Abstract
Three different modified SBA-15 have been synthesized by a post-grafting method using 3,4-dihydroxy benzaldehyde (OHBA), 2,4-dihydroxy benzaldehyde (MHBA), 2,4-dihydroxy acetophenone (RATP). These materials were characterized by NMR, TEM, FT-IR and elemental analysis. Batch experiments have been conducted to study the effects of pH, temperature, adsorbent dosage, ionic strength, shaking time, initial concentration of metal ion, and coexisting ions on uranium(VI) sorption behaviors of pure and functional SBA-15. In addition, the adsorption of U(VI) could be well-described by the Langmuir, Freundlich isotherms and pseudo-second kinetic models. The results suggest the sorption capacity and selectivity of SBA-15 were improved after functionalization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, a shift towards renewable energy supplies is urgently needed in order to sustain global economic growth and mitigate the climate change caused by burning fossil fuels [1] so as to decrease the level of the atmospheric carbon dioxide. Nuclear energy is an only mature, clean and large-scale power to satisfy the growing energy demand in the future. With rapid development and large utilization of nuclear power, uranium is one of the main radionuclides in nuclear industry [2] which starts as a source and ends up as a radioactive waste component [3]. Owing to its chemical and radio toxicity, uranium is one of major concern with regard to environmental clean up [4, 5]. Uranium can cause potentially serious health hazards to humans if it was released into soil and water bodies [6, 7].
The efficient methods for removal of uranium from wastes are imperative. Until now, a variety of methods have been developed for uptake of uranium, such as ion exchange, chemical precipitation, co-precipitation, solvent extraction, chromatographic extraction, hyperfiltration and adsorption [8–12]. Among them, sorption is a widely used method as regard to its easy-operation, rapidity, and high-efficiency [13, 14].
Because mesoporous silicas with well-ordered pore channels are currently the focus of a great deal of research interest [15–18]. Ordered mesoporous silicas have high porosities, large surface areas, excellent mechanical property, and adjustable pore size. They are usually used as adsorbents for the separation of organics, heavy metal ions, dyes, radionuclides, light hydrocarbons, and gases [19, 20]. Among them, SBA-15 mesoporous silica material possesses thicker pore walls, larger pore volume, high surface area and better hydrothermal stabilities, so it is usually chosen as a potential sorption material for some heavy metal ion sorption. At the same time, low chemical activity of the SBA-15 should be taken into account, which could decrease the sorption ability of SBA-15. In recent years, in order to improve the sorption ability and selectivity, many people have functionalized SBA-15 with organic groups. Wang YL et al. reported that mesoporous silica SBA-15 functionalized with iminodiacetic acid derivatives have a good sorption ability and selectivity for UO2 2+ from aqueous solution [21]. Mesoporous silica modified with 2-mercaptopyrimidine showed excellent adsorption capability for Cd(II) [22]. Mureseanu et al. reported that Mesoporous silica functionalized with 1-furoyl thiourea urea for Hg(II) adsorption from aqueous media [23]. Phosphonate derivatives functionalized SBA-15 material showed not only a good sorption ability and a desirable selectivity for U(VI) over a range of competing metal ions but also an excellent reusability [24], and 1,2-formylsalicylic acid have a high affinity for lanthanides (La, Ce, Pr, Nd, Eu, Gd and Lu) from aqueous solution [25].
Dihydroxy bezladely derivatives are known to form stable metal complexes with different metal ions in the solid state under proper conditions. Anupama et al. reported that the silica gel functionalized with bezladely derivatives resacetophenone: synthesis of a new chelating matrix and its application as metal ion collector [26]. As reported from previous work [27], silica gel modified with formylsalicylic acid has been used for extraction of iron(III) from aqueous solution. The results showed that high adsorption values are recorded at neutral pH, which may be due to the strong coordination ability of hydroxy benzaldehyde derivatives to metal ions. However, the relevant reports about the sorption of uranium on modified silica by dihydroxy bezladely derivatives were quite scarce.
Driven by the above realization, we modified SBA-15 to remove uranium by using three dihydroxy bezladely derivatives which are 3,4-dihydroxy benzaldehyde, 2,4-dihydroxy benzaldehyde and 2,4-dihydroxyacetophenone as ligands. The products exhibit high adsorption affinity for U(VI) ions, resulting from complexation of the U(VI) ions by surface dihydroxy bezladely derivatives groups. Furthermore, the effects of different operational parameters on U(VI) adsorption, such as solution adsorbent dosage, pH value, shaking time, and the temperature have been studied. In addition, the adsorption kinetics and thermodynamics have also been investigated. We also compared the selective sorption ability of pure and functional SBA-15 on U(VI) sorption behavior. Additionally, the reports focused on developing SBA-15 modified with dihydroxy bezladely derivatives for U(VI) sorption are quite rare to date, so we anticipate that this work could provide a good example for the removal of U(VI).
Experimental
Reagents and materials
All chemicals used in the experiments are purchased as analytical purity or highest purity available. All solutions are prepared with deionized water by means of a Millipore Milli-Q-system. SBA-15 mesoporous molecular sieve were purchased from Unicarbonshanghai Co. Ltd. Organic solvents (toluene, diethyl ether and ethanol) were distilled and dried before use according to conventional literature methods [22]. 3-aminopropyltriethoxysilane (APTES) and arsenazo III were obtained from Kermel and used as received.
Sorbent synthesis
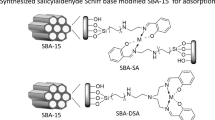
The dihydroxy bezladely derivatives modified SBA-15 were synthesized by post-grafting methods and the procedures are as follows (Fig. 1).
Preparation for SBA-15-NH2
SBA-15-NH2 was synthesized according to the literature methods [28]. 7 g activated SBA-15 which was treated 12 h at 100 °C under vacuum was dispersed in 100 mL anhydrous toluene. After that, 10 mL of APTES was added to the admixture right away at room temperature under N2 atmosphere, and the resulting mixture was brought to a boiling temperature in a reflux system glass for 9 h with magnetic sticking. After cooling down, the mixture was filtered, and the solid product was washed several times with toluene and ethanol. At last, the solid was dried at 80 °C under vacuum overnight. The product was named SBA-15-NH2.
Preparation for SBA-15-RATP
SBA-15-RATP was prepared according to a previously described method [30]: 5 g SBA-15-NH2 was treated with 7.5 g RATP dissolved in 100 mL of dry toluene and the mixture was refluxed with constant stirring for 12 h under nitrogen atomosphere. Rest of the procedure was same as described for SBA-15-NH2. The synthesis of SBA-15-MHBA and SBA-15-OHBA are conducted in the same way as SBA-15-RATP (see Fig. 1).
Characterization
SAB-15 and functional SBA-15 were characterized by X-ray powder diffraction (XRD), Fourier Transform Infrared Spectrometer FT-IR, nitrogen adsorption and desorption experiments, transmission electron microscopy (TEM), and solid-state magic-angle spinning (MAS) nuclear magnetic resonance (NMR) spectra.
XRD pattern is obtained from a D/Max-2400 X-ray diffractometer equipped with a monochromator, using Cu Kα radiation at a wavelength of 0.154 nm, for 2 h from 1° to 60° with a scan speed of 5° per min. The XRD device is operated at 40 kV and 100 mA. Infrared spectra were collected using a Nexus-670 FT-IR spectrometer in the range of 4000–400 cm−1 with a resolution of 4 cm−1 by using spectral quality KBr powder. N2 adsorption–desorption isotherm were conducted using a Micromeritics ASAP 2020 instrument at 77 K. The samples are pretreated at 373 K for 24 h. The pore size was obtained from the maximum of the pore size distribution curve calculated by the Barrett–Joyner–Halenda (BJH) method using the sorption branch of the isotherm. Transmission electron microscopy (TEM) measurements were carried out on a FEI Tecnai F30. Solid-state magic-angle spinning (MAS), nuclear magnetic resonance (NMR) spectra were obtained with a Bruker AV400WB NMR spectrometer with the spinning rate of 10.0 kHz and under magic-angle-spinning (MAS). C, H, and N contents of all samples were measured by combustion oxidation method using a Vario cube elemental analysis apparatus.
Uranium sorption experiment
Batch adsorption experiments were performed to obtain the sorption capacity of U(VI) from aqueous solution of unmodified and modified SBA-15. A representative experiment were carried out by contact of appropriate volume of suspension functionalized SBA-15 sorbent with 0.6 mL UO2(NO3)2 · 6H2O stock solutions, 0.16 mL NaNO3 solutions (5 mol L−1) and some water (the total volume is 8.00 mL) in polyethylene centrifuge tubes. Then adjusting the pH values of the suspension using negligible volumes of NaOH and HNO3 solution and the samples were agitated at 25 ± 1 °C for 72 h. After that, the solid and liquid phases are separated by centrifugation with a speed of 10,000 rpm for 30 min (H2050R-1, Xiang Yi Centrifuge Instrument Co. Ltd). Residual U(VI) concentration of the supernatant were analyzed by arsenazo(III) method as a complexing agent at a wavelength of 652 nm [28, 29]. In each set, uranium solution without sorbent was used as a control. Percentage removal and uptake (q) were defined by using the following expressions:
where q e (mg U/g sorbent) is the sorption capacity of U(VI), c 0 and c e (mol L−1) are the concentration of the U(VI) in the solution before and after sorption, respectively. V s is the volume of the aqueous solution and m s (g) is the dry weight of the sorbent.
Results and discussion
Characterization of the adsorbents
Low-angle X-ray powder diffraction pattern of SBA-15 and grafted SBA-15 were shown in Fig. 2. In the XRD pattern of SBA-15, there was an intense diffraction peak at about 0.8 degree and two plane and weak signals at about 1.5° and 1.75° respectively. The presence of (100) diffraction peaks as well as the weak (110) and (200) reflections indicate that all samples preserve the mesoscopic order after functionalization process [30].
Figure 3 shows FTIR spectra of SBA-15 before and after the modification. The typical peaks at 1080 and 804 and 461 cm−1 appear in the IR spectra of SBA-15, which are assigned to the Si–O–Si asymmetric stretching vibration, the Si–O–Si symmetric stretching vibration and Si–O–Si bending mode vibration respectively. The bands at 3435 and 1630 cm−1 are attributed to the hydrated silane group and bending vibrations of surface hydroxide [22, 31]. After the modification with amino groups, the FT-IR spectrum of modified SBA-15 displays a new peak at 2950–2850 cm−1, which belongs to the stretching vibrations of C–H bond in alkyl chains [32]. They also presented characteristic bands for C–H of benzene ring stretching vibrations for pendant alkyl chains around 3000–2800 cm−1. The new appeared several peaks at 1650–1430 cm−1 are related to C=C of benzene ring and C=N which indicated successful grafting of dihydroxy bezladely derivatives.
FT-IR spectra of adsorbents after adsorbed uranium are shown in Fig. 4. Compared with the materials before sorption, for SBA-15-OHBA, we can observe the UO2 2+ symmetric stretching vibrations at 944 cm−1 and the other two are at 950 and 949 cm−1 respectively. The peak of stretching vibrations of O=Si=O group at 1384 cm−1 have become more sharp. However, the rest adsorption bands remain constant.
The N2 adsorption–desorption isotherm and BJH plot were exhibited as Fig. 5. In Fig. 5a, we could find that the isotherms of four kinds of materials have H1 hysteresis loop characteristic which belong to typical type IV. All isotherm exhibit a sharp capillary condensation step with the increasing p/p 0. According to the IUPAC classification, the characteristic of mesoporous materials [33, 34] indicate that all functionalized SBA-15 materials have reserved SBA-15 structure successfully. However, the BET, pore volume and pore size of modified SBA-15 have decreased gradually compared with pure SBA-15 (Table 1) [35, 36] because of the grafting reaction. The results indicate the successful grafting of organic groups onto the surfaces of SBA-15. The BJH plot of the derivatives of the pore volume per unit weight with respect to the pore diameter (dV/dD) versus the pore diameter is shown in Fig. 5b. A very narrow pore size distribution with an average pore diameter of 16 Å is observed.
Transmission electron microscopy (TEM) images are shown in Fig. 6. The TEM images suggested that the pure SBA-15 and functionalized SBA-15 particles have a well-ordered hexagonal array of mesoporous structure when the electron beam is paralled to the main axis of the cylindrical pores. When the electron beam is perpendicular to the main axis, the parallel nanotubular pores can be seen. It has been reported which the hexagonal array of SBA-15 materials is highly ordered and stable and these images also support this point. The TEM images confirmed that one-dimensional channels was made up of the hexagonal crystal structure [37–39]. What’s more, it was demonstrated that the porous structure was not disrupted after the post-grafting reaction.
The contents of C, H and N for the mesoporous silica and the amount of functional groups grafted on the SBA-15 surface have been evaluated by element analysis. As can be observed from Table 1, the concentrations of functional groups for modified SBA-15 based on N elemental analysis for SBA-15-OHBA, SBA-15-MHBA and SBA-15-RATP were 1.539, 2.007 and 1.661 mmol g−1 respectively.
Figure 7 shows the 13C CP-MAS NMR spectra of SBA-15-MHBA, SBA-15-OHBA and SBA-15-RATP. All the denoted peaks in the spectra can be assigned to appropriate C atoms. From the result, it can confirm that the mesoporous silica SBA-15 was functionalized by dihydroxy bezladely derivatives successfully [40, 41].
Sorption behavior studies of uranium
The effect of pH value on sorption of uranium
We have studied the parameters which affect the U(VI) adsorption properties of pure SBA-15 and grafted SBA-15 mesoporous silica. Figure 9 shows the effect of pH on sorption. From Fig. 9, the sorption ability of uranium by SBA-15 and composite materials are significantly dependent on the pH value. Because it affects the speciation of metal ions (Fig. 8), the surface charge and binding sites of the adsorbent [42].
In our experiment, we choose the pH ranging from 1.5 to 7.0 [43]. The adsorption ability increases slightly with increasing pH ranging from 1.5 to 3 and then increases sharply to a maximum value with the rise of pH from 3.0 to 5.0. At last, sorption remain constant with further increase in pH. The effect of pH on uranium adsorption could be explained by the surface characteristics of the adsorbents as well as the degree of ionization and speciation of the solute. When the pH was low, the U(VI) was present in the solution mainly in the form of free UO2 2+ ions and the binding sites of materials may become positively charged because of the protonation reaction resulting in the increase of electrostatic repulsion between U(VI) ions and materials. This effect decreases the adsorption capacity of U(VI) ions on the materials. With pH increasing, the surface of mesoporous silica are progressively deprotonated, exhibiting the anionic character. The attractive forces between the anionic surface sites and cationic uranyl ions easily result in the formation of metal–ligand magnetic composite complexes. However, when pH value is more than 5.0, schopite is formed along with other hydroxo species. On the basis of the above results, the optimum pH value is found to be 4.00 ± 0.02.
The ascending curves of the modified SBA-15 in Fig. 9 were wider, and the sorption percentage was higher than SBA-15. The sorption percentage increased with the increasing pH, which might be attributed to the presence of hydroxyl in 3, 4-dihydroxy benzaldehyde (OHBA), 2,4-dihydroxy benzaldehyde (MHBA) and 2,4-dihydroxyacetophenone (RATP) is suitable for coordination with U(VI).
Effect of the solid-to-liquid ratio
The effect of the solid-to-liquid ratio on the adsorption of U(VI) was investigated. Different amounts of solid-to-liquid ratio (from 0.125 to 3.5 g L−1) were added to U(VI) solution with all other parameters keeping constant. It can be seen from Fig. 10 that the adsorption capacity increases rapidly with solid-to-liquid ratio value increasing at a lower value, while it increases slightly at a higher solid-to-liquid ratio for all materials. At last, the sorption rate keep constant with the solid-to-liquid ratio value increasing. The increase in adsorption capacity with increasing in the amount of adsorbent might be attributed to an increase in the surface area and the availability of adsorption sites of all materials for U(VI) [44, 45]. In addition, functionalized SBA-15 have a higher sorption ability than pure SBA-15 owing to more coordination sites in organic groups they have. SBA-15-OHBA, SBA-15-MHBA and SBA-15-RATP exhibit the similar adsorption efficiency, which could be due to the similar structural characteristics of functional groups on their surface.
Effect of ionic strength
Figure 11 shows the effect of ionic strength on the U(VI) sorption ability of four kinds of materials. Ionic strength is also an important parameter on the adsorption of U(VI). In Fig. 10, the percentage sorption of uranium decreases with the NaNO3 concentration increasing from 0.01 to 0.10 mol L−1. Then remains constant when the concentration of NaNO3 was higher than 0.1 mol L−1. From these phenomena, we could see that the adsorption rate remains nearly constant indicating negligible effect of ionic strength on the adsorption process. This suggests that the sorption process follows inner sphere complex formation between U(VI) and surface sites on mesoporous silica. The reason why small decrease in the percentage sorption with increasing ionic strength (I) up to I = 0.1 M might be the ionic strength dependence of the binding constant of U(VI) with silica [46, 47].
Time-dependent sorption
For the economic efficiency, the sorption ability is an important factor in evaluating the overall performance of sorbents. As shown in Fig. 12a, the sorption rate of U(VI) on pure and grafted SBA-15 increases rapidly in the contact time of 180 min and then increases slightly until the sorption equilibrium is attained. Application of proper kinetic models to fit the experimental kinetic data can offer useful information to identify the sorption mechanism type. Therefore, we have used pseudo first-order and pseudo second-order models (Fig. 12b) for their validity with the experimental sorption data for U(VI). The linear forms of pseudo first-order and pseudo second-order kinetic models are given as follows [48]:
where q t (mol g−1) and q e (mol g−1) are the sorption amounts of U(VI) ions at contact time of t (min) and at equilibrium time, respectively; k 1 (min−1) and k 2 (g mol−1 min−1) represent the pseudo first-order and the pseudo second-order sorption rate constants. The factors of the kinetic models and the correlation coefficient (R 2) are listed in Table 2. It could be found that the experimental data was fitted very well to the pseudo second-order model from the R 2 values. The results of pseudo second-order kinetics further imply that the sorption mechanism of adsorbate (U(VI)) and adsorbent are chemisorption.
Adsorption isotherms
The influence of temperature on sorption of U(VI) was investigated at different temperatures 298, 318 and 338 K (Fig. 13). Figure 13 shows that the sorption ability for all materials increases with the increasing concentration of U(VI). In addition, the U(VI) sorption ability of SBA-15 decreased with the increasing temperature, demonstrating that the sorption process is an exothermic process. For functionalized SBA-15 materials, the sorption ability decreased with the temperature increasing, indicating that the sorption was an exothermic process for these materials too. Adsorption isotherm plays a crucial role in the physicochemical behavior of metal ions. Adsorption isotherm can prove how the adsorbate molecules distribute between the liquid and the solid phases when the sorption process reaches an equilibrium state. Therefore, using Freundlich (Fig. 14a) and Langmuir (Fig. 14a) equations which are most common mathematical models to describe the correlation of equilibrium data are important to the practical design and operation of adsorption systems.
The expression of the Langmuir model is given by Eq. (5) [49]:
where K L is a adsorption equilibrium constant, C e is the equilibrium concentration (mol L−1), q e is the amount adsorbed at equilibrium (mol g−1), q m is the the maximum sorption capacity (mol g−1).
The Freundlich model can be represented in the linearized form as Eq. (6):
where K F (mol1−n Ln g−1) represents the sorption capacity when the adsorbate equilibrium concentration equals 1, and n is the degree of sorption dependence at equilibrium concentration [50].
The adsorption constants of the Langmuir and Freundlich equation and their correlation coefficients (R 2) at 298, 318, and 338 K are calculated and presented in Table 3. From the results, we can find that there is a good improvement in maximum sorption capacity compared with pure SBA-15 for functionalized SBA-15.
Selectivity studies
In order to evaluate the selectivity of pure and functionalized SBA-15 materials, selective sorption of U(VI) studies were carried out in aqueous solution containing other competitive metal ions. In the research of selectivity, the tests were performed in aqueous solution containing UO2 2+, Ca2+, Co2+, K+, Mg2+, Sr2+, Cd2+ at pH 4.00 ± 0.02. The amount of residual coexisting metal ions in supernatants after adsorption were determined by ICP-AES. The results in Fig. 15 show that SBA-15-MHBA, SBA-15-OHBA and SBA-15-RATP exhibit a better sorption efficiency for U(VI) than pure SBA-15. From the results, it indicates that the presence of Ca2+, K+, Co2+, Cd2+, Sr2+ and Mg2+ have no significant effect on adsorption of U(VI) on modified SBA-15. The results suggest that all mesoporous materials have higher sorption ability for U(VI) and lower sorption ability for competing metal ions. It is worth mentioning that, the selective sorption ability of SBA-15-MHBA and SBA-15-RATP may have promising application in U(VI) separation field.
Competitive sorption of coexisting ions. C 0U(VI) = 1.25 × 10−4 mol L − 1, C 0K(I) = 2.58 × 10−3 mol L−1, C 0Ca(II) = 3.58 × 10−3 mol L−1, C 0Mg(II) = 3.42 × 10−3 mol L−1, C 0Cd(II) = 2.50 × 10−3 mol L−1, C 0Co(II) = 3.48 × 10−3 mol L−1, C 0Sr(II) = 3.65 × 10−3 mol L−1, \(C_{{{\text{NaNO}}_{ 3} }}\) = 0.1 mol L−1, m/V = 0.5 g L−1, pH = 4.00 ± 0.02, T = 298 ± 1 K, t = 48 h
Desorption and reusability experiments
To get a better study of the modified mesoporous silicas, the tests on desorption and reusability of SBA-15-OHBA, SBA-15-MHBA and SBA-15-RATP are essential. Desorption experiments were operated after the adsorption equilibrium, both of them were adopted under the same adsorption experimental conditions: C 0U(VI) = 4.00 × 10−4 mol L−1, m/V = 2.0 g L−1, \(C_{{{\text{NaNO}}_{ 3} }}\) = 0.1 mol L−1, T = 298 ± 1 K.
The U(VI) desorption rate from modified SBA-15 were studied as a function of pH value (pH values of the solution were adjusted using NaOH and HNO3 solutions). From Fig. 16, it is clear that when the pH value was 1.5, release percent of U(VI) was the highest. The reusability test of three materials were conducted in the same operating conditions of sorption study at first. Then doing desorption study in HNO3 solutions (pH = 1.5). As seen from Fig. 17, the capacities of three modified materials reduced with the increase of repeating times.
Conclusions
In this study, three different dihydroxy bezladely derivatives functionalized mesoporous silica SBA-15 materials have been successfully synthesized and their sorption for U(VI) from aqueous solutions were studied by batch sorption experiment. From the results, it could be seen that higher adsorption affinity for aqueous U(VI) was achieved after the modification for pure SBA-15, the sorption ability for U(VI) relies heavily on pH values before and after the functionalization. The sorption mechanism of U(VI) might be inner-sphere complexation from the research of effect of ionic strength. The adsorption process for all materials were follow pseudo-second-order type sorption kinetics. The sorption isotherm for pure SBA-15 has been successfully modeled by the Freundlich isotherm which indicated that different sites with several sorption energies were involved. However, for three different modified SBA-15 the Langmuir isotherm was better and it revealed a monolayer chemical sorption of U(VI) on modified SBA-15. SBA-15-MHBA and SBA-15-RATP showed a good sorption ability and a desirable selectivity for U(VI) over a range of competing metal ions.
References
DeCanio SJ, Fremstad A (2011) Economic feasibility of the path to zero net carbon emissions Original. Energy Policy 39:1144–1153
Zhang YY, Zhao HG, Fan QH, Zheng XJ, Li P, Liu SP, Wu WS (2011) Sorption of U(VI) onto a decarbonated calcareous soil. J Radioanal Nucl Chem 288:395–404
Akyil S, Aslani MAA, Eral M (2003) Sorption characteristics of uranium onto composite ion exchangers. J Radioanal Nucl Chem 256:45–51
Wazne M, Korfiatis GP, Meng XG (2003) Carbonate effects on hexavalent uranium adsorption by iron oxyhydroxide. Environ Sci Technol 37:3619–3624
Ilton ES, Wang ZM, Boily JF, Qafoku O, Rosso KM, Smith SC (2012) The effect of pH and time on the extractability and speciation of uranium(VI) sorbed to SiO2. Environ Sci Technol 46:6604–6611
Bazykav DA, Prysyazhnyuk AY, Romanenko AY, Fedorenko ZP, Gudzenko NA, Fuzik MM, Khukhrianska OM, Trotsyuk NK, Gulak LO, Goroch YL, Sumkina YV (2012) Cancer incidence and nuclear facilities in ukraine: a community-based study. Exp Oncol 34:116–120
Brugge D, Lemos JL, Oldmixon B (2010) Exposure pathways and health effects associated with chemical and radiological toxicity of natural uranium: a review. Rev Environ Health 20:177–194
Kumari N, Prabhu DR, Pathak PN, Kanekar AS, Manchanda VK (2011) Extraction studies of uranium into a third-phase of thorium nitrate employing tributyl phosphate and N, N-dihexyl octanamide as extractants in different diluents. J Radioanal Nucl Chem 289:835–843
Hu BW, Cheng W, Zhang H, Sheng GD (2010) Sorption of radionickel to goethite: effect of water quality parameters and temperature. J Radioanal Nucl Chem 285:389–398
Hanif HA, Nadeem R, Bhatti HN, Ahmad NR, Ansari TM (2007) Ni(II) biosorption by Cassia fistula (Golden Shower) biomass. J Hazard Mater 139:345–355
Satapathy D, Natarajan GS (2006) Potassium bromate modification of the granular activated carbon and its effect on nickel adsorption. Adsorption 12:147–154
Wang XJ, Xia SQ, Chen L, Zhao JF, Chovelon J, Nicole J (2006) Biosorption of cadmium(II) and lead(II) ions from aqueous solutions onto dried activated sludge. J Environ Sci 18:840–844
Manos MJ, Kanatzidis MG (2012) Layered metal sulfides capture uranium from seawater. J Am Chem Soc 134:16441–16446
Comarmond MJ, Payne TE, Harrison JJ, Thiruvoth S, Wong HK, Aughterson RD, Lumpkin GR, Muller K, Foerstendorf H (2011) Uranium sorption on various forms of titanium dioxide—influence of surface area, surface charge, and impurities. Environ Sci Technol 45:5536–5542
Ying YL, Mehnert CP, Wong MS (1999) Synthesis and applications of supramolecular-templated mesoporous materials. Angew Chem 38:56–77
Øye G, Sjöblom J, Stöcker M (2001) Synthesis, characterization and potential applications of new materials in the mesoporous range. Adv Colloid Interface Sci 89:439–466
On DT, Desplantier-Giscard D, Danumah C, Kaliaguine S (2001) Perspectives in catalytic applications of mesostructured materials. Appl Catal A 222:299–357
Kresge CT, Leonowicz ME, Roth WJ (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359:710–712
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, Cullen SBM, Higgins JB, Schlenker JL (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114:10834–10843
Li M, Pham PJ, Pittman CU, Li T (2009) SBA-15-supported ionic liquid compounds containing silver salts: novel mesoporous π-complexing sorbents for separating polyunsaturated fatty acid methyl esters. Microporous Mesoporous Mater 117:436–443
Wang YL, Song LJ, Zhu L, Guo BL, Chen SW, Wu WS (2014) Removal of uranium(VI) from aqueous solution using iminodiacetic acid derivative functionalized SBA-15 as adsorbents. Dalton Trans 43:3739
Quintanilla DP, Hierro ID, Fajardo M, Sierra I (2006) 2-Mercaptothiazoline modified mesoporous silica for mercury removal from aqueous media. J Hazard Mater 134:245–256
Mureseanu M, Reiss A, Cioatera N, Trandafir I, Hulea V (2010) Mesoporous silica functionalized with 1-furoyl thiourea urea for Hg(II) adsorption from aqueous media. J Hazard Mater 182:197–203
Wang YL, Zhu L, Guo BL, Chen SW, Wu WS (2014) Mesoporous silica SBA-15 functionalized with phosphonate derivatives for uranium uptake. New J Chem 38:3853
Yantasee W, Fryxell GE, Addleman RS, Wiacek RJ, Koonsiripaiboon V, Pattamakomsan K, Sukwarotwat V, Xu J, Raymond KN (2009) Selective removal of lanthanides from natural waters, acidic streams and dialysate. J Hazard Mater 168:1233–1238
Anupama G, Ajai KS (2002) Silica gel functionalized with resacetophenone: synthesis of a new chelating matrix and its application as metal ion collector for their flame atomic absorption spectrometric determination. Anal Chim Acta 454:229–240
Mahmoud ME, Soliman EM (1997) Silica-immobilized formylsalicylic acid as a selective phase for the extraction of iron(III). Talanta 44:15–22
José A, Jesús MA, Amaya A, Montaña L, Victoria G (2009) Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J Hazard Mater 163:213–221
Gao L, Yang Z, Shi K, Wang X, Guo Z, Wu W (2010) U(VI) sorption on kaolinite: effects of pH, U(VI) concentration and oxyanions. J Radioanal Nucl Chem 284:519–526
José A, Jesús MA, Amaya A, Montaña L, Victoria G (2009) Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J Hazard Mater 163:213–221
Tao Q, Xu ZY, Wang JH, Liu FL, Wan HQ, Zheng SR (2010) Adsorption of humic acid to aminopropyl functionalized SBA-15. Microporous Mesoporous Mater 131:177–185
Kleitz F, Bérubé F, Guillet-Nicolas R, Yang CM, Thommes M (2010) Probing adsorption, pore condensation, and hysteresis behavior of pure fluids in three-dimensional cubic mesoporous KIT-6 silica. J Phys Chem C 114:9344–9355
Kim TW, Kleitz F, Paul B, Ryoo R (2005) MCM-48 like large mesoporous silicas with tailored pore structure: facile synthesis domain in a ternary triblock copolymer-butanol-water system. J Am Chem Soc 127:601–7610
Sevimli F, Yilmaz A (2012) Surface functionalization of SBA-15 particles for amoxicillin delivery. Microporous Mesoporous Mater 158:281–291
Njoku VO, Foo KY, Asif M, Hameed BH (2014) Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem Eng J 250:198–204
Wang L, Yang RT (2011) Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J Phys Chem C 115:21264–21272
Hernández-Morales V, Nava R, Acosta-Silva YJ, Macías-Sánchez SA, Pérez-Bueno JJ, Pawelec B (2012) Adsorption of lead(II) on SBA-15 mesoporous molecular sieve functionalized with –NH2 groups. Microporous Mesoporous Mater 160:133–142
Yang Y, Zhang Y, Hao SL, Kan QB (2011) Tethering of Cu(II), Co(II) and Fe(III) tetrahydro-salen and salen complexes onto amino-functionalized SBA-15: effects of salen ligand hydrogenation on catalytic performances for aerobic epoxidation of styrene. Chem Eng J 171:1356–1366
Gao ZF, Wang LN, Qi T, Chu JL, Zhang Y (2007) Synthesis, characterization, and cadmium(II) uptake of iminodiacetic acid-modified mesoporous SBA-15. Colloids Surf A 304:77–81
Przybylski P, Schilf W, Brzezinski B (2005) 13C, 15N NMR and CP-MAS as well as FT-IR and PM5 studies of Schiff base of gossypol with l-phenylalanine methyl ester in solution and solid. J Mol Struct 734:123–128
El-Nahhal IM, Zaggout FR, Nassar MA (2003) Synthesis, characterization and applications of immobilized iminodiacetic acid-modified silica. J Sol-Gel Sci Technol 28:255–265
Nie BW, Zhang ZB, Cao XH, Liu YH, Liang P (2012) Sorption study of uranium from aqueous solution on ordered mesoporous carbon CMK-3. J Radioanal Nucl Chem 295:663–670
Yang Y, Zhang Y, Hao S, Kan Q (2011) Tethering of Cu(II), Co(II) and Fe(III) tetrahydro-salen and salen complexes onto amino-functionalized SBA-15: effects of salen ligand hydrogenation on catalytic performances for aerobic epoxidation of styrene. Chem Eng J 171:1356–1366
Shao DD, Fan QH, Li JX, Niu ZW, Wu WS, Chen YX, Wang XK (2009) Removal of Eu(III) from aqueous solution using ZSM-5 zeolite. Microporous Mesoporous Mater 123:1–9
Chen CL, Wang XK (2007) Influence of pH, soil humic/fulvic acid, ionic strength and foreign ions on sorption of thorium(IV) onto γ-Al2O3. Appl Geochem 22:436–445
Kar AS, Kumar S, Tomar BS∗, Manchanda VK (2011) Sorption of curium by silica colloids: effect of humic acid. J Hazard Mater 186:1961–1965
Dzombak DA, Morel FMM (1990) Surface complexation modeling: hydrous ferric oxide. Wiley, New York
Tian G, Geng JX, Jin YD, Wang CL, Li SQ, Chen Z, Wang H, Zhao YS, Li SJ (2011) Sorption of uranium(VI) using oxime-grafted ordered mesoporous carbon CMK-5. J Hazard Mater 190:442–450
Chen CL, Li XL, Zhao DL, Tan XL, Wang XK (2007) Application of oxidized multi-wall carbon nanotubes for Th(IV) adsorption. Radiochim Acta 95:261–266
Semnani F, Asadi Z, Samadfam M, Sepehrian H (2012) Uranium(VI) sorption behavior onto amberlite CG-400 anion exchange resin: effects of pH, contact time, temperature and presence of phosphate. Ann Nucl Energy 48:21–24
Acknowledgments
This work was supported by National Natural Science Foundation of China (21101082, J1210001) and Fundamental Research Funds for the Central University (Lzujbky-2013-55).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, L., Sun, Y., Song, L. et al. Dihydroxy bezladely derivatives functionalized mesoporous silica SBA-15 for the sorption of U(VI). J Radioanal Nucl Chem 310, 125–137 (2016). https://doi.org/10.1007/s10967-016-4779-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4779-4