Abstract
We are developing electron linear accelerator 100Mo(γ,n)99Mo technology as a replacement to nuclear reactor 235U(n,f)99Mo production. We report irradiation of natural molybdenum disks (25 MeV, 10 kW) and 100Mo-enriched disks (35 MeV, 2 kW), their dissolution and the extraction of 99mTc-pertechnetate. Up to 6.2 GBq 99Mo was produced, solvent extraction was performed at >90 % yields of 99mTc, and quality control showed that a product with high radionuclidic and radiochemical purity could be obtained. Irradiated natural molybdenum products showed more impurities (91mNb, 92mNb, 95mNb and 95Nb) than enriched target material. Linear accelerator technology is feasible for production of quality 99Mo/99mTc, particularly when paired with 100Mo-enriched targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The radioisotope technetium-99m is the most widely used worldwide in diagnostic nuclear medicine, thanks to its near-optimal characteristics (short half life [T 1/2 = 6.01 h], low-energy γ emission [E γ = 140 keV], versatile chemistry, and relatively low cost). It is used in about 80 % of medical diagnosis imaging procedures to detect and monitor pathologies of diverse physiological conditions such as myocardial perfusion, kidney disorders and bone metastases [1]. 99mTc is obtained exclusively from the β-decay of its parent molybdenum-99 (half life = 66.02 H). Prior to 2010, 75 % of the world’s supply of 99Mo was produced from the National Research Universal (NRU) reactor in Chalk River, Canada and the High Flux Reactor (HFR) in Petten, Netherlands. These reactors are over 50 years old and use highly enriched uranium (HEU), typically greater that 85 % 235U, in a neutron-induced fission process for production of 99Mo. The advanced age led to a number of recent critical shutdowns, most notably in 2009–2010 that affected both reactors [2–4]. In addition, HEU use and the highly radioactive waste generated are major proliferation and security concerns in the use of reactors for medical isotope production [5]. The age-related NRU shutdown also prompted the Government of Canada’s decision to end NRU isotope production by Oct. 31, 2016.
Several measures have been taken in recent years to mitigate proactively the type of crisis experienced in the 2009–2010 shutdowns and to resolve concerns on future 99Mo supply. There has been a ramp-up of capacity at smaller reactor suppliers (SAFARI in Pelindaba, South Africa; ANSTO in Lucas Heights, Australia; OSIRIS in Saclay, France; LVR 15 in Rez, Czech Republic and MARIA in Otwock, Poland). There has been establishment of 99Mo production at the Missouri University Research Reactor. And in Canada, there has been a push to develop new 99Mo/99mTc production technologies in Canada that do not involve uranium fission [6]. The Canadian endeavors are focused on two alternative non-reactor approaches: (i) the direct production of 99mTc via 100Mo(p,2n)99mTc nuclear reaction in cyclotrons [7] and the production of the 99Mo parent radionuclide by electron linear accelerator transmutation of 100Mo following the 100Mo(γ,n)99Mo scheme [8–11]. The Government of Canada has supported these approaches through two funding project phases—the Non-Reactor Isotope Supply Program (2010) and the Isotope Technology Acceleration Program (2012) [12].
The Health Sciences Centre (Winnipeg) has partnered with others at the not-for-profit Prairie Isotope Production Enterprise Inc. (PIPE), and with the Canadian Light Source Inc. in the development and evaluation of linear accelerator 100Mo(γ,n)99Mo transmutation technology. In this paper, we report on our experiences since 2009 on linear accelerator irradiations of natural molybdenum (composition 9.6 % of 100Mo) and of enriched 100Mo molybdenum; on dissolutions of the metal disks, separations of 99mTc-pertechnetate using an automated solvent extraction generator, and on quality control evaluations of the 99Mo and 99mTc products. Irradiations during this period were performed at two available sites with different linear accelerator configurations; at Mevex Corporation and at the National Research Centre. These accelerators were used pending the installation and commissioning of an electron linear accelerator at the Canadian Light Source that will be primarily dedicated to 100Mo(γ,n)99Mo isotope production. The solvent extraction technology deployed to the project had previously been utilized at the Health Sciences Centre for routine clinical 99Mo/99mTc separations of fission-reactor produced 99Mo [13].
Materials and methods
Reagent grade chemicals were purchased from Sigma-Aldrich (Oakville, ON) and used as received unless otherwise stated. Methylethyl ketone (MEK, ACP Chemicals, Montreal, QC) was distilled before use and pretreated with up to 5.5 % of 30 % hydrogen peroxide. The solvent generator purification column was packed with 15 g of acidic aluminum oxide (Brockmann #1TM, Sigma-Aldrich, Oakville, ON) previously pretreated by running 5 mL of physiological saline followed by 40 mL of pretreated MEK through the column. Radioactivity activity was determined using a dose calibrator (CRC®-55tR, Capintec, Ramsey, NJ) calibrated with 57Co and 137Cs sealed reference sources.
Molybdenum irradiation and processing
Sintered natural molybdenum disks (1-mm thick, 21-mm diameter, 2.55-g, Acsion Industries, Pinawa, MB) were pressed from natural molybdenum powder, which had in turn had been obtained by reducing molybdenum trioxide (MoO3, 9.6 % 100Mo isotopic abundance) in a furnace under hydrogen atmosphere. The disks were irradiated in a stack of 20 disks at 20 MeV, 10 kW, 24 h in an electron linear accelerator (Mevex, Stittsville, ON). Sintered enriched molybdenum disks (3-mm thick, 5-mm diameter, 0.5-g, per pellet, Industrial Materials Institute, National Research Centre, Boucherville) were pressed from enriched molybdenum powder 97.39 % 100Mo isotopic abundance, Trace Sciences International, Richmond Hill, ON). The disks were irradiated in a stack of 6–10 at 35 MeV, 2 kW, 12 h in an electron linear accelerator (Institute of Material Standards, National Research Council, Boucherville, QC).
One disk from each irradiation batch (usually the distal, lowest activity one) was retained and dedicated for time-lapse high purity germanium (HPGe) spectroscopy. The irradiated molybdenum disks were dissolved in Erlenmeyer flasks using hydrogen peroxide (60 mL, 30 % in water), the solvent was evaporated to a constant weight pellet, and the resulting molybdenum trioxide (MoO3) powder was dissolved in sodium hydroxide (40 mL, 5 M) solution. The final sodium molybdate (Na2MoO4, total volume 310 mL) solution was loaded into the solvent generator for 99mTc-pertechnetate extractions [9]. A sample of the Na2MoO4 solution was retained for HPGe gamma spectroscopy.
99Mo/99mTc solvent generator extraction
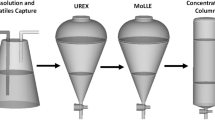
All 99mTc extractions were performed using refurbished solvent extraction technology that we in previous years had employed for fission 99Mo solutions, with the 99mTc products applied to clinical use [13]. The 99Mo/99mTc solvent extraction generator (Fig. 1) is controlled by software with real-time monitoring. It has two sections within the shielded core—the reaction vessel, and column housing; and two sections that are individually shielded and external to the core—the molybdenum inlet chamber and a 99mTc-pertechnetate collection vessel or a lead shielded transport pot on the rotating collection platform. The required amount of methylethyl ketone (MEK) is dispensed from a reservoir into the reaction vessel that already contains the 99Mo-sodium molybdate/99mTc-sodium pertechnetate mixture. After appropriate mixing and settling, the MEK layer carrying the 99mTc-sodium pertechnetate is siphoned by vacuum suction through an acidic aluminum oxide column that captures 99Mo-sodium molybdate remnants from the solution. Eluate from the alumina column goes into a Pb-shielded, heat-enabled stainless steel evaporation pot where the MEK is evaporated at 70–75 °C under reduced pressure to leave a white 99mTc-sodium pertechnetate powder. A variable user-defined volume of physiological saline is added to the evaporation vessel to dissolve the dried pertechnetate pellet for a colorless pertechnetate solution; and a liquid transfer mechanism moves the 99mTc-pertechnetate solution through a 0.22 m filter (terminal sterilization) into a sterile vial in the shielded collection pot. Multiple extractions of 99mTc- can be done from the same aqueous 99Mo stock in the reaction vessel, with replacement of the alumina column before each run. Samples of 99mTc-pertechnetate solutions (50 μL) from each extraction were taken for quality control, including HPGe spectroscopy.
Molybdenum breakthrough (the ratio of 99Mo to 99mTc) was assayed for all stock 99mTc eluates using a calibrated lead shield (Biodex Medical Systems, Shirley, NY). The levels of the organic solvent (MEK) in all sodium pertechnetate extracts were determined by an iodoform test. Inductive Conduction Plasma Optical Emission Spectroscopy (ICP-OES) analysis was performed on all 99mTc-pertechnetate extracts to determine chemical molybdenum levels.
HPGe spectroscopy
HPGe spectroscopy was performed regularly at fixed 15, 45 and 75 from the detector for a period up to 60 days. Distance used depended upon level of radioactivity, given the high sensitivity of the equipment. HPGe spectra for 99Mo samples from the dissolution and from the extracted 99mTc samples were similarly obtained.
ICP-OES analysis
ICP-OES analyses were performed on all 99mTc-pertechnetate extracts to determine chemical molybdenum levels (EM1 Gas-Solution Analytical Centre, Stanford, CA; detection limit for molybdenum 4 µg/L). The emission line use for sample analysis was 202.032 nm wavelength. Testing parameters were 1.5 kW power, 15.0 L/min plasma flow, 1.5 L/min auxiliary flow, and 0.75 L/min nebulizer flow. A reference standard calibration graph (0.005 to 1 mg/L) was used to correlate and calculate molybdenum concentration for all test samples. Three measurements are performed for every sample.
Results and discussion
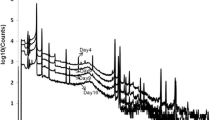
Irradiations of the natural molybdenum disks (25 g total weight) at 20 MeV, 10 kW yielded 1.2–1.5 GBq of 99Mo while irradiations of the enriched molybdenum disks (3 g total weight) at 35 MeV, 2 kW yielded 5-6.2 GBq 99Mo. Figure 2 shows the HPGe spectrum obtained for irradiated enriched 100Mo disks. The spectrum show peaks attributable only to Mo99 (peaks at 140, 180, 366, 739, 777, 822 and 960 keV). For irradiated natural molybdenum disks (Fig. 3), impurity peaks of 96Nb show up initially but decay rapidly due to shorter half-life (23.35 h). At 15 days, 96Nb peaks disappear and peaks for 91mNb, 92mNb, 95mNb and 95Nb are clearly prominent (t 1/2 = 60.86 days, 10.15 days, 86.6 h, 35.15 days, respectively). Although amounts were observed as being very low due with the predominant decay of the shorter-lived 99Mo, quantification of these impurities was not prioritized within this project as it is planned that irradiation at clinically relevant stages will be performed using the enriched 100Mo; a starting material that did not produce these radionuclidic impurities in detectable amounts (Fig. 2).
Dissolutions of the natural molybdenum disks were complete within 2 h, and were more rapid for the enriched molybdenum disks (approximately 30 min because of higher surface area-to-volume ratio). Average drying times were approximately 1 and 2 h for the natural and enriched molybdenum solutions, respectively.
Enriched 100Mo is currently available in North America only from two commercial sources, Isoflex (USA) and Trace Sciences International (USA, Canada). The lack of diverse commercial sources of enriched 100Mo is a potential vulnerability for alternative production technologies that require 100Mo as starting material. However, enriched 100Mo is non-radioactive and therefore can be stockpiled, only low amounts of the product would be needed at least in the initial stages of market adoption (approx. 1 kg in Canada), and recycling processes that allow for reutilization of stock have been reported to be effective and high-yielding [14].
The specific activity of 99Mo disks from the 100Mo(γ,n)99Mo in our irradiation runs were as low as 20 MBq 99Mo/gram molybdenum. This is very low compared to the specific activity obtained by thermal neutron fission of 235U (>370 TBq/g). Such low specific activity 99Mo/99mTc preparations require separation technologies different from the current commercial alumina columns as alumina has a capacity of only up to 20 mg/g molybdenum. Alternative technologies for separation, including solvent extraction, have been variously reviewed in literature [15–22]. MEK solvent extraction was described first by Gerlit in 1956 [23].
Due to the relatively low 99Mo stock radioactivity levels, generator elutions were generally performed once daily. 99mTc-pertechnetate extraction yields from the solvent generator were 91.7 ± 4.96 % (n = 26, range 83.8–99.3 %), with higher values obtained as the operators became more experienced with the procedures. After each extraction, a colorless pertechnetate solution in saline was obtained that can be sterilized within the generator by filtration.
Table 1 provides the quality control parameters of the 99mTc-pertechnetate eluate from the solvent extraction generator. The tests and limits are as stipulated by the United States Pharmacopeia (USP) and include the limits for MEK defined specifically for solvent–solvent generator extraction systems and a test for chemical purity (molybdenum composition).
All 99mTc-pertechnetate solutions (n = 35) from the passed the alumina test (less than 10 ug/ml). MEK levels were consistently less than 0.1 % (v/v). Figure 4 shows the typical spectra we obtained for the 99mTc-pertechnetate from the solvent generator. There are no radionuclidic impurities seen in these spectra, confirming 99mTc radionuclidic purity even after a decay of 24 h.
Three extractions failed the molybdenum breakthrough tests (average 0.329 MBq 99Mo/GBq 99mTc) and were not used for radiopharmaceutical preparation, and their HPGe spectra were confirmatory in showing corresponding 99Mo peaks. The challenge of very low 99Mo/total molybdenum specific activities in our preparations could logically be perceived to contribute to the few molybdenum failures observed. However, although the extraction process is dealing with very small radioactivities in large molybdenum mass amounts, we found that the more direct explanation was a deficiency in the extraction protocol. The molybdenum breakthrough failures were associated with higher pH values for the extracts (>7.5), suggesting that aqueous fractions of sodium molybdate (in 5 M NaOH) were eluting together with the organic MEK extraction layer. An adjustment of protocol to eliminate run-through of sodium molybdate during the loading phase seemed to have eliminated the breakthrough and higher-pH failure instances.
Conclusions
These proof-of-concept studies under non-optimized targeting and irradiation parameters show that linear accelerator technology is feasible for production of quality 99Mo/99mTc, particularly when paired with enriched 100Mo disks as targets for increased yields. 99Mo post-irradiation yields improved from a low of about 4–5 MBq/h/g/mA for natural molybdenum to 2.4–3 GBq/h/g/mA with enriched 100Mo. The larger target diameter for the natural molybdenum runs was almost certainly less efficient than the small enriched molybdenum disks and the enhanced yields are higher than can be solely attributed to the increased composition of 100Mo in the enriched target material. However, it is difficult to do an apples-to-apples comparison as the target and converter geometries and the beam energies and configurations were very different for the two linear accelerators. We expect to perform a more linear comparison between natural and enriched molybdenum yields using the recently commissioned 35 MeV, 40 kW linear accelerator at the Canadian Light Source dedicated to isotope production [24]. Future development of near-optimized production at the Canadian Light Source will also provide a clearer insight into the viability of this technology to have sufficient capacity to meet or substantially address Canadian patient demand. HPGe spectroscopic analyses of the 99Mo products (either as disks or after dissolution prior to extraction) showed differences in radionuclidic purity profiles for the two target materials, with the irradiated natural (but not the enriched) molybdenum showing 91mNb, 92mNb, 95mNb and 95Nb impurities. Significantly, solvent generator separation of the 99Mo/99mTc mixture is very efficient, with a pure 99mTc product obtained even from the more radioisotopically impure irradiated natural molybdenum disks. Extractions also gave average yields of 91.7 %, and no significant methylethyl ketone or alumina breakthroughs. Evaluation of the efficiency 99mTc-pertechnetate in the radiolabeling of commercial radiopharmaceutical kits and the quality of the resulting complexes is ongoing.
References
International Atomic Energy Agency (2008) Technetium-99m Radiopharmaceuticals: Manufacture of Kits. Technical Report Series 466. http://http://www-pub.iaea.org/mtcd/publications/pubdetails.asp?pubid=7867. Accessed 1 Feb 2015
MacLeod I. Secret U.S. weapon-grade uranium shipment to Chalk River near end. Ottawa Citizen, May 28, 2013. http://ottawacitizen.com/news/local-news/secret-weapons-grade-uranium-shipments-to-canada-near-end. Accessed 1 Feb 2015
International Atomic Energy Agency (2009) IAEA addresses global radioisotope shortage. J Nucl Med 50:13N–14N
Van Noorden R (2013) The medical testing crisis. Nature 504:202–204
Mitka M (2009) Medical isotope shortage. JAMA 302:732
Natural Resources Canada, Report of the Expert Review Panel on Medical Isotope Production, 30 November 2009. www.cins.ca/docs/panrep-rapexp-eng.pdf. Accessed February 1, 2015
Guérin B, Tremblay S, Rodrigue S, Rousseau JA, Dumulon-Perreault V, Lecomte R, van Lier JE, Zyuzin A, van Lier EJ (2010) Cyclotron production of 99mTc: an approach to the medical isotope crisis. J Nucl Med 51:13N
Bennet RG, Terry WK, Christian JD, Kirkham RJ, Petti DW (1999) A system of 99mTc production based on distributed electron accelerators and thermal separation. Nucl Technol 126:102–121
Mang’era K, Alina M, Barnard J, Omotayo A, Brown P, Martin J, Carlson P, Saunders C, Hayward P (2011) Production, separation and evaluation of 99mTc and Mo99 from accelerator transmutation of 100Mo. J Nucl Med 52(Suppl 1):1439
Naik H, Suryanarayana SV, Jagadeesan KC, Thakare SV, Joshi PV, Nimje VT, Mittal KC, Goswami A, Venugopal V, Kailas S (2013) An alternative route for the preparation of the medical isotope 99Mo from the 238U(ϒ, f) and 100Mo(ϒ, n) reactions. J Radioanal Nucl Chem 295:805–816
Pillai MRI, Knapp FF Jr (2011) Overcoming the 99mTc shortage: are options being overlooked? J Nucl Med 15:15N–28N
Natural Resources Canada, Government of Canada’s Action on our Medical Isotopes Supply. http://www.nrcan.gc.ca/energy/uranium-nuclear/7793. Accessed February 1, 2015
Billinghurst MW, Abrams DN, Dupont J (1992) A comparison of radiopharmaceutical labelling efficiency of chromatographic generator vs MEK extraction [99mTc] pertechnetate. Appl Rad Isot 43:1045–1049
Gagnon K, Wilson JS, Holt CM, Abrams DN, McEwan AJ, Mitlin D, McQuarrie SA (2012) Cyclotron production of 99mTc: recycling of enriched 100Mo metal targets. Appl Radiat Isot 70:1685–1690
Dash A, Knapp FF Jr, Pillai MRA (2013) 99Mo/99mTc separation: an assessment of technology options. Nucl Med Biol 40:167–176
Boyd RE (1987) Technetium generators: status and prospects. Radiochim Acta 41:59–63
Molinski VJ (1982) A review of technetium-99m generator technology. Int J Appl Radiat Isot 33:811–819
Mushtaq A (2012) Future of low specific activity molybdenum-99/technetium-99m generator. Curr Radiopharm 5:325–328
Chattopadhyay S, Sujata SD, Das M, Goomer NC (2008) Recovery of 99mTc from Na2[99Mo]MoO4 solution obtained from reactor-produced (n, γ) 99Mo using a tiny Dowex-1 column in tandem with a small alumina column. Appl Radiat Isot 66:1814–1817
Narasimham DVS, Mani RS (1976) Chemical and radiochemical evaluation of the purity of 99mTc extracted by MEK. J Radioanal Chem 33:81
Chattopadhyay S, Das SS, Barua L (2010) A simple and rapid technique for recovery of 99mTc from low specific activity (n, γ) 99Mo based on solvent extraction and column chromatography. Appl Rad Isot 68:1–4
Tsuchiya K, Mutalib A, Chakrov P, Kaminaga M, Ishihara M, Kawamura H (2012) Status of 99Mo-99mTc Production Development by (n,γ) Reaction. In: Proceeding of the 4th International symposium in material Testing Reactors JAEA-Conf 2011–003, p 137–141
Gerlit TB (1955) Some chemical properties of technetium. In: Proceedings of International Conference on the Peaceful uses of Atomic Energy, Geneva, 7:145
CTV News Saskatoon (2014) Saskatoon scientists make medical isotopes without nuclear reactor. http://saskatoon.ctvnews.ca/saskatoon-scientists-make-medical-isotopes-without-nuclear-reactor-1.2102945. Accessed 1 Feb 2015
Acknowledgments
This research was supported by funding from Natural Resources Canada under the Non-Reactor Isotope Supply Program and the Isotope Technology Acceleration Program. Irradiation work at Mevex Corporation and the National Research Council, Ottawa is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mang’era, K., Ogbomo, K., Zriba, R. et al. Processing and evaluation of linear accelerator-produced 99Mo/99mTc in Canada. J Radioanal Nucl Chem 305, 79–85 (2015). https://doi.org/10.1007/s10967-015-3997-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-3997-5