Abstract
The elemental characterization of coal fly ash samples is required to estimate the coal burning emissions into the environment and to assess the potential impact into the biosphere. Fly ash samples collected from a coal fired power plant in center Java, Indonesia were characterized by instrumental neutron activation analysis at two different facilities (BATAN, ANSTO) and synchrotron based techniques at Elettra Italy. Assessment of thirty (30) elements and an investigation of the potential toxicity of As species in coal fly ash were presented. The results obtained are discussed and compared with those reported from other regions of the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy has become an important prerequisite for the economic development of a country including Indonesia. Increasing electricity consumption in Indonesia due the rapid economic growth encourages the Indonesian government issued Mix Energy Utilization program to meet and to secure the electricity supply, where one of is 35,000 MW coal power plant crash program. The government also launched a national diversification of energy sources from fossil fuels to coal due to the sizeable coal reserves in Indonesia. These programs will hopefully improve the reliability of electricity services, utilize low grade coal, enhance local economy and support community development. The increasing of coal utilization as power fuel has gained many interests due to its adverse effect on environment. Coal combustion waste products whether in the form of gas and solids are known as sources of pollution that can deliver considerable impact on the environment [1–3]. Coal combustion exhaust gases such as SOx, NOx and CO2 are directly discharged into the air. Solid wastes of coal combustion are composed of fly ash of 75–80 % and bottom ash by 20–25 % [4, 5].
One of coal combustion by-products, coal fly ash, is characterized by the small particle size therefore it can be emitted to the atmosphere, and the released fine particulates can travel long distances. Coal fly ash particles contribute to increasing the concentration of fine atmospheric particulate matter (PM2.5) mass and that of the potentially toxic elements contained by them as well. The chemical composition of coal ash varies dependent on coal characteristic/quality, degree of pulverization, combustion technique and boiler operational conditions as well as mode of collection [3, 5, 6]. Elemental characterization of coal fly ash is usually the first step taken towards subsequent evaluation of associated environmental and biological risks. Determination of trace element concentrations in fly ash has always been an interesting area of environmental research. Several instrumental techniques were proposed for elemental analysis of fly ash such as atomic absorption spectrometry, inductively coupled plasma-atomic emission spectrometry, inductively coupled plasma-mass spectrometry, X-ray fluorescence and neutron activation analysis [3, 5–10]. Furthermore, the leaching of elements from fly ash into environment and also the behavior of the elements generated by coal combustion were also extensively studied [11–13]. However, the characterization of coal fly ash generated from Indonesian characteristic coal combustion is still lacking and limited.
Instrumental neutron activation analysis (INAA) is a sensitive analytical technique which is useful for performing both qualitative and quantitative characterization of major, minor, and trace elements in samples from almost every field of scientific or technical interest. It is one of the most mature analytical methods currently used and yet remains highly competitive with others in terms of accuracy, detection limits and multi-elemental capabilities [14–16]. This paper focuses on the elemental characterization of coal fly ash using the NAA method applied in an Indonesian facility. In order to further validate the obtained elemental concentrations comparative measurements were carried out at the INAA facility of the Australian Nuclear Science and Technology Organisation (ANSTO). Synchrotron radiation (SR) induced micro X-ray fluorescence (micro-XRF) was also utilized in a complementary manner to expand the compositional results for those elements that cannot be measured by INAA such as Si, S, Ni and Pb, but also to provide for the common detected elements an additional set of compositional data. The measurements were performed at the International Atomic Energy Agency (IAEA) endstation facility installed at the X-ray fluorescence (XRF) beamline of the Elettra Sincrotrone Trieste (Trieste, Italy).
Synchrotron radiation based techniques such as micro-XRF analysis and X-ray absorption near-edge structure (XANES) spectroscopy have been demonstrated to provide elemental compositional analysis of single fly-ash particles [17] and chemical speciation of key importance elements such as Cr and As in respective bulk samples [18]. In the present study, arsenic was studied by XANES because of its potential carcinogenic and non-carcinogenic health risks associated with its emission. The most toxic arsenic species are considered to be the inorganic ones, As (III) being more toxic than As(V). Organic compounds containing arsenic (e.g., arsenobetaine and arsenocholine) usually contained in food and plant materials are much less toxic [19]. XANES spectroscopy delivers non-destructive information on the chemical state of the element of interest. A more oxidative chemical environment results in deeper core state binding energies, which in turn cause an absorption edge shift towards higher energies in the XANES spectrum. The magnitude of the shift is around 2 eV per valence change for As K edge. The shape and energy position of pre-edge and near-edge structures are characteristic for the chemical state of the absorbing atom, the XANES spectra can be used as fingerprints for different speciation of a given element. The method uses high intensity monochromatic X-ray beams usually originating from synchrotron radiation. The oxidation state of As can be determined by XANES spectrometry with 5 % (absolute) error for concentrations as low as 10 μg/g [18].
Experimental
Sampling and sample preparation of coal fly ash
The coal fly ash was obtained from electrostatic precipitator of coal fired power plants in Central Java, Indonesia. The starting materials were air-dried, blended, grinded using mortar and then sieved using the 150 mesh sieve. To improve the homogeneity, the materials that cannot pass through the sieve then were grinded again using mortar grinder pulverisette. This would made the samples on <105 µm size fraction. Afterwards, the coal fly ash was homogenized by mixing and repetitive quartering, before being placed in HDPE containers and identified.
Moisture analysis
The loss on drying method was used for measuring the moisture level in coal fly ash (CFA) samples. The moisture content was determined by weighing 2 g (triplicate) of CFA samples and dried in the oven at 105–110 °C for 4 h or until it has constant weight, cooled in dry atmosphere and then reweighed.
Elemental characterization by INAA
At the BATAN facilities, INAA was carried out for elemental analysis of the samples using the relative method of standardization. About 25 mg of samples placed in the 0.3 mL polyethylene vials. SRM NIST 1633b Coal Fly Ash used to validate the method was weighed also about 25 mg and placed in the 0.3 mL polyethylene vials. Mix standards for relative method were prepared from certified multi-elements standard solutions and placed in the same size vials. The samples, SRM and standards are ready for irradiation. The samples, SRMs and standards were irradiated in the rabbit system of The Multi-Purpose Reactor G.A. Siwabessy with neutron flux ~1013 n cm−2 s−1 [20]. Short, medium and long irradiations were performed in order to determine various radionuclides with different half-lives. The irradiation times of samples were 1 min for short-lived radionuclide (Al, Mg, Mn, Ca, Ti, V), 15 min for medium short-lived radionuclide (Na, K, As, U, Sm, La) and 120 min for long-lived radionuclides (Co, Cr, Fe, Se, Zn). After appropriate cooling, samples were counted for about 5 min for short-lived radionuclide, and within 30–60 min for medium and long-lived radionuclides using HPGe gamma spectrometer. Software GENIE 2000 was utilized for spectrum analysis.
At ANSTO, INAA was performed by the k 0-method of standardization, using certified reference material IRMM-530RC, Al-0.1 %Au wire alloy, as the standard. Samples were irradiated in the 20 MW OPAL research reactor. Sub-samples were subjected to a short irradiation (60 mg sub-sample for 30 s at a thermal neutron flux of 2.1 × 1013 cm−2 s−1) and a long irradiation (160 mg sub-sample for 9 h at a thermal neutron flux of 2.6 × 1012 cm−2 s−1). Following the short irradiation, the sub-sample was counted for 3 min after a 9 min decay and again for 15 min after an 18 min decay. Following the long irradiation, the decay periods were 4 and 18 days and the counting times were 1.5 and 24 h respectively. High purity germanium detectors were coupled to ORTEC DSPEC-Pro digital spectrometers and the data were analyzed using proprietary software. By combining all the measurements, a total of 36 elements were quantified in the fly ash sample. The moisture content of the fly ash was determined using a 5 g sample taken at the same time that the sub-samples were weighed for irradiation. The two sets of INAA results from the BATAN and ANSTO facilities were compared.
SR micro-XRF and XANES measurements
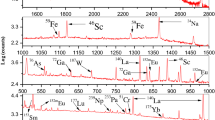
The synchrotron radiation measurements were carried out at the IAEA end station of the XRF beamline at Elettra Sincrotrone Trieste. The facility has been recently commissioned and it is jointly operated by Elettra and the IAEA being accessible to end-users from the beginning of 2015. The general features of the XRF beamline are described in Ref. [21]. Currently, the XRF beamline delivers tunable monochromatic exciting radiation by means of Si(111) double crystal monochromator in the energy range from about 3.6 to 14 keV. The beam spot size at the sample position is about 260 μm (H) × 110 μm (V) defined by a pair of horizontal and vertical slits located downstream. The samples were inserted for analysis in an Ultra-high vacuum (UHV) environment and area scanning measurements can be performed using a motorized sample manipulator. For minimizing accidental contamination of the UHV chamber, the fly-ash pellet was protected with a Kapton foil of 0.5 mil thickness. A silicon drift detector (SDD) with energy resolution of about 131 eV at Mn-Kα was utilized to detect the fluorescence radiation emitted from the fly-ash constituent elements. The SDD is equipped with an ultrathin polymer window and a photoelectron trap which due to its geometrical design and the incorporated apertures reduces the solid angle of detection. Considering the fact that practically the Kapton foil prevents the detection of fluorescence radiation emitted from elements lighter than Al (Z = 13), the photoelectron trap was replaced with a Be 1/3 mil window that it is adequately thick to absorb photoelectrons up to 14 keV. The Be window was mounted directly on the SDD snout together with a defining aperture for the incident X-rays. This modified SDD configuration increases the solid angle of detection more than three times optimizing the detection of trace elements in the tender and hard X-ray region. The typical reflection 45°/45° irradiation geometry was employed for both the micro-XRF and XANES measurements. In particular, the micro-XRF scanning analyses were performed at 13,500 eV incident beam energy and a total area of about 4 mm × 4 mm was analyzed by performing 9 × 9 measurements with a step size of 0.5 mm with 10 s acquisition time per step. These micro-XRF measurements showed a level of heterogeneity less than 10 % for certain elements (Si, K, Ca, Mn, Fe, Cu, Zn, Ge, Pb), however a more systematic investigation is required for assessing properly the possible inhomogeneity of the samples at different concentration level and local scale. Spectrum analysis and quantification was carried out by means of the PyMCA software package [22]. For the calibration of the experimental set-up (incident flux and solid angle of detection), a multi-elemental certified thin standard, custom prepared by AXO Dresden GmbH was utilized [23].
XANES spectroscopy was used to determine the oxidation state of arsenic in fly ash samples. The XANES spectra were collected in fluorescence mode through detecting the As-Kα fluorescence intensity while tuning the Si(111) monochromator around the K absorption edge of As (11,868 eV) in small energy steps (0.5–1 eV). The XANES spectra were normalized to the edge jump. CFA samples were prepared as pressed pellets having a diameter of 13 mm using wax binder in a percentage of 20 wt%. Chemical compounds containing arsenic in known oxidation states such as As2O3 [for As(III)] and NaHAsO4 [for As(V)] were also prepared as pressed pellets and used as XANES standards,. These standard compounds contained 1 wt% arsenic and boric acid used as diluting agent having negligible absorption around the As K edge.
Results and discussion
Moisture analysis
The moisture content of coal fly ash sample analyzed at BATAN and ANSTO were 0.20 and 0.17 wt%, respectively. The moisture content was used to adjust the measured elemental concentrations to a dry-mass basis.
Analytical performance of INAA
To assure that the analytical results generated by INAA were accurate and precise, the NIST standard reference material (SRM) 1633b Coal Fly Ash was analyzed in the BATAN facility in the same experimental conditions used for the sample analysis. Several elements in the SRM 1633b were detected and the results were then compared with its certificate values. The ratio between the analysis results obtained with INAA methods and the certificate values of Ti, Al, Mg, V, Ca, Mn, U, Ba, Na, K, Sm, La, As, Cr, Fe, Co, Th, Sc and Zn are shown in Fig. 1. These analysis results had a good agreement with the value quoted in the NIST certificate. The ratio between the analysis value and the certificate indicates the values with a ratio between 0.94 and 1.16. The concentrations of most elements are consistently within the uncertainties of the certified values.
The precision of INAA was assessed by analyzing of five replicates of SRM NIST 1633b coal fly ash samples. The bias obtained for 19 elements ranged from 1.82 to 8.26 %. Most of elements showed a good reproducibility.
In order to ensure the reliability of the BATAN INAA concentrations, a comparison exercise was carried out with the NAA facility in ANSTO, Australia. Figure 2 shows the correlation of the results obtained by both NAA facilities. For the most of the elements, the differences are less than 5 %, whereas Ti, Zn, K, Th, Fe, Na, La and As showing a ratio of 0.95, 0.96, 1.00, 1.00, 1.02, 1.02, 1.04 and 1.04, respectively. There are some elements Mg and Sb that exhibit a difference more than 20 %. The different for Mg is due to the competing nuclear interference reaction or the possible strong overlapping peak from Mn, while for Sb is still acceptable since the concentration in the trace levels. In general, the ratio of the two sets of data is 0.89. The coefficient of determination R 2 = 0.99 for the least squares fitting indicates an excellent agreement of the results obtained at two different laboratories.
Elemental composition of coal fly ash by INAA and SR micro-XRF
The elemental analysis results of CFA samples obtained by both INAA and SR micro-XRF, expressed on a dry-mass basis, are provided in Table 1. The INAA results presented on Table 1 were average of the results obtained from two different neutron activation analysis facilities from Indonesia (BATAN) and Australia (ANSTO). While for the uncertainty sources of the INAA method were identified according to international accepted instructions [16]. The INAA characterization resulted of 25 elements with quantified concentration where Na, Mg, Al, K, Ca, Ti and Fe were major constituents of coal fly ash, while Sc, V, Cr, Mn, Co, Zn, As, Sb, Ba, La, Ce, Ga, Br, Sm, Th and U were minor and trace constituents. The results also showed that concentrations of minor and trace elements (Cu and Se) were below detection limits of INAA methods. This is due to the low sensitivities of this elements, and to increase the sensitivities of Se could be done by using epithermal NAA. The detection limit were calculated based on the signal-to-noise ratio, the selectivity of determining with a certain degree of confidence, a peak in the gamma-ray spectrum [16]. In addition of INAA method, concentrations of major elements Si and S, minor and trace constituents as Ni, Cu, Ge, Se and Pb were determined by SR micro-XRF analysis. There is generally a good agreement between concentrations obtained by the two complementary techniques, however SR micro-XRF delivered substantially lower concentrations for V (~20 %) and Cr (~35 %). Both elements characteristic X-ray lines are influenced by various spectral interferences including those arising from the co-existence in the CFA samples of rare earth elements such as Ba and to a less extent by La and Ce (due to their lower concentration). In addition, the Cr main peak (Kα) is also affected by the precision in the Fe-Kβ escape peak correction. The results are compared to concentration ranges in coal fly ash reported for different countries/regions in the world, such as Europe [24], Turkey [25], North America [4, 26], Argentina [3], India [27], Korea [28], Australia [29], Japan [29], India [30], China [31] and Malaysia [6] (Table 1, last column). Concentrations of most of the elements fall within the range reported in the literatures. It should be mentioned that the concentrations of trace elements Cu, As, Se, Sb, Pb, Th and U in Indonesian coal fly ash are close to the minimum reported values. The content of rare earth elements also falls into the lower end of the world fly ash concentration range.
Oxidation state of As using XANES
The speciation of As in coal fly ash was examined using XANES spectroscopy to identify possible toxic and less toxic oxidation state forms through comparison with standards. The toxicity of the inorganic arsenic species depends on the oxidation state, As(III) being more toxic than As(V). As K-edge XANES spectrum of a coal fly ash pellet sample compared to As(III) and As(V) spectra is shown in Fig. 3. The spectrum of the fly ash sample shows absorption edge shift and peak position (so-called white line) identical to those in the As(V) standard spectrum indicating that most of arsenic (>95 %) is present in its less toxic inorganic form [As(V)] in coal fly ash. The present result is similar to those found in studies conducted by other research groups. Huggins and Goodarzi [32] and Goodarzi et al. [33] reported that arsenic is predominantly present (>90 %) as arsenate in coal fly ash. However, Sanchez-Rodas et al. [34] and Gonzalez-Castanedo et al. [35] stated that even though As(III) was present in lower concentration in airborne fine particulate matter (PM2.5), the presence of As(III) is an added risk for human health since it accumulates in finer particles with greater capability to enter the organism.
Conclusions
The INAA and SR micro-XRF analysis of coal fly ash samples from an Indonesian coal fired power resulted to the determination of thirty (30) elements. INAA due to its s high selectivity, sensitivity and good reproducibility is well suited for an accurate routine analysis of coal fly ash samples. SR micro-XRF analysis can complement significantly the elemental characterization of coal fly ash providing the concentration of several major, minor and trace elements not determined by INAA. For the commonly detected elements, good agreement was observed between the two techniques. The results obtained from two different INAA facilities in BATAN and ANSTO showed good agreement. The XANES speciation of As in the coal fly ash revealed that the less toxic inorganic form As(V) represented the main arsenic species. Further studies are planned to be conducted to enlighten the chemical state of other potential toxic elements in coal fly ash samples and also to investigate the potential to develop reference materials.
References
Choudry MAF, Nurgis Y, Sharif M, Mahmood AA, Abbasi HN (2010) Composition, trace element contents and major ash constituents of Thar coal, Pakistan. Am J Sci Res 11:91–102
Alonso-Hernandez CM, Bernal-Castillo J, Bolanos-Alvarez Y, Gomez-Batista M, Diaz-Asencio M (2011) Heavy metal content of bottom ashes from a fuel oil power plant and oil refinery in Cuba. Fuel 90:2820–2823
Marrero J, Polla G, Rebagliati RJ, Pla R, Gomez D, Smichowski P (2007) Characterization and determination of 28 elements in fly ashes collected in a thermal power plant in Argentina using different instrumental technique. Spectrochim Acta Part B 62:101–108
Goodarzi F (2006) Assessment of elemental content of milled coal, combustion residues, and stack emitted materials: possible environmental effects for Canadian pulverized coal-fired power plant. Int J Coal Geol 65:17–25
Rautray TR, Behera B, Badapanda T, Vijayan V, Panigrahi S (2009) Trace element analysis of fly ash samples by EDXRF technique. Indian J Phys 83(4):543–546
Al-Areqi WM, Majid AA, Sarmani S (2008) Analysis of trace elements in power plant and industrial incinerator fly ashes by instrumental neutron activation analysis (INAA). Malays J Anal Sci 12:375–379
Levandowski J, Kalkreuth W (2009) Chemical and petrographical characterization of feed coal, fly ash and bottom ash from the figueira power plant, Parana, Brazil. Int J Coal Geol 77:269–281
Reinik J, Irha N, Steinnes E, Urb G, Jefimova J, Piirisalu E (2014) Release of 22 elements from bottom and fly ash samples of oil shale fueled PF and CFB boilers by two-cycle standars leaching test. Fuel Process Technol 124:147–154
Iwashita A, Nakajima T, Takanashi H, Ohki A, Fujita Y, Yamashita T (2007) Determination of trace elements in coal and coal fly ash by joint-use of ICP-AES and atomic absorption spectrometry. Talanta 71:251–257
Chand P, Kumar A, Gaur A, Mahna SK (2010) Elemental analysis of ash using x-ray fluorescence technique. Asian J Chem 21:S220–S224
Jones MR, McCarthy A, Booth APPG (2006) Characteristics of the ultrafine component of fly ash. Fuel 85:2250–2259
Izquierdo M, Querol X (2012) Leaching behavior of elements from coal combustion fly ash. An overview. Int J Coal Geol 94:54–66
Goodarzi F, Sanei H (2009) Plenosphere and its role in reduction of emitted fine fly ash particles from pulverized coal-fired power plants. Fuel 88:382–386
Greenberg RR (2008) Pushing the limits of NAA: accuracy, uncertainty and detection limits. J Radioanal Nucl Chem 278:231–240
Alamin MB, Spyrou NM (1997) Elemental characterization of different matrices including coal, sawdust, fly-ash and landfill waste samples using INAA and PIXE analyses. J Radioanal Nucl Chem 216:41–45
Greenberg RR, Bode P, Fernandes E (2011) Neutron activation analysis: a primary method of measurement. Spectrochimica Acta Part B 66:193–241
Vincze L, Somogyi A, Osán J, Vekemans B, Török S, Janssens K, Adams F (2002) Quantitative trace element analysis of individual fly ash particles by means of X-ray microfluorescence. Anal Chem 74:1128–1135
Osán J, Török B, Török S, Jones KW (1997) Study of chemical state of toxic metals during the life cycle of fly ash using X-ray absorption near-edge structure. X-Ray Spectrom 26:37–44
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16
Lestiani DD, Santoso M, Adventini N (2009) Application of neutron activation analysis in characterization of environmental SRM samples. Indones J Chem 9:231–235
Jark W, Eichert D, Luehl L, Gambitta A (2014) Optimisation of a compact optical system for the beamtransport at the X-ray Fluorescence beamline at Elettra for experiments with small spots. In: Morawe C, Khounsary AM, Goto S (eds) Advances in X-Ray/EUV optics and components IX, Proceedings of SPIE, vol 9207, 92070G. SPIE, USA
Solé VA, Papillon E, Cotte M, Walter P, Susini J (2007) A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim Acta Part B 62:63–68
Krämer M, Dietsch R, Holz T, Weißbach D, Falkenberg G, Simon R, Fittschen U, Krugmann T, Kolbe M, Müller M, Beckhoff B (2011) Ultrathin layer depositions—a new type of reference samples for high performance XRF analysis. Adv X-Ray Anal 54:299–304
Moreno N, Querol X, Andres JM, Stanton K, Towler M, Nugteren H, Janssen-Jurkovicova M, Jones R (2005) Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 84:1351–1363
Vassilev SV, Vassileva CG, Karayigit AI, Bulut Y, Alastuey A, Querol X (2005) Phasemineral and chemical composition of composite samples from feed coals, bottom ashes and fly ashes at the Soma power station, Turkey. Int J Coal Geol 61:35–63
Rivera N, Kaur N, Hesterberg D, Ward CR, Austin RE, Duckworth OW (2015) Chemical composition, speciation, and elemental associations in coal fly ash samples related to the Kingston ash spill. Energy Fuels 29:954–967
Shreya N, Valentim B, Paul B, Guedes A, Pinho S, Ribeiro J, Ward CR, Flores D (2015) Multi-technique study of fly ash from the Bokaro and Jharia coalfields (Jharkhand state, India): a contribution to its use as a geoliner. Int J Coal Geol 152:25–38
Lim JM, Jeong JH, Lee JH (2013) Instrumental neutron activation analysis of coal and its combustion residues from a power plant. J Radioanal Nucl Chem 298:201–208
Vassilev SV, Eskenazy GM, Vassileva CG (2000) Contents, modes of occurrence and origin of chlorine and bromine in coal. Fuel 79:903–921
Tiwari M, Sahu SK, Bhangare RC, Ajmal PY, Pandit GG (2014) Elemental characterization of coal, fly ash, and bottom ash using an energy dispersive X-ray fluorescence technique. Appl Radiat Isot 90:53–57
Li J, Zhuang X, Querol X, Font O, Moreno N, Zhou J (2012) Environmental geochemistry of the feed coals and their combustion by-products from two coal-fired power plants in Xinjiang Province, Northwest China. Fuel 95:446–456
Huggins FE, Goodarzi F (2009) Environmental assessment of elements and polyaromatic hydrocarbons emitted from a Canadian coal-fired power plant. Int J Coal Geol 77:282–288
Goodarzi F, Huggins FE, Sanei H (2008) Assessment of elements, speciation of As, Cr, Ni and emitted Hg for a Canadian power plant burning bituminous coal. Int J Coal Geol 74:1–12
Sanchez-Rodas D, Sanchez de la Campa AM, Oliveira V, de la Rosa J (2012) Health implications of distribution of arsenic species in airborne particulate matter. J Inorg Biochem 108:112–114
Gonzales-Castanedo Y, Sanchez-Rodas D, Sanchez de la Campa AM, Pandolfi M, Alastuey A, Chachorro VE, Querol X, de la Rosa JD (2015) Arsenic species in atmospheric particulate matter as tracer of the air quality of Donana Natural Park (SW Spain). Chemosphere 119:1296–1303
Acknowledgments
The authors acknowledge the National Nuclear Energy Agency for the financial support throughout this work. The authors also gratefully acknowledge the International Atomic Energy Agency (IAEA) Coordination Research Project G42005 (CRP) on Experiments with Synchrotron Radiation for Modern Environmental and Industrial Applications for supporting the project through the contract No. 18251 and the Elettra Sincrotrone Trieste, Trieste, Italy for providing access through the beamtime proposal 20145327. The IAEA and the BATAN co-authors would like to acknowledge the XRF beamline staff for facilitating the delivery of optimum operational conditions for the synchrotron experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santoso, M., Lestiani, D.D., Damastuti, E. et al. Trace elements and As speciation analysis of fly ash samples from an Indonesian coal power plant by means of neutron activation analysis and synchrotron based techniques. J Radioanal Nucl Chem 309, 413–419 (2016). https://doi.org/10.1007/s10967-016-4755-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4755-z