Abstract
The extraction efficiency for thorium followed the trend: Cyanex-923 > Cyanex-272 > DHOA > TBP. In case of TBP and DHOA the extraction proceeded via ‘solvation mechanism’ through Th(NO3)4·2L, while for Cyanex-923 and Cyanex-272 it proceeded via ‘ion exchange’ mechanism through (Th(NO3)2·2L)2+. The extraction process followed slower kinetics while change in Gibb’s energy revealed the spontaneity of the process. These ionic liquid based systems were found to be radiolytically stable, highly efficient and selective for Th. Oxalic acid was found to be suitable for almost quantitative stripping of Th from extracted ionic liquid phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Room temperature ionic liquid has gained world-wide acceptance as the ‘green’ alternative to the volatile organic compounds and finds application in the field of chemical synthesis, catalysis, electro chemistry and drug delivery [1–8]. In nuclear industry, ionic liquid based research is aiming at the processing of radioactive waste [9–12]. Apart from the ‘green’ aspect, the overall extraction/complexation chemistry of lanthanides and actinides differs in ionic liquids compared to that in molecular diluents [13, 14]. It is very interesting to note that ionic liquid based systems not only improve the extraction efficiency or selectivity but also the extraction mechanism, kinetics, thermodynamics and species involved in extraction show interesting features which are unconventional and definitely not the same as observed in case of molecular diluents based systems. The ionic liquid based solvent systems were found to be radiolytically more stable than the conventional solvent due to the formation of resonance stabilized (mainly imidazolium based) organic radical [15–18]. A small structural modification in either cationic or anionic part of ionic liquid led to drastic change in their physico-chemical properties and as a consequence the extraction kinetics and mechanism can be modified [19, 20]. In view of these advantages ionic liquid was chosen as diluent for the extraction of thorium in the present investigation.
To fulfil the world-wide ever increasing energy demand, nuclear energy is one of the prime sources of energy. In a country like India, the nuclear energy programme largely depends on the utilization of the vast thorium resources due to the unavailability of large amount of high quality uranium [21, 22]. Significant efforts were found in literature on the extraction and complexation of thorium using varieties of ligands in molecular diluents like dodecane, xylene etc. [23–27]. Though several literatures are available on Am3+, Pu4+, UO2 2+, Sr2+, Cs+ in ionic liquid [28–30], to the best of our knowledge the extraction, complexation properties of Th have not yet been explored widely [31, 32]. So there is a need to put efforts on development of highly efficient, selective and ‘green’ method for the extraction of thorium.
In view of these, phosphorous based ligands like TBP (tri-n-butyl phosphate), Cyanex-923 (tri-n-alkyl phosphine oxide) and Cyanex-272 (Bis(2,4,4-trimethyl) pentyl phosphinic acid); and non-phosphorous based ligand DHOA (di-n-hexyl octanamide) in C8mimNTf2 (1-octyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide) were investigated for the extraction/complexation behaviour of thorium [33–35].
TBP, Cyanex-923 and Cyanex-272, having phosphorous atom in their structure, are routinely employed in nuclear industries for the extraction of uranium, plutonium and thorium. It is proposed that any ligand constituting C, H, N, O is only completely incinerable and therefore environmentally benign. The ligands containing atoms other than C, H, O, N does not meet the above criteria of being environment friendly. Since our aim was to find out an environmentally benign method for efficient and selective extraction of thorium; we were looking for both green diluent and green ligand. In view of these ionic liquid was chosen to serve the purpose of green solvent while the established ligands for actinide separation namely TBP, Cyanex-272, Cyanex-923 are not so. Therefore, a non phosphorous based ligand is only constituted of C, H, O, N atoms; Di-n-hexyl octanamide (DHOA) which showed potential in Lab scale experiment, was employed along with TBP, Cyanex-923 and Cyanex-272 for the present investigation. The study includes the identification of the species involved in the extraction, kinetics, radiolytic stability and stripping behaviour. Finally, these solvent systems were applied for processing of simulated high level waste solutions (SHLW) obtained from the fast breeder reactor (FBR) and research reactor (RR) [36].
Experimental
Instrument and operating conditions
The analysis was carried out using atomic emission spectroscopy (AES) with inductively colupled argon plasma as an excitation source and capacitatively coupled device (CCD) as detector system. Operating conditions and instrumental specifications are listed in Supplementary Table 1.
Reagents and standard solutions
Standard solutions for all the elements were prepared from CertiPUR® ICP solutions of individual elements (E-Merck, Darmstadt, Germany) by proper dilution. Supra- pure HNO3 (E-Merck, Darmstadt, Germany) and quartz double distilled water were used throughout the study. Multi-point standardization was carried out using standard in the range of 0.05–500 mg/L for each analytical line after proper peak search, auto attenuation etc. For the analyses of each sample 5 replicated measurements were carried.
Th(IV) stock solution was prepared by dissolving spectra pure ThO2. For dissolution HF–HNO3 was used primarily. To avoid the interference from fluoride ion, it was removed by repeated evaporation to dryness and finally, the feed was adjusted to the required acidity. Xylene was procured from Prabhat Chemicals, Gujrat Mumbai, whereas oxalic acid and Na2CO3 were produced from Thomas Baker Chemical limited and Qualigens fine Chemicals, Mumbai, India, respectively. TBP was procured from Koch-Light Laboratories, USA. DHOA was synthesized by previously reported method and its purity was determined by 1H NMR, elemental analysis and distribution coefficient measurement for UO2 2+ [37, 38]. C8mimNTf2 has been procured from Global Nanotech, India with purity more than 99 % and was used for extraction process without further purification. Bis(trifluoromethane)sulfanilamide lithium salt (LiNTf2) has been procured from Aldrich Chemistry, USA. The structures of TBP, DHOA, Cyanex-923 Cyanex-272 and C8mimNTf2 have been shown in Fig. 1.
Method
For all the experiments the 10 mg L−1 thorium solution was taken in the required feed acidity. Then it was allowed to be contacted with same volume (5 mL) of organic phase containing ligands of suitable concentration in ionic liquid. Then both the phase were equilibrated for 2 h in a thermostated water bath at 300 K. Then this reaction mixture was allowed for centrifugation for 2 min for complete phase separation. Finally, suitable aliquot of the aqueous phase was fed into the plasma directly for the analysis. The distribution ratio for any metal ion can be expressed as
The subscripts org and aq refer to the organic and aqueous phases respectively. Since the initial concentration of thorium was known (10 mg L−1), and the final concentration after solvent extraction was evaluated, the concentration of the thorium in the organic phase was calculated. All the experiments were carried out in triplicate to evaluate the reproducibility of the data.
For the stripping purpose, these thorium loaded organic phase was allowed to equilibrate for 2 h with equal volume of aqueous phase containing stripping solution at 300 K temperature. The again after complete phase separation, the aqueous phase was analyzed for thorium concentration and thus the stripping percentages were evaluated.
Spectroscopic studies
UV–Visible spectroscopic studies were carried out using a JASCO V 530 double beam spectrophotometer using quartz cells and suitable reference solutions. The aqueous phase containing different concentration of thorium was allowed to equilibrate with 0.35 M ligand in ionic liquid for 2 h at 300 K with a phase ratio of 1. After complete phase separation, 2 mL of such aqueous phase was collected and the UV–Vis spectra were recorded for the absorbance. The blank solution showed no absorbance in the range 200–350 nm.
Results and discussion
Extraction profile of thorium at various feed acidities
The DTh value was found to decrease gradually with increase in feed acidity up to 3 M HNO3 followed by a sharp decrease both in case of Cyanex-923 and Cyanex-272, whereas for TBP and DHOA, the DTh values decrease marginally up to 0.1 M HNO3 followed by increase in DTh values (Fig. 2). The decrease in DTh values can be attributed to the strong competition of H+ ion with Th4+ and suggesting the predominance of ‘ion-exchange mechanism’ while for TBP and DHOA systems beyond 0.1 M HNO3 solvation mechanism seems to be predominating. Extraction of UO2 2+ using TBP in C8mimNTf2 also showed similar trend as seen in case of Th extraction by TBP or DHOA [39]. It was also reported that for most of the diglycolamide based ligands in C8mim NTf2 followed ion exchange mechanism for extraction of actinides throughout the feed acidity range as seen in case of Cyanex-923 and Cyanex-272 for the extraction of thorium [40–42]. It was revealed from the literature that the extraction mechanism changed from ion exchange to solvation on increasing the length of alkyl substituent in the alkyl methyl imidazolium ring of the ionic liquid for the extraction of Sr2+ using crown ether in ionic liquids [43–45]. Therefore, the nature of extraction mechanism in ionic liquid based systems depends on the nature of the ligands and ionic liquids. In case of solvation mechanism the Th complex needs to be neutral in nature as suggested by Eq. (1). In case of cation exchange mechanism the overall Th complex is to be cationic in nature and will be transferred to the organic phase while to make overall charge balance equivalent amount of cations from ionic liquid phase i.e. C8mim+ ion will come to the aqueous phase Eq. (2). Similarly, if it proceeds through anion exchange mechanism the overall charge of the complex will be anionic and it will be transferred from aqueous phase to organic phase while equivalent amount of anion from ionic liquid medium i.e. \( {\text{NO}}_{3}{^{ - }} \) will come to the aqueous phase Eq. (3). Therefore, to ascertain the nature of species which is extracted into the ionic liquid phase and the mechanism of extraction, it needs to investigate the effect of different species like \( {\text{NO}}_{3}{^{ - }} \), C8mim+, \( {\text{NTF}}_{2}{^{ - }} \) and ligands on the extraction properties of thorium. The variation in feed acidity only gives an indication that the overall extraction mechanism of thorium is ion exchange for Cyanex-923 and Cyanex-272 while for TBP and DHOA it is solvation beyond 0.1 M HNO3.
where, ‘aq’ and ‘IL’ referred to the aqueous and ionic liquid phase, ‘m’ and ‘n’ referred to the number of nitrate ion and ligand molecules attached to the thorium atom.
The DTh values obtained in the present investigation were compared with the ionic liquid based systems reported in the literature [46–49] and presented in Table 1. 0.04 M TODGA in CnmimPF6 (where n = 4, 8) were employed for the extraction of Th [46]. The ionic liquids with \( {\text{PF}}_{6}{^{ - }} \) anion were reported to be unstable in presence of even small amount of acid. There is liberation of fluoride ion in the systems leads to the interference during separation and problem of corrosion and hence not advisable [50]. Moreover, TODGA was reported to have very high D values for specially trivalent lanthanides and actinides and hence used for actinide partitioning [51, 52]. Application of N1923 ligand in C8mimPF6 showed appreciable extraction of Th only in lower feed acidity. In case of a series of amides and beta di-ketone, higher DTh values were reported only from less than 1 M feed acidity. The acidity of high level waste solution encountered is mostly ~3 M HNO3 [36, 51, 52]. Therefore it is desirable to develop extraction method from the feed of similar acidity.
Determination of metal–ligand stoichiometry
In order to examine the metal–ligand stoichiometry, the DTh value was varied as a function of free ligand concentration (Fig. 3). The increase DTh value on increasing the free ligand concentration primarily revealed the participation of ligand molecule into the extracted complex. The equilibrium constant for the extraction process can be expressed asFor solvation mechanism
For cation exchange mechanism
For anion exchange mechanism
If the free ligand concentration is varied at a particular feed acidity and at particular temperature then Eqs. (4), (5) and (6) can be simplified as Eq. (7), where \( k^{\prime}_{\text{ex}} \) is conditional extraction constant. It is assumed that at a particular temperature the partition coefficients for C8mim+ and \( {\text{NTf}}_{2}{^{ - }} \) were constants.
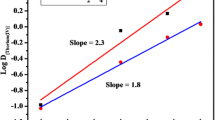
In accordance with Eq. (8), a plot of log DTh versus log [L] gave straight line with a slope of ‘n’ which is nothing but the number of ligand molecule associated with a Th atom. For all the solvent systems, one Th molecule is associated with two ligand molecules which is found to be same as that observed in case of molecular diluent based systems [55]. Similar metal–ligand stoichiometry but different extraction mechanism was also reported in case of uranyl-TBP complex in molecular diluent and ionic liquid [56]. The uranyl complex of CMPO in dodecane and in ionic liquid also showed same metal–ligand stoichiometry [20].
If we consider the complexation of Th4+ with two ligands then the conditional formation constant (k f) can be expressed as follows [57].
where, PTh is the partition coefficient of thorium between 3 M HNO3 and C8mimNTf2 (This is the distribution ratio of thorium between 3 M HNO3 and C8mimNTf2 without ligands. The aqueous phase containg 10 mg L−1 of Th in 3 M HNO3 was allowed to equilibrate with C8mimNTf2 for 2 h. After complete phase separation by centrifugation, the aqueous phase was analyzed by ICP-AES for thorium and D value for thorium was calculated using the equation shown in experimental section and this is denoted as \( {\text{P}}_{\text{Th}} \)). Table 2 summarized the conditional extraction constants, formation constants of thorium by TBP, DHOA, Cyanex-923, Cyanex-272 in ionic liquid. The overall ‘Gibb’s free energy change during the extraction can also be evaluated by the following equation
The \( k^{\prime}_{\text{ex}} \) and k f values were found to follow the trend Cyanex-923 > Cyanex-272 > DHOA > TBP. The extraction processes were found to be energetically favorable with the similar trend as above.
Participation of \( {\text{NO}}_{3}{^{ - }} \) anion in the extraction
To ascertain the nature of extraction mechanism, it is essential to understand the participation of \( {\text{NO}}_{3}{^{ - }} \) anion in the extracted species. For the extraction of Th using constant concentration of ligands and at constant temperature, Eqs. (4), (5) and (6) can be simplified as
Figure 4 depicted the plots of log DTh versus log [\( {\text{NO}}_{3}{^{ - }} \)] for different systems. The increase in DTh values with increase in \( {\text{NO}}_{3}{^{ - }} \) concentration in the aqueous phase primarily confirmed the participation of \( {\text{NO}}_{3}{^{ - }} \) in the extracted Th complex. Slope values revealed the participation of four \( {\text{NO}}_{3}{^{ - }} \) ion in the extracted species of TBP and DHOA, revealing the possibility of neutral extracted complex (Th(NO3)4·2L) via solvation mechanism (provided there is no participation of \( {\text{NO}}_{ 3}{^{ - }} \) anion). In case of Cyanex-923 and Cyanex-272, only two \( {\text{NO}}_{3}{^{ - }} \) were found to participate in the extracted complex making the overall charge of the extracted species 2+ ((Th(NO3)2·2L)2+). The slope and intercept values alongwith the errors and regression coefficients were summarized in Supplementary Table 2.
Participation of \( {\text{NTF}}_{2}{^{ - }} \) anion in the extracted complex
It is clear from the above studies that it is essential to understand the participation of \( {\text{NTF}}_{2}{^{ - }} \) into the extracted species to ascertain the extraction mechanism. The concentration of \( {\text{NTF}}_{2}{^{ - }} \) in aqueous phase was varied in the form of water soluble LiNTf2 salt. If \( {\text{NTF}}_{2}{^{ - }} \) is participating in the extracted species, then with increase in \( {\text{NTF}}_{2}{^{ - }} \)concentration in the aqueous phase should favour the extraction of Th and hence increase in DTh values. If the overall extraction mechanism is anion exchange, then with increase in \( {\text{NTF}}_{2}{^{ - }} \) ion concentration in aqueous, DTh values are expected to decrease. But no changes in DTh values were observed during a vast change of \( {\text{NTF}}_{2}{^{ - }} \) ion concentration in the aqueous phase in the range of 0.1–10 mM (Fig. 5). The above fact revealed that there was no participation of \( {\text{NTF}}_{2}{^{ - }} \) ion in the extracted species which confirmed the extracted species of Th(NO3)4·2L for TBP and DHOA via salvation mechanism and (Th(NO3)2·2L)2+ for Cyanex-923 and Cyanex-272 via ‘cation exchange’ mechanism.
Effect of C8mim+ on extraction of thorium
As the above study revealed that the extraction of Th by TBP and DHOA was proposed to follow through ‘solvation mechanism’ while that for Cyanex-923 and Cyanex-272 followed through ‘cation exchange mechanism’. To ascertain the facts a variation of DTh as a function of C8mim+ concentration in aqueous phase was investigated (Fig. 6). In case of TBP and DHOA systems the DTh values were found to remain unchanged with C8mim+ concentration in the aqueous phase, suggesting the non participation of C8mim+ in the extraction process which was expected from the solvation mechanism. In case of Cyanex-923 and Cyanex-272, on increasing C8mim+ concentration in the aqueous phase the DTh values were found to decrease. Since in cation exchange process, Th-ligand complex in overall cationic form was exchanged from aqueous phase to organic phase by C8mim+ ion. This exchange process would likely to be hampered with increase in C8mim+ cation in aqueous phase. Hence the reverse reaction was favoured. Hence DTh values were found to decrease on increasing C8mim+ ion concentration in aqueous phase.
To confirm the extraction mechanism another approach has also been adopted. The aqueous phase was loaded with various concentrations of Th and after extraction, the raffinates were analyzed for UV–Vis spectroscopy to monitor the absorption due to C8mim+ ion. If the extraction proceeds via ‘cation exchange’ mechanism involving the exchange of C8mim+ in place of Th-extracted cationic species, then more loading of Th leads to more concentration of C8mim+ in the raffinate and the absorption in UV–Vis spectra should increase without changing the absorption maxima [1]. In the present investigation, it was observed that on increasing Th concentration the absorption increased for Cyanex-923 and Cyanex-272 confirming the ‘cation exchange’ nature of the extraction through C8mim+ ion (Supplementary Fig. 1). On the other hand no such observation was noticed confirming the predominance of ‘solvation mechanism’ for them.
Ionic liquid based all these solvent systems were found to be much more efficient than the common molecular diluents like xylene (Supplementary Table 3). At such lower ligand concentration (0.35 M), TBP and DHOA were not able to extract Th practically into xylene phase while in C8mimNTf2 more than 50 % Th can be extracted. This fact revealed that though in both the cases the extraction proceeds through ‘solvation mechanism’ some additional driving force was responsible for higher extraction of Th into ionic liquid phase. For Cyanex-923 and 272, from molecular diluents to ionic liquid, the extraction mechanism changed from salvation to ion exchange which might be responsible for higher extraction efficiency of Th in ionic liquid.
Scheme 1 revealed the ‘cation exchange’ mechanism predominated for Cyanex-272 and Cyanex-923. In this mechanism, Th4+ ion from aqueous phase came to the ionic liquid phase by formation of species [Th(NO3)2·2L]2+. To maintain the overall charge balance, two C8mim+ ion from the ionic liquid phase came to the aqueous phase. In the above species two nitrate ion coordinated in a bidentate fashion, while Cyanex-923 coordinated through the phosphoryl oxygen in a monodentate fashion. Most probably two water molecules from the medium also coordinated to the thorium ion to fulfill the coordination saturation of 8 for tetravalent actinides. In case of Cyanex-272, there is a chance that two oxygen atoms from the ligand would coordinate to the thorium ion which removes the possibility of coordination of two water due to coordination un-saturation.
Scheme 2 represented the ‘solvation mechanism’ predominately operating for the TBP and DHOA. For these ligands thorium form neutral complex with two ligand molecules associated by four nitrate ions. In these case, two ligand molecules coordinated in a monodentate fashion. Out of four nitrate ions, two coordinated in bidentate and one coordinated in monodentate fashion to fulfill the 8 coordination numbers for the tetravalent actinides.
Extraction kinetics
The DTh values were investigated as a function of equilibration time for all the solvent systems. For TBP the DTh values were found to increase up to 60 mintutes followed by a plateau with DTh ~1.2. The similar trend was also seen for Cyanex-923, Cyanex-272 and DHOA with DTh values of ~150, 100 and 1.9 at the plateau. The investigation revealed that 60 min was required for achieving maximum D values for all the ligands in ionic liquid (while 15 min. was found to be sufficient for the extraction using molecular diluent, doecane (Supplementary Fig. 2). Since for all the solvent systems same time was required, it indicated that probably the diluent, C8mimNTf2 was playing the role for such slower kinetics of extraction. Earlier reports revealed that such slower kinetics were also observed in ionic liquid based systems due to the higher viscosity coefficient of the ionic liquid during the extraction of Am3+ [30]. In our previous investigation a correlation was made for methylimidazolium based ionic liquids, how the structural modification in ionic liquid led to increase in viscosity coefficient which subsequently made the extraction kinetics of Sr2+ even slower [13]. Figure 7 showing the extraction kinetic behavior of Th by these ionic liquid based systems attributed to the viscous nature of ionic liquid.
Stripping of Th(IV) from organic phase
With room temperature ionic liquids, the stripping of the metal ions from the ionic liquid phase is one of the major challenges. Usually, the extraction is carried out at higher acidity (3–6 M HNO3), while the stripping is done at lower acidity (pH −2.0) for molecular diluents based systems. However, with ionic liquids as the diluent the D values at lower feed acidity are too high to back extract. Therefore, the problem of stripping of Th from ionic liquid phase was overcome by using aqueous phase complexing agents like 0.5 M Na2CO3 and 0.5 M oxalic acid.15 These solutions were employed in the present study for the stripping of Th(IV) from TBP, DHOA, Cyanex-923 and Cyanex-272. The results are shown in Fig. 8, which suggest that 0.5 M oxalic acid solution are effective as strippant where close to 95 % stripping of Th was observed in a single stage in case of TBP, Cyanex-272, DHOA while in case of Cyanex-923 oxalic acid could extract nearly 75 %. On the other hand, Na2CO3 was not very effective as a strippant as only 80 % stripping was obtained in a single stripping case of TBP while dealing with DHOA, Cyanex-272 and Cyanex-923 stripping was nearly 60, 38 and 30 %.The results are encouraging by using oxalic acid for stripping. It was also observed that 2 contacts of oxalic acid with phase ratio were required for the quantitative back extraction of Th from ionic liquid phase containing TBP, Cyanex-272 and DHOA while for Cyanex-923, which is a stronger extractant needs 6 such contacts. Using Na2CO3 as strippant, 6 contacts can led to quantitative stripping of Th from TBP ionic liquid phase while even after 6 contacts, 88, 94 and 99.5 % Th were found to be back extracted into the aqueous phase from complexes of Cyanex-923, Cyanex-272 and DHOA, respectively (Supplementary Fig. 3).
Effect of Gama Irradiation on extraction of Th(IV)
Though the TBP, DHOA, Cyanex-923 and Cyanex-272 ligands are highly promising for actinide ion extraction, their actual use for actinide ion separation requires their prolonged reusability, which means their good radiolytic stability. This is because all actinide ions emit high LET (linear energy transfer) alpha particles, β particles and γ radiation. Therefore, a systematic study was carried out to investigate the radiolytic stability of the TBP, DHOA, Cyanex-923 and Cyanex-272 in C8mimNTf2. It was revealed that after exposing the organic phase to 500 kGy of the absorbed gamma dose, the extraction efficiency becomes 88, 85, 73, and 78 % of the original DTh values (with the unirradiated ligand solution) for TBP, DHOA, Cyanex-923 and Cyanex-272, respectively. On exposure to 1000 kGy, DTh values decreased to 70, 60, 60, and 67 %, respectively of the original values (Fig. 9). The results suggested that after 1000 kGy gamma exposure, TBP-ionic liquid system was the most radio resistant (DTh becomes ~70 % of its original value) and the radiolytic sability of all the solvent systems were good up to 500 kGy of gamma exposure.
Application of ionic liquid based solvent systems for processing simulated high level waste solutions (SHLW) from fast breeder reactor (FBR) and research reactor (RR) origin
The main aim of developing these ionic liquid based solvent systems was to process the radioactive waste generated from different streams. The selectivity of these solvent based systems also needs to be investigated. In the present case, SHLW solutions of FBR and RR origin were contacted with these ionic liquid based systems for 2 h and the raffinate was directly fed into the plasma. All these ionic liquid based solvent systems were found to be highly selective for thorium. Only U and very small amount of Zr were found to co-extracted into the ionic liquid phase with the trend in extraction efficiency Cyanex-923 > Cyanex-272 > TBP > DHOA. Tables 3 and 4 summarized the analytical results obtained after processing the SHLW of FBR and RR origins.
Conclusions
In the present investigation TBP, DHOA, Cyanex-923, Cyanex-272 in ionic liquid have been explored for the selective separation of thorium from aqueous acidic waste solution and even simulated high level waste solutions of Fast Breeder and Research reactor. The extraction process was found to be kinetically slower but energetically favourable. For all the cases the metal–ligand stoichiometry was found to 1:2 with four \( {\text{NO}}_{3}{^{ - }} \) coordinated to Th for TBP and DHOA and 2 for Cyanex-923 and Cyanex-272. In case of TBP and DHOA, the extraction was found to proceed via ‘solvation mechanism’ while ‘cation exchange’ mechanism was found to be predominating for Cyanex-923 and 272. The extraction efficiency was found to follow the trend Cyanex-923 > Cyanex-272 > DHOA > TBP while stripping of thorium followed the reverse trend. Oxalic acid was found to be suitable for the quantitative back extraction of Th from ionic liquid phase. Up to 500 kGy, all the solvent systems were found to be radiolytically stable with the trend TBP > DHOA > Cyanex-272 > Cyanex-923.
References
Pei Y, Wang J, Wu K, Xuan X, Lu X (2009) Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep Purif Technol 64(3):288–295
Soto A, Arce A, Khoshkbarchi MK (2005) Partitioning of antibiotics in a two-liquid phase system formed by water and a room temperature ionic liquid. Sep Purif Technol 44(3):242–246
Park S, Kazlauskas RJ (2003) Biocatalysis in ionic liquids—advantages beyond green technology. Curr Opin Biotechnol 14(4):432–437
Sengupta A, Murali MS, Mohapatra PK, Iqbal M, Huskens J, Verboom W (2015) An insight into the complexation of UO2 2+ with diglycolamide-functionalized task specific ionic liquid: kinetic, cyclic voltammetric, extraction and spectroscopic investigations. Polyhedron 102:549–555
Sengupta A, Murali MS, Mohapatra PK (2013) Role of alkyl substituent in room temperature ionic liquid on the electrochemical behavior of uranium ion and its local environment. J Radioanal Nucl Chem 298:209–217
Sengupta A, Murali MS, Mohapatra PK, Iqbal M, Huskens J, Verboom W (2015) Extracted species of Np(IV) complex with diglycolamide functionalized task specific ionic liquid: diffusion, kinetics and thermodynamics by cyclic voltammetry. J Radioanal Nucl Chem 304:563–570
Shamshin JL, Barber PS, Rogers RD (2013) Ionic liquids in drug delivery. Expert Opin Drug Deliv 10(10):1367–1381
Ziyauddin S, Qureshi KM, Deshmukh BM (2014) Applications of ionic liquids in organic synthesis and catalysis. Clean Technol Environ Policy 16(8):1487–1513
Mudring AV, Tang S (2010) Ionic liquids for lanthanide and actinide chemistry. Eur J Inorg Chem 18:2569–2581
Sun X, Luo H, Dai S (2012) Ionic liquids-based extraction: a promising strategy for the advanced nuclear fuel cycle. Chem Rev 112:2100–2128
Rao PR, Venkatesan KA, Rout A, Srinivasan TG, Nagarajan K (2012) Potential applications of room temperature ionic liquids for fission products and actinide. Sep Sci Technol 47:204–222
Dietz ML (2006) Ionic liquids as extraction solvents: where do we stand? Sep Sci Technol 41:2047–2063
Singh M, Sengupta A, Murali MS, Kadam RM (2016) Selective separation of uranium from nuclear waste solution by Bis(2,4,4-trimethyl) pentyl phosphinic acid in ionic liquid and molecular diluents: a comparative study. J Radioanal Nucl Chem. doi:10.1007/s10967-016-4691-y
Singh M, Sengupta A, Murali MS, Kadam RM (2015) Comparative Study on the Radiolytic stability of TBP, DHOA, Cyanex-923 and Cyanex-272 in ionic liquid and molecular diluent for the extraction of thorium. J Radioanal Nucl Chem. doi:10.1007/s10967-015-4624-1
Shkrob IL, Sergey D (2007) Chemerisov, the initial stages of radiation damage in ionic liquids and ionic liquid-based extraction systems. J Phys Chem B 111:11786–11793
Shkrob IL, Marin TW, Bell JR, Luo H, Dai S, Hatcher JL, Rimmer RD, Wishart JF (2012) Radiation-induced fragmentation of diamide extraction agents in ionic liquid diluents. J Phys Chem B 116(7):2234–2243
Sengupta A, Mohapatra PK, Patil AB, Kadam RM, Verboom W (2016) Radiation stability of diglycolamide functionalized calix[4]arenes in ionic liquid: solvent extraction, EPR and GC–MS studies. Sep Purif Technol 162:77–83
Yuan L, Peng J, Xu L, Zhai M, Li J, Wei G (2009) Radiation effects on hydrophobic ionic liquid [C4mim][NTf2] during extraction of strontium ions. J Phys Chem B 113(26):8948–8952. doi:10.1021/jp9016079
Sengupta A, Mohapatra PK (2012) Extraction of radiostrontium from nuclear waste solution using crown ethers in room temperature ionic liquids. Supramol Chem 24(11):771–778
Visser AE, Rogers RD (2003) Room-temperature ionic liquids: new solvents for f-element separations and associated solution chemistry. J Solid State Chem 171(1–2):109–113
Singha RK, Kakodkar A (2006) Design and development of the AHWR—the Indian thorium fuelled innovative nuclear reactor. Nucl Eng and Des 236:683–700
Lung M, Gremm O (1998) Perspectives of thorium fuel cycle. Nucl Eng and Des 180:133–146
Peppard DF, Mason GW, McCarty S (1960) Extraction of thorium(IV) by di esters of orthophosphoric acid, (GO)2PO(OH). J Inorg Nucl Chem 13(1–2):138–150
Preston JS, du Pree AC (1995) Solvent extraction of uranium(VI) and thorium(IV) from nitrate media by carboxylic acid amides. Solv Extr Ion Exch 13(3):391–413
Yaftian MR, Taheri R, Zamani AA, Matt D (2004) Thermodynamics of the solvent extraction of thorium and europium nitrates by neutral phosphorylated ligands. J Radioanal Nucl Chem 262:455–459
Sahu SK, Chakravortty V, Reddy MLP, Ramamohan TR (2000) The synergistic extraction of thorium(IV) and uranium(VI) with mixtures of 3-phenyl-4-benzoyl-5-isoxazolone and crown ethers. Talanta 51:523–530
Amaral JCBS, Morais CA (2010) Thorium and uranium extraction from rare earth elements in monazite sulfuric acid liquor through solvent extraction. Miner Eng 23:498–503
Giridhar P, Venkatesan KA, Subramaniam S, Srinivasan TG, Rao PV (2008) Extraction of uranium (VI) by 1.1 M tri-n-butylphosphate/ionic liquid and the feasibility of recovery by direct electrodeposition from organic phase. J Alloys Comp 448(1–2):104–108
Dietz ML, Stepinski DC (2008) Anion concentration-dependent partitioning mechanism in the extraction of uranium into room-temperature ionic liquids. Talanta 75(2):598–603
Rout A, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2012) Liquid–liquid extraction of Pu(IV), U(VI) and Am(III) using malonamide in room temperature ionic liquid as diluent. J Hazard Mater 221–222:62–67
Zuo Y, Chen J, Li D (2008) Reversed micellar extraction and separation of thorium (IV) from rare earth (III) by primary amine N1923 in ionic liquid. Sep Purif Technol 63:684–690
Zuo Y, Liu Y, Chen J, De Li Q (2008) The separation of cerium(iv) from nitric acid solutions containing thorium(iv) and lanthanides(iii) using pure [C8mim]PF6 as extracting phase. Ind Eng Chem Res 47(7):2349–2355
Billard I, Ouadi A, Jobin E, Champion J, Gaillard C, Georg S (2011) Understanding the extraction mechanism in ionic liquids: UO2 2+/HNO3/TBP/C4mimTf2N as a case study. Solv Extr Ion Exch 29(4):577–601
Kumari N, Prabhu DR, Pathak PN, Kanekar AS, Manchanda VK (2011) Extraction studies of uranium into a third-phase of thorium nitrate employing tributyl phosphate and N,N-dihexyl octanamide as extractants in different diluents. J Radioanal Nucl Chem 289:835–843
Cocalia VA, Jensen MP, Holbrey JD, Spear SK, Stepinski DC, Rogers RD (2005) Identical extraction behavior and coordination of trivalent or hexavalent f-element cations using ionic liquid and molecular solvents. Dalton Trans 11:1966–1971
Sengupta A, Murali MS, Thulasidas SK, Mohapatra PK (2014) Solvent system containing CMPO as the extractant in a diluent mixture containing n-dodecane and isodecanol for actinide partitioning runs. Hydrometallurgy 147–148:228–233
Kumari N, Prabhu DR, Pathak PN (2013) Uranium extraction studies employing tributyl phosphate and N,N-Dihexyl octanamide as extractants: counter-current centrifugal contactors Runs. Sep Sci Technol 48(16):2479–2485
Sengupta A, Kulkarni MJ, Godbole SV (2011) Analytical application of DHOA for the determination of trace metallic constituents in U based fuel materials by ICP-AES. J Radioanal Nucl Chem 289(3):961–965
Giridhar P, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2005) Extraction of uranium(VI) from nitric acid medium by 1.1 M tri-n-butylphosphate in ionic liquid diluent. J Radioanal Nucl Chem 265:31–38
Sengupta A, Mohapatra PK, Iqbal M, Huskens J, Verboom W (2012) A highly efficient solvent system containing functionalized diglycolamides and an ionic liquid for americium recovery from radioactive wastes. Dalton Trans. 41(23):6970–6979
Mohapatra PK, Sengupta A, Iqbal M, Huskens J, Godbole SV, Verboom W (2013) Remarkable acidity independent actinide extraction with a both-side diglycolamide-functionalized calix[4]arene. Dalton Trans 42:8558–8562
Sengupta A, Mohapatra PK, Kadam RM, Manna D, Ghanty TK, Iqbal M, Huskens J, Verboom W (2014) Diglycolamide-functionalized task specific ionic liquids for nuclear waste remediation: extraction, luminescence, theoretical and EPR investigations. RSC Adv 4:46613–46623
Dietz ML, Dzielawa JA (2001) Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: implications for the ‘greenness’ of ionic liquids as diluents in liquid–liquid extraction. Chem Commun 20:2124–2125
Dietz ML, Dzielawa JA, Laszak I, Young BA, Jensen MP (2003) Influence of solvent structural variations on the mechanism of facilitated ion transfer into room-temperature ionic liquids. Green Chem 5:682–685
Dietz ML, Stepinski DC (2005) A ternary mechanism for the facilitated transfer of metal ions into room-temperature ionic liquids (RTILs): implications for the “greenness” of RTILs as extraction solvents. Green Chem 7:747–750
Zhang Y, Liu Z, Fan F, Zhu L, Shen Y (2014) Extraction of uranium and thorium from nitric acid solution by todga in ionic liquids. Sep Sci Technol 49:1895–1902
Rao A, Tomar BS (2016) Extraction of thorium employing N,N-dialkyl amide into room temperature ionic liquid followed by supercritical carbon dioxide stripping. Sep Purif Technol 161:159–164
Fu J, Chen Q, Sun T, Shen X (2013) Extraction of Th(IV) from aqueous solution by room-temperature ionic liquids and coupled with supercritical carbon dioxide stripping. Sep Purif Technol 119:66–71
Mohapatra PK, Sengupta A, Iqbal M, Huskens J, Verboom W (2013) Diglycolamide-functionalized calix[4]arenes showing unusual complexation of actinide ions in room temperature ionic liquids: role of ligand structure, radiolytic stability, emission spectroscopy, and thermodynamic studies. Inorg Chem 52(5):2533–2541
Freire MG, Neves CMSS, Marrucho IM, Coutinho JAP, Fernandes AM (2010) Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J Phys Chem A 114(11):3744–3749
Ansari SA, Mohapatra PK, Rout DR, Kumar M, Rajeswari B, Manchanda VK (2009) Performance of some extractants used for ‘actinide partitioning’ in a comparative hollow fibre supported liquid membrane transport study using simulated high level nuclear waste. J Membr Sci 337(1–2):304–309
Gujar RB, Ansari SA, Prabhu DR, Pathak PN, Sengupta A, Thulasidas SK, Mohapatra PK, Manchanda VK (2012) Actinide partitioning with a modified TODGA solvent: counter-current extraction studies with simulated high level waste. Solv Extr Ion Exch 30:156–170
Shen Y, Wang S, Zhu L, Wang J, Wu W (2011) Extraction of Th(IV) from an HNO3 solution by diglycolamide in ionic liquids. Ind Eng Chem Res 50(24):13990–13996
Zuo Y, Liu Y, Chen J, Li D-Q (2009) Extraction and recovery of cerium(IV) along with fluorine(I) from bastnasite leaching liquor by DEHEHP in [C8mim]PF6. J Chem Technol Biotechnol 84:949–956
Sengupta A, Ippili T, Sk Jayabun, Singh M, Thulasidas SK (2016) ICP-AES determination of trace metallic constituents in thorium matrix after preferential extraction of thorium using TBP, TOPO and DHOA: a comparative study. J Radioanal Nucl Chem. doi:10.1007/s10967-016-4790-9
Murali MS, Bonville N, Choppin GR (2010) Uranyl ion extraction into room temperature ionic liquids: species determination by ESI and MALDI-MS. Solv Extr Ion Exch 28(4):495–509
Sengupta A, Mohapatra PK, Iqbal M, Verboom W, Huskens J, Godbole SV (2012) Extraction of Am(III) using novel solvent systems containing a tripodal diglycolamide ligand in room temperature ionic liquids: a ‘green’ approach for radioactive waste processing. RSC Adv 2:7492–7500
Acknowledgments
The author wish to acknowledge Dr. R.M.Kadam, Head, Actinide Spectroscopy Section and Dr. P.K.Pujari, Head, Radiochemistry Division for their constant support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, M., Sengupta, A., Jayabun, S. et al. Understanding the extraction mechanism, radiolytic stability and stripping behavior of thorium by ionic liquid based solvent systems: evidence of ‘ion-exchange’ and ‘solvation’ mechanism. J Radioanal Nucl Chem 311, 195–208 (2017). https://doi.org/10.1007/s10967-016-4994-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4994-z