Abstract
In calcareous soils from Yakutia only 0.1 % or less 239Pu and 241Am exist in water-soluble form, i.e., the mobility of these radionuclides is relatively low. In the top humus-containing layer (0–4 cm) 239Pu and 241Am are distributed uniformly between organic and inorganic soil components. In the bottom soil layer (20–30 cm) the radionuclides are present mainly in inorganic soil components. The estimation of the radionuclide mobility demonstrates that 241Am is potentially a more mobile element than 239Pu. In the considered calcareous soils collected from the top layer 239Pu and 241Am exist both in humic and fulvic acids (FA). 241Am is much stronger bound to the group of mobile FA than 239Pu. In the bottom soil layer 239Pu and 241Am have been found mainly in FA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
12 underground nuclear explosions (UNE) were performed in Sakha (Yakutia) republic in 1974–1987 for intensification of gas and oil influx, deep seismic sounding of the earth crust and contruction of tailings dam for a mining processing plant. Accidental Kraton-3 UNE (1978) resulted in appearance of a lot of long-living radionuclides (such as 239,240Pu, 90Sr, 137Cs) in northern taiga landscapes of Yakutia. Content of long-living radionuclides in soils varies in a wide range depending on the depth and distance from the point of Kraton-3 UNE and it exceeds the worldwide background of these radionuclides in the soils of Northern hemisphere by an order or two orders [1–3]. Radioecological monitoring of contaminated soils in Yakutia demands the information about the content and distribution of radionuclides in soils. However, long-term forecast of radionuclide behavior in this region requires knowledge and account of both the data concerning their total content in the environment objects and their speciation, which determines the intensity and direction of radionuclide migration in the environment along with the natural factors. Nevertheless, there are very little data concerning the speciation of radionuclides in soils affected by Kraton-3 UNE in Yakutia.
This research aimed to study the speciation of 239,240Pu and 241Am in soils collected in the zone, which has been affected by Kraton-3 UNE.
Experimental
The objects of the research on speciation of radionuclides were two soil samples collected from soil pit at the depth of 0–4 and 20–30 cm. The samples were collected in 2012 in the zone affected by Kraton-3 accidental underground nuclear explosion in Sakha (Yakutia) Republic by researchers from Institute for Biological Problems of Cryolithozone, Siberian Branch of Russian Academy of Sciences. The soils belong to soddy calcareous destructive type. Content of 238−240Pu in the 0–4 and 20–30-cm-deep soil layers is 194.0 and 1.7 Bq/kg respectively, content of 241Am is also low and insufficient for the study of its speciation in the native soil samples. For this reason we added 239Pu and 241Am isotopes to these soil samples in laboratory conditions. For this purpose 0.2 mL portions of concentrated solution of radionuclide nitrates were spiked into air-dry soils samples (30 g) which were thoughtfully mixed after that (at least during 60 days). Content of 239Pu and 241Am in the soils was 90–110 and 80–90 Bq/g, respectively. The experiment aiming to reveal the speciation of 239Pu and 241Am was carried out 11–14 months after the addition of isotopes to the soils.
The study of speciation of radionuclides and chemical elements is a difficult problem. Soil is a thermodynamically open multiphase system involving various physicochemical, chemical and biological processes. Soil has high absorption capacity including mechanical, physical, physicochemical, biological absorption etc. In addition, one should take into account the conventional nature of the methods used for studying the speciation of radionuclides, which are usually based on selective dissolution of soil compounds during chemical treatment, each step of which becomes more aggressive. This situation is associated with the difficulty of choosing the reagents for extraction of certain groups of radionuclides from soils in laboratory conditions; a group is considered as a entity of compounds with similar properties. Usually the division of compounds and associated radionuclides into groups is based on the differences in their solubility as well as their bonds with organic and inorganic soil components. Despite certain problems, the methods for revealing radionuclide speciation are widely used in radioecology and the results allow understanding their pathways in the environment [4–9].
We used several methods for studying the speciation of radionuclides in soils from Yakutia. In all cases P.a. grade chemicals were used.
(1) Distribution of radionuclides between organic and inorganic soil components was studied by stepwise extraction with the following reagents: distilled H2O (water-soluble form), 0.5 M Ca(NO3)2·(pH 5.5) (exchangeable form); 1 M CH3COONH4, pH 4.8 (easily soluble form); 0.1 M NaOH (pH 10) (organic matter-associated form); 0.18 M (NH4)2C2O4 + 0.1 M H2C2O4 (pH 3.5) (the form which was associated with amorphous oxides of Fe, Al and Mn), residue (poorly soluble form). The duration of soil incubation with the solution was 20–24 h, the ratio of solid and liquid phases was 1:10.
(2) The bond between radionuclides and different groups of soil organic matter (OM) was studied using the method of Orlov [10], which was based on stepwise treatment of the samples with acids and alkalines.
A group of substances composing OM usually implies the entity of compounds which have similar structure and properties. Soil OM includes the compounds of non-specific (individual) nature, which belong to different classes recognized by organic chemistry (cellulose, proteins, amino acids, monosugars, proteins, carbohydrates, waxes, resins, organic oxiacids and etc.) and specific humic substances (HS). The latter are deep-colored organic substances found in humus. There are several different groups of HS: (1) humic acids (HA), which are soluble only in alkaline media; (2) fulvic acids (FA), which are soluble in water, alkaline and acidic media. The latter include true FA as defined by Forsyth [11], which are extracted from acid-soluble fraction using activated carbon (BAU, activated carbon from birch). The groups are divided into fractions. Generally, fraction is any part of group, which differs from the other parts of the same group in terms of the form of bond with mineral soil components. For this reason, the solubility and potential mobility of radionuclides depends on the fraction they are associated with. In this study both for HA and FA the mobility of radionuclides decreases in the row F1a > F1 > F2 > F3.
Analysis scheme
Fraction 1 was extracted from a separate sample portion treated with 0.1 M NaOH during a day. The suspension was centrifuged at 6000 rpm and filtered through a Blue ribbon filter. For separating HA and FA 6 M HCl was added to alkaline solution to achieve pH 1–2, the solution was then heated to 70–80 °C, the precipitate was allowed to settle and then centrifuged. The precipitate of HA in the centrifuge tube was dissolved using hot solution of 0.1 M NaOH. The treatment was repeated until complete dissolution of the precipitate; FA and substances of non-specific nature remained in the acidic solution.
HA and FA (free or bound predominantly with mobile R2O3·nH2O (hydroxides of iron, manganese and aluminum)) and some substances of non-specific nature were transferred into fraction 1.
Isolation of fraction 1a (decalcified fraction)
Decalcification followed by isolation of fractions of organic substances was performed in a single soil portion. To perform decalcification, a weighed portion of the sample was treated several times with 0.1 M HCl or 0.1 M H2SO4, incubated and periodically stirred for 12–16 h, and the supernatant was separated from the soil using a centrifuge at 6000 rpm. The acidic treatment of the solid phase was repeated until the reaction for Ca+2 in the filtrate became negative (murexide indicator was used for this test). Low molecular weight acids of nonspecific nature and a part of FA (free or bound to mobile R2O3·nH2O) were transferred into the decalcified fraction.
The residue obtained after decalcifying was incubated with 0.1 M solution of NaOH during 12–18 h in order to isolate of the sum of fractions (1 + 2). Deep-colored alkaline solution was centrifuged and the extraction of HS was continued until poorly colored filtrate was obtained. 6 M HCl was added to alkaline deep-colored solution in order to achieve pH 1–2. Free or bound to mobile R2O3·nH2O and Ca+2 HA and FA were transferred into fraction (1 + 2). Acidic solution appearing after separation of HA was actually FA with admixture of low molecular organic substances of non-specific nature. The latter were separated using BAU carbon.
In order to obtain fraction 3, the soil residue after isolation of fraction (1 + 2) was treated with 0.02 M NaOH (the ratio of solid and liquid phases was 1:10) during 6-h heating using a water bath. After settling the suspension was centrifuged and filtered. HA and FA in the filtrate were separated as described above. HA and FA, which were strongly bound to hydroxides and mineral particles of the sample, were transferred into fraction 3.
The residue of the soil was ignited in a muffle at 500° and boiled with 7.5 M HNO3. The residue contained humic acids, which were strongly bound to hydroxides of chemical elements, mineral particles of soils, humines and various mineral compounds.
Non-specific low molecular organic compounds, the majority of which are found in FA1a and to less extent in FA (1 + 2), are an important group in soil organic matter. This group includes organic products generated by decomposing plant or animal material, root exudates, and the substances produced by numerous soil microorganisms. Non-specific compounds have the most rapid reaction towards the change of environment conditions, are easily digested and decomposed by microorganisns and therefore are the active component of soil humus.
In order to study the bond between radionuclides and the components of FA fractions (non-specific low molecular compounds of individual nature and FA), we separated them using BAU active carbon [12, 13]. BAU carbon was ground and sieved (pore size 1 and 0.5 mm) and granules 0.5–1 mm in size was collected. Carbon was twice heated with concentrated HF using a water bath to reduce ash content and each time the acid was evaporated to dryness, then it was twice treated with concentrated HCl which was then evaporated to wet residue and then carbon was washed with distilled water until the reaction for Cl− became negative.
Chromatographic columns (height 150 mm, diameter 10 mm) were filled with purified BAU carbon (the weight of air-dry carbon portion was 1.2 g). Acid solution of FA was passed through the columns with BAU carbon at the flow rate of 1–1.5 mL/min. The columns were flushed stepwise with 0.1 M solution (fraction A), 10 % solution of H2O in acetone followed by desoption with H2O and combined with acetone desorbate (fractions B + C) and final desorption with 0.1 M NaOH (fraction D). Fraction A included carbonic, oxicarbonic acids, aminoacids, purine bases, fraction B + C contained phenols, polyphenols, mono- and polysaccharides; fraction D desorbed FA, which were purified from low-molecular organic compounds of individual nature.
239Pu and 241Am were determined in alkaline and acidic soil extracts, using 0.5–1 mL aliquots of each of the resultant fractions. For radionuclide determination the aliquots were treated with concentrated HNO3 and H2O2 in order to remove the OM. 239Pu and 241Am isotopes were recovered by their coprecipitation with LaF3 in weakly acidic medium onto a track membrane (pore size 0.1 μm) followed by target measurement using an ANALIST spectrometer. The chemical yield was controlled using the spiked 242Pu and 244Cm labels. The error of radionuclide registration did not exceed 10–15 %. After the extraction of radionuclide-containing forms the soil residues were boiled with 7.5 M HNO3. VP-1AP anionite was used for preconcentration and radiochemical purification of 239Pu; after its separation 241Am was absorbed using polyarsenazo-n. Permanganate oxidation was used for the determination of organic carbon (Corg) in acidic extracts and humic acids, and bichromate method was used for the same purpose in case of soils [14, 15]. Total content of chemical elements in soils was determined using X-ray fluorescence.
Results and discussion
Homogenization of the considered samples is very important for revealing the speciation of radionuclides. The main difficulty of sample homogenization arises from the possible presence of highly active ‘hot’ particles, consisting of mixed uranium oxides, constructional materials, plutonium, and fission products [16]. For this reason, in the beginning we made a detailed γ-spectroscopy study of the samples using quartering procedure and found no’hot’ particles.
Tables 1 and 2 show the composition of the samples of soddy calcareous destructive soil, which were collected at the depth of 0–4 and 20–30 cm from a soil pit for the studying the speciation of 239Pu and 241Am.
The soil samples collected from the top and bottom horizons had significantly different physical and chemical properties. The top (0–4 cm) layer contained more total Si, Al, Fe and Mn, the bottom one (20–30 cm) had more alkali earth elements CaO, MgO and CO2 carbonate. Due to the very high content of the latter in the bottom layer treating the soil with acid led to intensive gas emission. Unlike the bottom horizon, the top horizon contained nearly six times more humus and more exchangeable Ca+2 and Mg+2 (Tables 1, 2).
We found that the most radionuclides were bound to inorganic soil components (mineral part) (Table 3). 33.4 % 239Pu and 25.3 % 241Am were in the OM (low molecular compounds, humic and FA, free and bound to Ca+2, R2O3·nH2O and other elements) of the top soil layer (0–4 cm). The content of radionuclides bound to OM in the bottom (20–30 cm) layer was low and did not exceed 3 % (Table 3). An interesting fact was the high stepwise recovery of 239Pu and 241Am from the soils with solutions of 0.5 M Ca(NO3)2 and then 1 M CH3COONH4, which could be probably associated with rather aggressive destruction of carbonate absorbing complex of soils with weakly acidic solutions of these reagents. The study of the soils of other genesis [7] found significantly less plutonium and americium in these fractions (3–5 % or less), but the value strongly depended on the duration of phase contact. Radionuclides in exchangeable and easily soluble form are probably retained in soils due to weak electrostatic interactions; their behavior is determined by the ion exchange processes and the change in the ionic composition of the medium and decrease in solution pH leads to mobilization (liberation) of the exchangeable ions. The content of 239Pu and 241Am in this group of substances was higher in the 20–30 cm soil layer than in the top layer.
Amorphous oxides of Fe+3, Al+3, Mn+2, and other elements in the considered soils contained mainly 239Pu (25–28 %), while 241Am was poorly bound to this group of compounds (Table 3).
The amount of 239Pu and 241Am in aqueous extract from the top and bottom soil horizon was low and did not exceed 0.1 %. (Table 3). This was a negligible fraction of radionuclides, which were bound to water-soluble metal compounds and soluble organic substances, mainly non-specific low molecular acids.
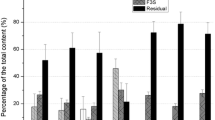
Distribution 239Pu, 241Am and Corg by groups and fraction of OM in the top and bottom soil layers was significantly different due to the difference in quality and quantity of OM in them (Fig. 1).
The content of 239Pu, 241Am and Corg in HA group of the top soil layer was higher than in the bottom layer and was 27.9, 7.8 and 57.3 %, respectively. The bottom soil layer nearly lacked HA (content of Corg <1 %) so no radionuclides were found in this fraction.
The content of 239Pu in FA group was lower in the top layer than in the bottom one (16.3 and 27.7 %, respectively). The content of 241Am in the top and the bottom layer did not differ significantly (66.7 % in the top layer and 54.5 % in the bottom one). The content of radionuclides and OM in the soil residue after multi-stage extraction of organic substances of various nature decreased in the rows 239Pu (44.2 %) > 241Am (25.5 %) > Corg (5.3 %) for the top layer and 239Pu (72.3 %) > 241Am (45.5 %) > Corg (31.8 %) for the bottom one.
Distribution of radionuclides by fractions of HA and FA, which had different forms of bond with mineral components of soils, also depended on the sample collection depth (Figs. 2, 3). In the top soil layer, which had more humus, the majority of 239Pu, 241Am and Corg was found in HA, which were mainly bound with Ca+2 (HA fraction 2): 78.8; 91.0 and 85.7 % of the content in the group, respectively.
In FA group of the considered soil the dominating content of radionuclides was present in fractions FA1a and FA1 (mobile fulvic and non-specific low molecular organic acids, free or bound mainly to mobile R2O3·nH2O and easily soluble mineral compounds of soils). For example, in the top soil layer (0–4 cm) the content of 239Pu and 241Am in these fraction was 54.6 and 84.2 %, in the bottom (20–30 cm) layer radionuclides and Corg also were mainly found in FA1a and FA1 fraction (74.3 and 90.3 % respectively).
The ratio of Corg in groups HA:FA in top and bottom soil layer was 1.5 and 0.01, such values demonstrating different nature of organic substance. In the top layer, which contained more humus, the OM had more humic properties while in the bottom soil layer the OM was depleted with HA and mainly consisted of free FA and mobile non-specific low molecular organic substances.
In order to reveal the bonds between radionuclides and FA and organic substances of non-specific (individual) nature in FA fraction (1 + 2), we separated them using BAU carbon (Fig. 4). We found that the acidic FA filtrate (1 + 2), absorbed by BAU carbon, contained a mixture of high molecular FA (fraction D) and low molecular organic substances of individual nature (fractions A and B + C). The content of organic carbon in fraction D was lower than its content in the sum of fractions A + B + C (Fig. 4).
As our data show, in the group of isolated FA in the soils of the considered type plutonium and americium were more bound with low molecular aminoacids, carbonic and oxicarbonic acids and purine bases than with FA themselves.
The data enable explaining the migration mechanisms of 239Pu and 241Am in calcareous soils of Yakutia. We found that 238−240Pu poorly migrated along the soil profile: 34 years after the contamination caused by Kraton-3 UNE only 9 % 238−240Pu was found in soil layers deeper than 0–4 cm, and as low as 0.8 % plutonium was found in the 20–30 cm-deep soil layer. One may assume that the dominating slow transfer of 239Pu and 241Am is actually their migration with soil fine and colloid particles during soil flushing with atmospheric precipitates. On the surface of soil particles with different sizes radionuclides can exist as poorly mobile organic compounds of humic nature, calcium humates, polymer complexes of HA and FA bound with stable hydroxides of Fe and Al. Slow transfer of radionuclides contributes to their dominating accumulation in humic layer of soil. Rapid transfer of 239Pu and 241Am in these soils can be associated with their migration as soluble complexes with low molecular organic acids of non-specific nature and FA (fraction 1a and 1), such assumption being supported by the absence of humate complexes of 239Pu and 241Am in the 20–30 cm soil layer. Slow transfer is the dominating type of migration in calcareous soil which is weakly alkaline.
Conclusions
The results evidence that 239Pu and 241Am introduced as labels into top and bottom layers of calcareous destructive soil collected in the zone affected by Kraton UNE (Yakutia) were strongly absorbed by soils and bound mainly with their solid phase consisting of various organic and inorganic components. As low as 0.1 % or less 239Pu and 241Am existed in water-soluble form in the calcareous soils from Yakutia, such value demonstrating the relatively low mobility of these radionuclides. Distribution of radionuclides between inorganic and organic soil components depended on their chemical composition (content of Corg, Ca+2, R2O3·nH2O etc.) and the properties of radionuclides. In the top (0–4 cm) humus-containing layer 239Pu was more uniformly distributed between organic and inorganic soil components (easily soluble compounds, OM and amorphous oxides of Fe and Al) than 241Am. In the bottom soil layer (20–30 cm) the radionuclides were mainly present in inorganic soil components. The estimation of the radionuclide mobility demonstrated that 241Am was potentially a more mobile element than 239Pu.
In terms of radionuclide migration in soils the most important natural organic compounds are HS and low-molecular substances of non-specific nature. HA and FA are known to have different migration pathways in soils. Fulvic substances and low-molecular substances of non-specific nature as well as their complexes with chemical elements (mainly Fe and Al) (fractions 1a and 1 FA) have the greatest migrational ability. In calcareous soils from Yakytia collected from the top layer (0–4 cm) 239Pu and 241Am exist both in HA and FA and 241Am is much stronger bound to FA group than 239Pu. As for the bottom soil layer, 239Pu and 241Am were found mainly in FA.
References
Erofeevskaya LA, Aleksandrov AR (2012) The influence of radio nuclides on the soil microbial community on the territory of the underground nuclear explosion 'Kraton- 3' (Yakutia). Arct North 8:1–11
Sobakin PI, Gerasimov YR, Chevychelov AP, Perk AA, Goryachenkova TA, Novikov AP (2014) Radioecological situation in the zone affected by accidental nuclear explosion Kraton-3 in Sakha (Yakutia) republic. Radiatsionnaya biologiya. Radioekologiya 54:1–9
Chevychelov AP, Sobakin PI, Molchanova IV (2006) Radioactive contamination of permafrost-affected soils with 137Cs and 90Sr, the products of an accidental underground nuclear explosion. Eurasian Soil Sci 39:1362–1369
Goryachenkova TA, Pavlotskaya FI, Myasoedov BF (1991) Forms of occurrence of plutonium in soils. J Radioanal Nucl Chem 147:153–157
Miller WP, Martens DC, Zelazny LW (1986) Effect of sequence in extraction of trace metals from soils. Soil Sci Soc Am J 50:598–601
Clark SB, Johnson WH, Malek MA, Serkiz SM, Hinton TG (1996) A comparison of se-quential extraction techniques to estimate geochemical controls on themobility of fission product, actinide, and heavy metal contaminants insoils. Radiochim Acta 74:173–179
Goryachenkova TA, Kazinskaya IE, Clark SB, Novikov AP, Myasoedov BF (2005) Comparison of methods for assessing plutonium speciation in environmental objects. Radiochemistry 47:599–604
Fedotov PS, Spivakov BY (2008) Fractionation of elements in soils, sludges and sediments: batch and dynamic methods. Russ Chem Rev 77:649–660
Novikov AP (2010) Migration of radionuclides in the environment. Geochem Int 48:1285–1398
Orlov DS (1992) Khimiya pochv [Chemistry of soils]. Moscow State University, Moscow
Forsyth W (1947) Studies on the more soluble complexes of soil organic matter. Biochem J 41:176
Varshal GM, Velyukhanova TK, Sirotkina IS, Yartseva RD (1973) Separation of fulvic acid by activated carbon. Fractionation, quantitative definition and study of certain major components of dissolved organic substances in natural waters. Gidrokhim Mat 59:143–151
Kazinskaya IE, Goryachenkova TA, Novikov AP, Vinokurov SE, Trkachev VV (2012) Association of radionuclides with components of fulvic acids isolated from soils. Radiochemistry 54:87–91
Pavlotskaya FI (1997) Main principles of radiochemical analysis of environment objects and methods for the determination of radionuclides of strontium and transuranium elements. Zh Anal Khim 52:126–143
Vorob’eva LA (1998) Khimicheskii analiz pochv [Chemical analysis of soils]. Moscow State University, Moscow
Dubasov YuV, Krivokhvatskii AS, Savonenkov VG, Smirnova EA (1991) Types pf fuel particles in the sediments of the near zone of Chernobyl nuclear station. Radiokhimiya 33:102–103
Tamm O (1934) Uber der oxalatmethode in der chemischen Boden analyse. Meda Fr Stat Shogsfürst 32:3–11
Acknowledgments
The research was financially supported by Russian Foundation for Basic research (Project No. 14-05-00181) and Ministry of Education and Science (Federal Targeted Program for Research and Development in Priority Areas of Development of the Russian Scientific and Technological Complex for 2014-2020 (Agreement No. 14.604.21.0095 UIS RFMEFI60414X0095).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novikov, A.P., Goryachenkova, T.A., Sobakin, P.I. et al. Speciation of plutonium and americium in the soils affected by Kraton-3 accidental underground nuclear explosion in Yakutia (Russia). J Radioanal Nucl Chem 307, 691–697 (2016). https://doi.org/10.1007/s10967-015-4195-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4195-1