spectra, are present in the counting source and they must be distinguished from each other. It is possible to quantify 89Sr by gamma spectrometry using its 909.0 keV peak, but this peak has a very low emission probability (0.00956%), which means it is not possible to get an accurate result if the sample is not active enough.
Several papers have been published on the simultaneous measurement of 89Sr and 90Sr activities in various types of environmental samples, such as soil, water and milk, etc. Vajda and Kim published a review paper on the determination of radiostrontium isotopes [3]. The oldest separation method was based on a few precipitation steps using nitric acid, hydroxide, chromate, and carbonate [4]. Currently, the most popular method is based on extraction chromatography using Sr Resin® developed by Horwitz et al. who found the di-t-butyl derivative of the di-cyclohexano-18-crown-6 ether dissolved in 1-octanol efficiently and selectively extracted strontium from nitric acid solutions [5, 6]. The crown ether was impregnated onto support material producing Sr Resin®. The Sr Resin® is commercially available from Eichrom or Triskem.
Cerenkov counting is one method of measuring beta emitting radionuclides. When charged particles, such as beta particles, travel through a media, they can travel at a speed greater than the phase velocity of light in that media if they initially possess sufficient energy. When this occurs, the charged particles produce Cerenkov photons. Beta particles with energy in excess of 263 keV in water demonstrate the Cerenkov effect. 89Sr and 90Y, which is the daughter of 90Sr, produce Cerenkov light because of their high beta energies exceeding the 263 keV threshold energy for the Cerenkov effect. Therefore the activities of 89Sr and 90Sr can be determined by Cerenkov measurements of 89Sr and 90Y [7, 8]. The measurements are usually carried out in one of three ways: (1) two separate Cerenkov measurements for the separated 90Y and 89Sr + 90Sr fractions assuming 90Sr and 90Y are in secular equilibrium in the sample [9, 10]; (2) Cerenkov count of the strontium fraction immediately after the purification to quantify 89Sr assuming 90Y ingrowth and 90Sr Cerenkov effect are negligible, followed by a second Cerenkov count after a few days to quantify 90Sr based on 90Y ingrowth [11–13]; (3) alternatively, the second Cerenkov measurement can be carried out for 90Y separated from the purified strontium after its ingrowth [8, 14, 15]. These methods are rapid, but either the first Cerenkov measurement needs to be carried out immediately after the purification, which limits the number of samples processed in a batch, or there is an additional purification step after the ingrowth for the second Cerenkov measurement. Compared to their near 100% liquid scintillation counting (LSC) efficiencies [3], the Cerenkov counting efficiencies for 89Sr and 90Y are low, typically ~40 and ~60% [16], which is also a concern.
The activities of 89Sr and 90Sr can be measured by LSC. Because 89Sr, 90Sr and 90Y have different beta endpoint energies, two- or three- window methods or spectrum deconvolution/unfolding methods can be applied. However, these methods often fail when the activity concentrations of 89Sr and 90Sr are significantly different (activity ratio greater than 5) [14, 17–20]. The simultaneous determination of 89Sr and 90Sr activities can also be achieved by sequential counting of Cerenkov followed by LSC. Kim et al. reported the rapid analysis of milk samples using this method [16]. The 90Y ingrowth is taken into account in the calculation, so the time for the first Cerenkov measurement is not very critical although it should still be carried out as soon as possible after the separation of strontium from yttrium to reduce the interference and improve the measurement uncertainty. This method combines the advantages of rapid measurement by Cerenkov counting and the high counting efficiencies offered by LSC, and it was used in this study.
Although this combined method is efficient, the published studies often involve the preparation of four calibration sources (89Sr, 90Sr, 90Y, and mixed 90Sr/90Y) and Cerenkov/LSC efficiency calibrations for each of them [16, 21]. The procedures are complicated and the preparation of separate 90Sr and 90Y sources with accurately known activities is difficult because of the immediate ingrowth of 90Y. In this study, the counting efficiencies were determined using two commercially available certified 89Sr and 90Sr (in equilibrium with 90Y) solutions without the need of 90Sr/90Y separation. Results can be available within 3–4 days after the radiochemical separation using this method and the method has been validated during two inter-laboratory comparison exercises involving the analysis of recently irradiated uranium samples.
Experimental part
Sample preparation
Two highly enriched uranium samples from two inter-laboratory comparison exercises were used in the experiments. The samples were irradiated with thermal neutrons and cooled for a couple of days before the analytical process. The first sample, Sample A, was dissolved by nitric acid digestion and the final solution was diluted to give an acid concentration of 3 mol L−1. AWE received a portion of this solution, which contained 1 × 1013 fissions. Five replicates were prepared and each of them contained (0.1–0.5) × 1013 fissions (approximately 0.7–3 g of solution). The second sample, Sample B, was also in 3 mol L−1 nitric acid. The portion received by AWE contained 1 × 1014 fissions. Four replicates were prepared and each of them contained 1 × 1013 fissions in approximately 1 g of solution.
In order to monitor the chemical recovery, 1 mg of strontium carrier containing naturally occurring stable isotopes from CPI International (Santa Rosa, CA, USA) was added to the samples. Strontium was initially separated from other bulk elements by two successive precipitations as strontium nitrate using ice cold fuming nitric acid. The solution containing other bulk elements was continued for the analysis of other fission products. The strontium precipitate was then dissolved in water and separated from residual actinides by ferric hydroxide precipitation using ammonia. The supernatant containing strontium was evaporated to dryness, dissolved in 8 mol L−1 nitric acid, and finally purified twice using Triskem (Bruz, France) 50–100 µm Sr Resin® (part number: SR-B100-S) in a 2 mL plastic column (part number: 104704) from Rockbourne Scientific (Hampshire, UK). The column was rinsed with 8 mol L−1 nitric acid, 3 mol L−1 nitric acid/0.05 mol L−1 oxalic acid and 4 mol L−1 nitric acid respectively to remove barium, lanthanum, yttrium and other residual elements. The strontium/yttrium separation time was recorded and strontium was finally eluted from the column using 0.05 mol L−1 nitric acid. This purification procedure using Sr Resin® was derived from Eichrom’s analytical procedure for the analysis of 89Sr and 90Sr in water [22].
The radiochemically purified strontium was dissolved in 5.0 mL of 0.1 mol L−1 nitric acid. An aliquot of 0.1 mL sample solution was diluted by a factor of 1 × 104 and the stable strontium concentration was measured using an X Series II Inductively Coupled Plasma—Mass Spectrometer (ICP-MS) (Thermo Fisher Scientific™, Waltham, MA, USA). The measured amount of stable strontium was compared to the amount added to calculate the chemical recovery. An aliquot of 4.5 mL sample solution was transferred into a 20 mL plastic liquid scintillation (LS) vial (part number: 6008117, PerkinElmer™, Waltham, MA, USA). A XPE205 balance from Mettler Toledo (Leicester, UK) was used to weigh the sample solutions.
Radiochemical purity check and measurement of 89sr by gamma spectrometry
The LS vial containing 4.5 mL of purified strontium was counted for 7000 s on a GR3019 High-purity Germanium Detector (Canberra Industries Inc, Meriden, CT, USA) to verify the sample was free from interferences for Cerenkov counting and LSC. The interferences are primarily 140Ba and its daughter 140La because 140Ba is one of the major fission products with high yield (6.2% for 235U thermal fission) and the chemistry of barium and strontium is very similar. After Cerenkov counting, the sample was counted by gamma spectrometry again with a longer count time to quantify 89Sr in the sample using its 908.96 keV peak. The measurement was only attempted for replicate 1 for each of the samples because of the very low emission probability of the peak (0.00956%) and the highest sample mass for replicate 1. The detector was calibrated using a mixed radionuclide standard from the National Physical Laboratory (Teddington, UK). Genie 2000 software (version 3.3, Canberra Industries Inc, Meriden, CT, USA) was used for the spectrum analysis. The nuclear data were taken from NuDat (2014) at the National Nuclear Data Center (NNDC) at Brookhaven National Laboratory (BNL) [23].
Measurement of 89Sr and 90Sr by Cerenkov and liquid scintillation counting
After the radiochemical purity had been checked by gamma spectrometry, the sample was measured on a 1220 Quantulus™ Liquid Scintillation Spectrometer (PerkinElmer™, Waltham, MA, USA) for Cerenkov counting with a count time of 30 min, window setting 10–450. Ultima Gold™ cocktail (part number: 6013321, PerkinElmer™), 15.5 mL, was then added to the purified strontium in the 20 mL plastic LS vial. The sample was dark-adapted and stored for at least 12 h before counted on the Quantulus™ again for LSC with a count time of 15 min, window setting 100–950.
Certified 89Sr and 90Sr (in equilibrium with 90Y) standard solutions from Eckert and Ziegler (Braunschweig, Germany) were used as the tracer radionuclides for counting efficiency calibration. The relative standard uncertainty of the activity concentration of these solutions were 1.33 and 0.36%, respectively. Six standard sources were prepared for each of the radionuclides by adding 0.1 mL of tracer, 0.1 mL of 5 mg mL−1 stable strontium carrier, and 3.3–5.8 mL of 0.1 mol L−1 nitric acid. A Mettler XPE205 balance was used to weigh the added solutions. Each standard vial contained either 50 Bq of 89Sr or 35 Bq of 90Sr. Six blanks containing the same components except the tracer were prepared for background determination. The two sets of 89Sr and 90Sr standards were counted on the Quantulus™ by Cerenkov counting with a count time of 30 min. The standards were then mixed with Ultima Gold™ cocktail to give a total volume of 20 mL and counted on the Quantulus™ again for 15 min by LSC.
89Sr/90Sr activity ratio investigation
When both 89Sr and 90Sr are present in a sample, if the activity of one radiostrontium isotope is much higher than the other isotope, it is difficult to accurately measure the activity of the minor isotope due to its high uncertainty. In order to investigate the activity ratio effect on the measurement and to understand the limitation of this method, two sets of the experiments were carried out. The ratios of 89Sr to 90Sr varied from 1 to 100 for the first set and the ratios were reversed for the second set. The samples were prepared by spiking with certified 89Sr and 90Sr standard solutions (Eckert and Ziegler).
Calculations in the method
The net count rates for 89Sr, 90Sr and 90Y in Cerenkov and liquid scintillation counting can be expressed by Eqs. (3) and (4):
where \(R_{t1}^{\text{CKV}}\) and \(R_{t2}^{\text{LSC}}\) are the net Cerenkov and LSC count rates; \(\varepsilon_{\text{CKV}}^{^{89}{\text{Sr}}}\), \(\varepsilon_{\text{CKV}}^{^{90}{\text{Sr}}}\) and \(\varepsilon_{\text{CKV}}^{^{90}{\text{Y}}}\) are the Cerenkov counting efficiencies for 89Sr, 90Sr and 90Y respectively; \(\varepsilon_{\text{LSC}}^{^{89}{\text{Sr}}}\), \(\varepsilon_{\text{LSC}}^{^{90}{\text{Sr}}}\) and \(\varepsilon_{\text{LSC}}^{^{90}{\text{Y}}}\) are the LSC efficiencies for 89Sr, 90Sr and 90Y respectively; \(A_{t1}^{^{89}{\text{Sr}}}\) is the total activity of 89Sr in source at Cerenkov counting time (t 1); \(A_{t0}^{^{90}{\text{Sr}}}\), \(A_{t1}^{^{90}{\text{Sr}}}\) and \(A_{t2}^{^{90}{\text{Sr}}}\) are the total activity of 90Sr in source at the Sr/Y separation time (t 0); Cerenkov counting time (t 1) and LSC counting time (t 2) respectively. f 1 is the ingrowth factor for 90Y from the separation time to the Cerenkov counting time (t 0 → t 1); f 2 is the ingrowth factor for 90Y from the separation time to the LSC counting time (t 0 → t 2); and f 3 is the decay factor for 89Sr from the Cerenkov counting time to the LSC counting time (t 1 → t 2). They are expressed in Eqs. (5), (6) and (7).
Because the time interval between t 0, t 1, and t 2 is much less than the half-life of 90Sr, \(A_{t1}^{^{90}\text{Sr}} \cong A_{t0}^{^{90}\text{Sr}} \cong A_{t2}^{^{90}\text{Sr}}\).
Therefore Eqs. (3) and (4) can be expressed as Eqs. (8) and (9):
\(\varepsilon_{\text{CKV}}^{^{89}\text{Sr}}\) and \(\varepsilon_{\text{LSC}}^{^{89}\text{Sr}}\) in Eqs. (8) and (9) can be easily obtained from the Cerenkov and LSC efficiency calibration using a 89Sr standard solution. \(\varepsilon_{\text{CKV}}^{^{90}\text{Y}}\) in Eq. (8) is not directly available, but \(\varepsilon_{\text{CKV}}^{^{90}\text{Sr}}\) + \(\varepsilon_{\text{CKV}}^{^{90}\text{Y}}\) can be easily determined from the Cerenkov efficiency calibration using a 90Sr (in equilibrium with 90Y) standard solution. Literature shows that \(\varepsilon_{\text{CKV}}^{^{90}\text{Sr}}\) is less than 1.4%, usually ~1% [24, 25], and this was confirmed as 0.7% by a single measurement in this study, therefore it is assigned as (1 ± 0.5)% (k = 2) here and not routinely measured, and \(\varepsilon_{\text{CKV}}^{^{90}\text{Y}}\) can then be calculated. \(\varepsilon_{\text{LSC}}^{^{90}\text{Sr}}\) + \(\varepsilon_{\text{LSC}}^{^{90}\text{Y}}\) in Eq. (9) can be easily measured from the LSC efficiency calibration using a 90Sr (in equilibrium with 90Y) standard solution. \(\varepsilon_{\text{LSC}}^{^{90}\text{Y}}\) is almost 100% due to its very high beta energy, so it is assigned as (99.75 ± 0.25)% (k = 2) in the calculation. Once all the efficiencies in the simultaneous equations, Eqs. (8) and (9), have been determined and Cerenkov and LSC count rates are available, \(A_{t1}^{^{89}\text{Sr}}\) and \(A_{t1}^{^{90}\text{Sr}}\) can then be calculated.
Each uncertainty component is assessed individually, and then propagated according to the ISO Guide to the Expression of Uncertainty in Measurement provided by United Kingdom Accreditation Service (UKAS) [26].
The detection limit is determined following Currie (1968) [27] and expressed as minimum detectable activity (MDA).
where L D is the detection limit in counts and B is the number of blank counts. When calculating the MDA of one radiostrontium isotope, the counts due to the other isotope are regarded as the background counts and taken into account in the calculation.
Results and discussion
Counting efficiencies
The Cerenkov and LS counting efficiencies obtained from the direct measurements of commercially available 89Sr and 90Sr (in equilibrium with 90Y) standard solutions are presented in Table 2, with the assigned values for 90Sr Cerenkov counting efficiency (1%) and 90Y LSC efficiency (99.75%). The calibrated SQP(E) (External Standard Quenching Parameter) ranges are also given. The obtained efficiencies are only applicable for a sample which has a SQP(E) value within the calibrated range. All the efficiencies required for the calculation were obtained without the need of radiochemical separation. This calibration process significantly reduced the time spent on the preparation of counting sources, simplified the efficiency calculations, and provided reliable results with reasonable uncertainties.
Inter-laboratory comparison Sample A
Gamma screening of the sample purified once using Sr Resin® showed the presence of 140Ba, which is the major interfering radionuclide due to its similar chemistry and high fission yields. After the second column purification, the gamma spectrum confirmed the radiochemical purity of the strontium source. It was free from any other gamma emitting radionuclides, including 140Ba. The MDA (not chemical recovery and decay corrected) was 2.3 Bq for 140Ba for 7000 s count time.
The measurement of 89Sr by gamma spectrometry is challenging because of its very low emission probability (0.00956%). For replicate 1 (A-1), a total of 3743 counts were collected in 163,000 s. The counting efficiency for the detector used was 2.8% for the 89Sr 908.96 keV peak. The activity concentration of 89Sr in A-1 was (9.73 ± 0.56) kBq g−1 (k = 1). The result was decay corrected to the reference date 07 November 2014 17:23 (GMT). The MDA for the measurement was approximately 150 Bq for 163,000 s count time.
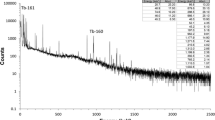
The Cerenkov and LSC spectra of the purified strontium for Sample A-1 are shown in Fig. 1. The background spectra have been subtracted. The spectra show the purified strontium was free from any observable alpha or low energy beta interference.
The 89Sr and 90Sr activity concentrations measured by Cerenkov counting and LSC for the five replicates of Sample A, together with sample masses and chemical recoveries, are presented in Table 3. Chemical recovery for a single step strontium nitrate precipitation was typically 80–85%. Although this recovery was not particularly high compared to those obtained by modern column separation, strontium nitrate precipitation did provide relatively pure strontium/barium fraction whereas other bulk fission products were also efficiently preserved for their analysis, which was especially important for the analysis of some fission products with very low fission yields. There was 30–35% of strontium lost during the two cycles of nitrate precipitation, but it was considered as acceptable due to the high fission yields of radiostrontium. Chemical recovery for a single column purification using Sr Resin® was typically 90–95%. The overall chemical recovery for the whole separation and purification process was approximately 50%. The 89Sr results for the five replicates were consistent and the measurement MDA (not chemical recovery and decay corrected) was approximately 1 Bq. The 90Sr activity in the counting source was above the measurement MDA, which was approximately 20 Bq, but the uncertainty was above 100%, therefore only upper limits are presented in the table. Results were obtained within a few working days of the radiochemical separation.
Inter-laboratory comparison Sample B
The purified strontium sources of replicates 1–4 for Sample B were free from any interferences as confirmed by gamma spectrometry. For replicate 1 (B-1), a total of 38,943 counts were collected in 73,954 s during gamma measurement. The activity concentration of 89Sr in B-1 was (636 ± 34) kBq g−1 (k = 1). The result was decay corrected to the reference date 21 July 2015 22:11 (GMT).
The background subtracted Cerenkov and LSC spectra of the purified strontium for Sample B-1 are shown in Fig. 2. The spectra are very similar to those for Sample A-1, but Sample B had a higher activity. There is no interference observed in the spectra.
Sample masses, chemical recoveries and the measured 89Sr and 90Sr activity concentrations for the four replicates of Sample B are presented in Table 4. Chemical recoveries were consistent, averaging around 50%. The results for 89Sr agree with each other very well. Similar to Sample A, the 90Sr activities could not be confidently determined.
The measurements of both Samples A and B show that the activity concentration of 90Sr in a sample cannot be accurately quantified if the 89Sr activity is significantly higher, such as in a freshly irradiated sample. In order to test the method applicability for samples with moderate 89Sr/90Sr ratios, replicates of Sample B were measured by LSC again six months (approximately four 89Sr half-lives) after the irradiation, when 89Sr in the sample had decayed sufficiently. Figure 3 shows the second LSC spectrum for Sample B-1. There is a peak shoulder between channels 600–700 which belongs to 90Sr, and there is a tail to channel 900, which belongs to 90Y. The recalculated results for 89Sr and 90Sr are presented in Table 5.
The 89Sr results are almost identical to those shown in Table 4. The 90Sr activity concentration was quantifiable with a reasonable accuracy. On the second LSC date, the 89Sr activity concentration was 30.3 kBq g−1, which was approximately 10 times of the 90Sr activity concentration, compared to 170 times on the irradiation date. Therefore this method performs well if a sample has a moderate 89Sr/90Sr ratio. The effect of activity ratio on the measurement has been investigated and will be discussed later. For a sample with an extreme 89Sr/90Sr ratio, the activity of the minor isotope is usually negligible for nuclear forensics purpose. Whereas the 90Sr activity is much lower but still needed, such as for radiation protection purpose, it can be determined by the second separation of strontium and yttrium after the 90Y ingrowth and the measurement of 90Y by either Cerenkov counting or LSC, but this is out of the scope of this paper.
Uncertainty budget
The uncertainty calculation is done on a sample specific basis. The uncertainty budget for a sample containing comparable amount of 89Sr and 90Sr is given in Table 6. The largest uncertainty component usually comes from the chemical recovery determination by ICP-MS, which is 4.3% overall attributed to solution dilution, standard deviation of replicates, linear calibration, potential drift, non-linear measurement and interference. The typical Cerenkov counting uncertainty is 2% and for LSC is 1%. In reality, the magnitude of these uncertainties can vary widely, according to concentration and count time. The relative standard uncertainty may be less than 1% or greater than 100%. Counting efficiency uncertainties attributes to the uncertainties on source preparation, counting and half-lives, varied 0.5–2.5%. A 1.7% efficiency drift is allowed for the Quantulus™ instrument. The weighing uncertainty is very small, 0.02%. The typical combined relative standard uncertainty is 6%. This uncertainty budget assumes the activity concentrations of 89Sr and 90Sr in a sample are comparable, otherwise the uncertainty for the minor isotope will be increased due to the interference from the major isotope, whereas the uncertainty for the major isotope still remains the same.
Results comparison
The US Pacific Northwest National Laboratory (PNNL) also participated in these inter-comparison exercises. The average results of 89Sr with their relative uncertainties u (%, k = 1) obtained by AWE and PNNL are presented in Table 7. The 90Sr result was not required for reporting. For Sample A, there is a very good agreement between AWE results obtained by Cerenkov counting and LSC, and by gamma spectrometry. The result ratio is 0.99. There is a 15% discrepancy between the AWE results and the PNNL result, but this is not significant when taking the uncertainties into account. For sample B, all results agree very well with result ratios 0.97 and 0.96.
89Sr/90Sr Activity ratio effect
As discussed previously, the 89Sr activities in both samples A and B were much higher than the 90Sr activities which prevented the accurate measurement of the 90Sr activities. Minor strontium isotope can only be measured when the 89Sr/90Sr activity ratio is moderate. Experiments were carried out to investigate the activity ratio effect on the measurement. The spiked activities and the measured activities with their associated relative uncertainties u (%, k = 1) are presented in Table 8.
Good results with gradually increased uncertainties for minor strontium isotope were obtained for samples with activity ratios between 1 and 20. When the ratios went up to 40, uncertainties for the minor isotope were continuously increased to 50% due to the severe interference from the major isotope. When the ratios were beyond 40, good results for the major isotope still remained, but unreliable results and uncertainties were observed for the minor isotope, which indicates its activity cannot be determined and only an upper limit should be reported in this case. In practice, the minor isotope in a nuclear forensics sample with an extreme activity ratio (>40) is usually considered as negligible, therefore the lack of result for the minor isotope is not a concern.
Conclusions
A rapid method for the measurement of 89Sr and 90Sr has been developed utilising the 1220 Quantulus™ Liquid Scintillation Spectrometer using a combination of Cerenkov counting and LSC. The counting efficiencies used in the calculation were determined using commercially available certified 89Sr and 90Sr (in equilibrium with 90Y) solutions without the need for 90Sr/90Y separation. Results were obtained within 3–4 working days after the radiochemical separation. The method was validated during two inter-comparison exercises involving the analysis of radiostrontium obtained from two freshly irradiated uranium samples. Further investigation showed that accurate determination of minor radiostrontium isotopes is dependent on the 89Sr/90Sr activity ratio in the sample. For a sample with an extreme activity ratio (>40), the activity of minor isotope can be considered as negligible and it is usually not required for nuclear forensics purpose.
References
England TR, Rider BF (1993) Evaluation and compilation of fission product yields. Los Alamos National Laboratory, New Mexico
DDEP, Decay Data Evaluation Project. http://www.nucleide.org
Vajda N, Kim CK (2010) Determination of radiostrontium isotopes: a review of analytical methodology. Appl Radiat Isot 68:2306–2326
Sunderman DN, Townley CW (1960) The radiochemistry of barium, calcium and strontium. Technical Report NAS-NS 3010. National Academy of Sciences – National Research Council. Nuclear Science Series, Radiochemistry Techniques
Horwitz EP, Chiarizia R, Dietz ML (1992) A novel and strontium-selective extraction chromatographic resin. Solvent Extr Ion Exch 10(2):313–336
Vajda N, Ghods-Esphahani A, Cooper E, Danesi PR (1992) Determination of radiostrontium in soil samples using a crown ether. J Radioanal Nucl Chem 162(2):307–323
Regan JGT, Tyler JFC (1976) The determination of Sr-90 and Sr-89 in water without separation of strontium from calcium. Analyst 101:32–38
Carmon B (1979) The use of Cerenkov radiation for the assay of radiostrontium in aqueous solutions. Appl Radiat Isot 30:97–100
Chu TC, Wang JJ, Lin YM (1998) Radiostrontium analytical method using crown-ether compound and Cerenkov counting and its applications in environmental monitoring. Appl Radiat Isot 49(12):1671–1675
Grahek Ž, Zečević N, Lulić S (1999) Possibility of rapid determination of low-level 90Sr activity by combination of extraction chromatography separation and Cherenkov counting. Anal Chim Acta 399:237–247
Günther K, Lange S, Veit M (2009) A rapid method for determining 89Sr and 90Sr by Cerenkov counting. Appl Radiat Isot 67:781–785
St-Amant N, Whyte JC, Rousseau ME, Lariviere D, Kurt Ungar R, Johnson S (2011) Radiostrontium and radium analysis in low-level environmental samples following a multi-stage semi-automated chromatographic sequential separation. Appl Radiat Isot 69:8–17
Kim CK, Al-Hamwi A, Törvényi A, Kis-Benedek G, Sansone U (2009) Validation of rapid methods for the determination of radiostrontium in milk. Appl Radiat Isot 67:786–793
Eikenberg J, Beer H, Rüthi M, Zumsteg I, Vetter A (2006) Precise determination of 89Sr and 90Sr/90Y in various matrices: The LSC 3-window approach. In: Chalupnik S, Schönhofer F, Noakes J (eds) Advances in liquid scintillation spectrometry, 2005. Radiocarbon Publishers, University of Arizona, Tucson, pp 237–249
Melin J, Suomela J (1995) Rapid determination of 89Sr and 90Sr in food and environmental samples by Cerenkov counting. Rapid instrumental and separation methods for monitoring radionuclides in food and environmental samples. International Atomic Energy Agency, Vienna
Kim CK, Tarján S, Bokori E, Molnár Z (2011) Improved rapid determination of 89Sr and 90Sr in milk using Cerenkov and scintillation counting. In: Cassette P (ed) Advances in liquid scintillation spectrometry, 2010. Radiocarbon Publishers, University of Arizona, Tucson, pp 125–138
Chobola R, Mell P, Daroczi L, Vincze A (2006) Rapid determination of radiostrontium isotopes in sample of NNP origin. J Radioanal Nucl Chem 267(2):297–304
Hong KH, Cho YH, Lee MH, Choi GS, Lee CW (2001) Simultaneous measurement of 89Sr and 90Sr in aqueous samples by liquid scintillation counting using the spectrum unfolding method. Appl Radiat Isot 54:299–305
Heilgeist M (2000) Use of extraction chromatography, ion chromatography and liquid scintillation spectrometry for rapid determination of strontium-89 and strontium-90 in food in cases of increased release of radionuclides. J Radioanal Nucl Chem 245(2):249–254
Heckel A, Vogl K (2009) Rapid method for determination of the activity concentration of 89Sr and 90Sr. Appl Radiat Isot 67:794–796
Groska J, Molnár Z, Bokori E, Vajda N (2012) Simultaneous determination of 89Sr and 90Sr: comparison of methods and calculation techniques. J Radioanal Nucl Chem 291(3):707–715
NuDat (2014) National Nuclear Data Center, Brookhaven National Laboratory, USA. http://www.nndc.bnl.gov/ensdf/
L’Annunziata MF (2012) Handbook of radioactivity analysis, 3rd edn. Elsevier, Oxford
Lehto J, Hou X (2011) Chemistry and analysis of radionuclides. Wiley-VCH, Weinheim
UKAS (2007) The expression of uncertainty and confidence in measurement, 2nd edn. UKAS, Middlesex
Currie LA (1968) Limits for qualitative detection and quantitative determination -application to radiochemistry. Anal Chem 40(3):586–593
Acknowledgements
The authors would like to thank the US Department of Energy’s Pacific Northwest National Laboratory for providing their results to allow comparison.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, J., Davies, A., Thorne, K. et al. Rapid analysis of 89Sr and 90Sr in nuclear forensics samples. J Radioanal Nucl Chem 311, 1417–1425 (2017). https://doi.org/10.1007/s10967-016-5095-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5095-8