Abstract
To explore the possible application of graphene oxide (GO) as a suitable adsorbent for the removal of U(VI) in solutions, sorption of uranium on GO was investigated as a function of pH, ionic strength, foreign ions and U(VI) concentrations using a batch technique. The results showed that the sorption of U(VI) was strongly pH-dependant and the influences of foreign ions were not significant. The sorption isotherm can be described by the Langmuir model, and the thermodynamic parameters indicated that the sorption process was endothermic and spontaneous. The results suggest that GO is a promising adsorbent for U(VI).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Graphene nanosheets (GNS), with hexagonal, sp2-hybridized and one-atom-thick layers structure, is a newly emerging member of carbon materials [1]. As one kind of graphene nanosheets derivatives, graphene oxide (GO), possessing multiple oxygen containing functional groups such as carbonyls and carboxyl groups presumably at the plane edges and hydroxyls and epoxides in the basal plane [2], can be easily prepared by chemical modification. The exist of the oxygen-containing functional groups of GO leads to its good dispersion in water [3, 4], which is essential for the binding of metal ions. Moreover, the two dimensional structure, with a lateral size extending up to several micrometers and one to several carbon atoms thickness, gives GO a high specific surface area [5]. Considering the abundant oxygen-containing functional groups and the high surface area, GO possesses a high adsorption capacity for some gas [6–9], metal ions [5, 10, 11] and radionuclides [12–14]. For example, Zhao et al. [5, 10] verified that the maximum sorption capacities of Pb(II), Cd(II) and Co(II) on few-layered graphene oxide nanosheets were higher than any other modern reported materials, and speculated that the abundant oxygen-containing functional groups on the surfaces of graphene oxide nanosheets played an important role on the sorption process. Romanchuk et al. [14] also demonstrated that GO is far more effective in removing transuranium elements from simulated nuclear waste than other used sorbents such as activated carbon and bentonite clays.
Uranium is an important nuclear material and contaminant, concerning the sites of uranium mining and the whole nuclear fuel cycles. Excessive amounts of uranium have entered into environment through the activities of nuclear industry [15]. Under oxidizing conditions, uranium is highly soluble due to the formation of a numerous U(VI) complexes in aqueous solutions [16]. Thus, the sorption of U(VI) on various minerals has been extensively studied [17–22]. Cheng et al. [22] measured the sorption capacity of U(VI) on GO, sepiolite, and sepiolite@GO, and found the sorption capacity decreased in the order of sepiolite@GO > GO > sepiolite. Zhao et al. [23] and Li et al. [24] studied the sorption behaviors of U(VI) onto GO with the background solution of NaClO4, and found that the sorption process was endothermic and spontaneous. Whereas, the effects of solid-to-liquid ratio (m/V), foreign ions, or ionic strength and so on has not been extensively investigated, leading to the status of not well understood the sorption behavior of uranium on GO.
In this paper, with the aim to highlight the possible applications of GO as a suitable adsorbent for the removal of U(VI) in solutions and enrich the database of GO and U(VI) sorption, we prepared few-layered graphene oxide nanosheets from natural flake graphite using a modified Hummers method, investigated the influence of pH, ionic strength, solid-to-liquid ratio (m/V) and foreign ions on the sorption of U(VI) onto GO with NaCl as the background solution. Furthermore, the sorption kinetics was studied, the sorption isotherms of U(VI) on GO were obtained, and the experiment data was fitted with the Freundlich and Langmuir models.
Experimental
Materials
The natural flake graphite (325 mesh) was supplied by alfa aesar (99.8 % metals basis). GO was synthesized from flake graphite by modified Hummers method [2, 25, 26], which has been presented in our previous work [27]. Uranium stock solutions were prepared from UO2(NO3)·6H2O (A.R. grade). All other chemicals used were of analytical grade. Deionized water was used in all experiments.
Sorption experiments
All sorption experiments were carried out at 25 ± 2 °C. A stock suspension of GO (m/V = 0.5 g L−1) was prepared in NaCl solution (0.01, 0.1, 0.5 mol L−1). The pH of the suspension was adjusted with negligible volumes of 0.1 or 0.01 M HCl or NaOH solutions to achieve the desired pH values. The tubes were then made up to 6 mL with U(VI) stock solutions and shaken for 2 days. The suspension was separated by centrifugation at 18,000×g for 30 min. Arsenazo(III) was used to determine the concentrations of U(VI) via the UV method, and the absorbance of the U(VI)-arsenazo III complex was measured at 652 nm against the reagent blank solution.
The formulas for calculating the solid phase concentration (q, mol g−1) and the distribution coefficient (K d , mL g−1) are identical to the previously employed formulas:
where C 0 (mol L−1) is the initial concentration of U(VI) in the solution, C aq (mol L−1) is the concentration of U(VI) in the supernatant after equilibrium, and V (L) is the volume of aqueous solution and m (g) is the mass of GO.
Results and discussion
Characteristics of the sorbents: GO
The GO was characterized by TEM, AFM, Raman, TGA, XPS and XRD. The pHpzc of GO was determined to be 2.09 from the mass titration technique, and the surface area of GO was 36.56 m2 g−1 by BET analysis. The pore size and pore volume calculated from the BET model were 4.70 nm and 4.30 × 10−2 cm3 g−1, respectively. It can be observed from the TEM and AFM image of GO [27] that the thickness of the sorbent is only several nanometers and the layers are nearly transparent with some crumples [28, 29], and the GO nanosheets have a size of 300 nm–2 μm with a height of 1 nm.
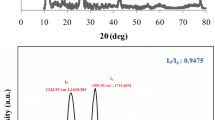
The Raman spectra of GO is shown in Fig. 1 [27]. The two prominent peaks at 1,322 and 1,595 cm−1 are corresponding to the D band [30] and the G band, respectively. The D band originates from the stretching vibration of sp3 carbon atoms, which induces disorders and defects, whereas the G band originates from the stretching vibration of sp2 carbon atoms, corresponding to the first-order scattering of the E2g mode [31]. Shen et al. [32] studied the Raman spectrum of graphite and GO, and found that the Raman spectrum of GO exhibited a D-band peak at 1,340 cm−1 due to the breathing mode of κ-point phonons of A1g symmetry, and a G-band peak at 1,560 cm−1 that corresponds to the first-order scattering of the E2g phonons.
The Raman spectra of GO [27]
The TG and differential thermal analysis (DTA) curves were measured using a SDT Q600 thermobalance (TA Instruments) under N2 gas flow (100 mL min−1) and at a heating rate of 10 °C min−1. As shown in Fig. 2, the mass loss is about 15 % below 110 °C, which is ascribed to the desorption of adsorbed free water on the GO. The second mass loss, also the main mass loss (~20 %), takes place around 230 °C, presumably due to the loss of CO, CO2 and steam from the pyrolysis of the labile oxygen-containing functional groups [4, 23]. And the third one is a steady mass loss process over the whole temperature range above 255 °C, which can be attributed to the removal of more stable oxygen-containing functionalities.
Figure 3 shows the X-ray powder diffraction (XRD) patterns of the GO and graphite samples. For natural flake graphite, the peak at 2θ = 26.5° is contributed to the (002) [28, 33], and the peak at a relative low angle, i.e., 2θ = 13.3°, indexed to the GO (001). Therefore, it can be speculated that the graphite had successfully transformed into GO. The sample before and after sorption was characterized by XPS (Table 1). The binding energies (BEs) of O1s before and after sorption were 532.79 and 532.63 eV [27], respectively, which can be explained as a decrease of electron density around the O atom resulting from the electron transfer to U(VI). The process can be described as follows:
Sorption kinetics of U(VI) on GO
U(VI) sorption percentage as a function of contact time is shown in Fig. 4. As can be seen from Fig. 4, sorption of U(VI) on GO increases very quickly at the initial stage, then the sorption maintains at a certain level with increasing time. The sorption behavior is similar to the sorption of Pd(II) and Cd(II) onto GNS [29], suggesting that the sorbent has high adsorption efficiency and high-value of potential industrial applications. The high adsorption rate within the initial 5 min was attributed to the nonporous laminated structure and large external surface of GNSs [1]. It is peculated that the sorption process is much more like a chemical one. Therefore, time of 24 h was selected to achieve sorption equilibration in the following experiments.
To analyze the sorption rate of U(VI) on GO, a pseudo-second-order rate equation was used to simulate the adsorption kinetics:
where \({k_{2}^{\prime}}\)/g mol−1 h−1) is the pseudo-second-order rate constant of sorption, q t (mol g−1 of dry weight) is the amount of U(VI) adsorbed on the surface of GO at time t. q e (mol g−1 of dry weight) is the equilibrium adsorption capacity of U(VI) on GO. A linear plot of t/q t versus t was achieved and shown in Fig. 4. The k′ and q e values calculated from the slope and intercept were 5.68 × 106 g mol−1 h−1 and 3.75 × 10−5 mol g−1, respectively. The low value of k′ suggests that the sorption equilibrium of U(VI) can be rapidly achieved [18], and the high correlation coefficient (R 2 = 0.9994) indicates that the adsorption kinetic is well described by a pseudo-second-order rate equation.
Effect of pH
pH is an important parameter for the sorption of U(VI) on the porous adsorbent, and it can influence the metal speciation both in aqueous and solid phases [34]. As shown in Fig. 5, the sorption of U(VI) on GO increases sharply from 30 % to nearly 100 % in the pH range of 2–5, and then maintains constant at about pH 5–7.5, whereas a decreased is observed after pH > 7.5. The sorption trends can be interpreted by the surface property of GO and the speciation distribution of U(VI) in solution.
Figure 6 shows the U(VI) speciation distribution at [U(VI)]TOT = 3.37 × 10−4 mol L−1 and [NaCl] = 0.1 mol L−1 in the absence of GO, calculated by CHEMSPEC [35]. It is not difficult to find that at pH < 4, the predominant uranium species is UO2 2+. As the pH of a uranium solution increases, the uranyl ions are easily hydrolyzed, and the hydrolysis products are also polymerized, consequently, the main species are multinuclear hydroxide complexes such as (UO2)3(OH) +5 , (UO2)4(OH) +7 and (UO2)3(OH) −7 . Particularly, solid species of schoepite can be observed in the pH range of 6.5–9. Moreover, the negative U(VI) species of (UO2)3(OH) −7 and UO2(OH) −3 are observed and their concentrations increase with pH increasing after pH > 7.5.
Moreover, the abundant oxygen-containing functional groups of GO were demonstrated to play crucial roles in the sorption process. Deng et al. [29] studied the effect of pH on the sorption of Pb(II) and Cd(II) on GNSPF6, and they found the pHpzc was 3.7 and there were 5.0 mmol g−1 acidic sites onto GNSPF6. pHpzc can be used to explain the effect of pH on the adsorption of metal ions, and the pHpzc of GO is determined to be 2.09 [27]. In aqueous systems, the concentrations of surface species (≡SOH uncharged surface groups, ≡SOH2 + positive charged surface groups, ≡SO− negatively charged groups) change at different pH values. When the pH value is higher than the pHpzc (2.09), the surface species of GO are the negatively charged groups ≡SO− and the electrostatic forces between the adsorbate ions and adsorbent surface are attractive, which is beneficial for the sorption of U(VI) on GO [27]. With pH increasing, the amount of negatively charged ≡SO− groups increases, and the hydrolysis of U(VI) is enhanced as well, leading to the increase of U(VI) sorption [19, 23].
It should be noted that when pH is between 2 and 7.5,the dominant species of U(VI) are all positively charged, and in the pH range of 2–5, the amount of the dominated species (bivalent cations UO2 2+ and (UO2)2(OH) 2+2 ) is nearly unchanged, thus the increased negative charge on GO surface dominated the sorption process and showed a significant increasing of sorption with pH increased. Whereas in the pH range of 5–7.5, monovalent cations (UO2)3(OH) +5 and (UO2)4(OH) +7 and solid species of schoepite are the main species of U(VI), it is probably that the combined interaction between the less positively charged uranium ions and the more negatively charged GO surface leading to the negligible change in the sorption percentage.
Specially, the suppressed sorption of U(VI) on GO at pH > 7.5 is due to the electrostatic repulsion forces between the negative U(VI) species ((UO2)3(OH) −7 and UO2(OH) −3 ) and the negative surface of GO, and also contributed by the decrease of the solid species of schoepite.
Sorption isotherms
Figure 7 shows the adsorption isotherms of uranium on GO at an initial concentration of 1.6 × 10−4–2.4 × 10−3 mol L−1 for UO2 2+. The experimental data was analyzed using Langmuir and Freundlich models, respectively. The Langmuir model assumes that there is no interaction between the sorbate molecules and the sorption is localized in a monolayer, whereas the Freundlich isotherm model is an empirical model allowing for multilayer adsorption on adsorbent [29]. The equations of the Langmuir (Eq. 5) and Freundlich (Eq. 6) models are expressed as follows:
q (mol g−1) is the amount of U(VI) sorbed per mass unit of GO. q m (mol g−1) is the maximum adsorption capacity of U(VI) per unit weight of adsorbent, C e (mol L−1) is the concentration of U(VI) in the supernatant after equilibrium, and k f and k d are isotherm constants. The separation factor R L [36] is a dimensionless constant used to predict if the adsorption is favorable or unfavorable, and is defined as.
The relative parameters and correlation coefficients (R 2) are listed in Table 2. The maximum adsorption capacity of U(VI) on GO is approximately 3.96 × 10−4 mol g−1 at pH 3.45 and T = 298 K calculated from the equation of Langmuir model. The value of R L is in the range 0 < R L < 1, indicating that the sorption is favorable. The higher correlation coefficients indicates that the sorption data can be better fitted by the Langmuir model than the Freundlich model, suggesting that the sorption of U(VI) on GO is monolayer adsorption.
The influence of temperature on U(VI) sorption isotherms on GO at pH 3.45 is also given in Fig. 7. As can be seen, the adsorption capacity of U(VI) on GO is the highest at T = 340 K and lowest at T = 298 K, indicating that higher temperature can promote the sorption process. The thermodynamic parameters (standard Gibbs free energy ∆G °, standard enthalpy ∆H °, and standard entropy ∆S °) for the sorption of U(VI) on GO can be evaluated by the sorption isotherm data obtained at different temperatures. The ∆G ° can be calculated from Eq. (8) [37].
where R is a constant (8.314 J mol−1 K−1) and T is temperature in Kelvin. The sorption equilibrium constant K ° can be obtained by plotting ln K ° versus Ce and extrapolating Ce to zero.
The ∆H ° and ∆S ° can be calculated by Eq. (9):
The obtained thermodynamic parameters of U(VI) sorption on GO are listed in Table 3. The negative values of ∆G ° (−23.54, −24.88, −25.93 kJ mol−1 at 298, 313 and 340 K, respectively) indicate that the sorption of U(VI) on GO is thermodynamically feasible and a spontaneous process [38, 39]. The values of ∆G ° decrease with increasing temperature, demonstrating that the sorption can be promoted at higher temperature, since at higher temperature, ions are more desolvated and the sorption becomes more favorable. The positive ∆H ° value confirms that the sorption of U(VI) on GO is endothermic in nature, which is in good agreement with the previous work [22–24, 37]. Generally, dehydration of the metal ions from their hydration sheath is an energy required process, thus it is an endothermic process and is favored at high temperature, while the attachment of ions to the surface of the adsorbent is an exothermic process [40]. Therefore, the positive ∆H ° value can be attributed to the energy of dehydration of metal ions exceeds the exothermicity of the ions attaching to the surface [40]. So it appears that the endothermicity of desolvation process of the U(VI) ions exceeds that of the enthalpy from U(VI) sorption on GO by a considerable extent. The positive value of ∆S ° suggests that the sorption process is spontaneous, implies that some structural changes may happen to GO during the sorption process, which leads to an increase in the disorder of the solid-solution system after sorption [37].
Effects of ionic strength and solid-to-liquid ratio
To study the influence of ionic strength on the sorption of U(VI) to GO, NaCl was used as a background electrolyte. As shown in Fig. 8, the sorption of U(VI) on GO is sensitive to the change of ionic strength, revealing that the sorption percentage decreased with increasing of ionic strength. It maybe due to that the ionic strength can affect the double layer thickness and interface potential, thus affecting the binding of the sorbed species. As the ionic strength increases, the generated double layer will be thinner, which will limit the interaction between the adsorbate and the adsorbent surface and thereby decrease the adsorption [41]. Theoretically, when the electrostatic forces between the adsorbent surface and the adsorbate ions are attractive, an increase in ionic strength will decrease the adsorption capacity. As is the case in our system, when studying the effect of ionic strength at pH 3.45 ± 0.05, the surface charge of GO (pHpzc 2.09) is negative, and the charge of U(VI) ions is positive, so the increase in ionic strength will decrease the attractive forces between the surface of GO and the U(VI) ions and decrease the adsorption capacity. Sahu et al. [42] found that the zeta potential decreased from 34.3 to 25.7 mV for 1 wt% Cu–TiO2 and from 28.3 to 16.9 mV for 3 wt% Cu–TiO2 respectively, when ionic strength increased from 0.001 to 0.1 M. The decreased zeta potential and increased hydrodynamic diameter (HD) with increasing ionic strength can be explained by the change in Debye length, which affects the electrostatic repulsive force. Generally, ion exchange and outer-sphere complexes may be affected more by ionic strength than inner-sphere complexes. However, in our previous study, we found that the sorption of U(VI) on goethite [19] and gamma-alumina [20] are insensitive to ionic strength.
The influence of solid-to-liquid ratio (m/V) on the sorption of U(VI) to GO is shown in Fig. 9. The result indicates that m/V plays an important role in the sorption process of U(VI) on GO, showing that when the m/V ratio is lower than 1 g L−1, the higher the m/V is, the higher the sorption percentage is, and the U(VI) sorption percentage reaches nearly 100 % and maintains constant after m/V ratio is larger than 1 g L−1. The increased sorption percentage of the U(VI) ions with increasing GO dose is due to the greater surface area and more available active sorption sites with increased GO dose.
Effects of foreign anions or cations
In order to investigate the influence of background electrolyte ions on U(VI) sorption, the sorption of U(VI) on GO was studied as a function of pH in 0.1 mol L−1 NaClO4, NaCl, NaNO3, KCl and LiCl, respectively, and the results are shown in Figs. 10 and 11. It can be seen that both the foreign anions and cations can affect the sorption of U(VI) on GO. From Fig. 10, it seems that the sorption percentage of uranium on GO is the lowest in 0.1 mol L−1 NaClO4 solution and highest in 0.1 mol L−1 NaCl solution at pH < 4.5, but the difference between the three electrolyte solutions is not significant. Although the inorganic acid radical radius order is Cl− < \({\rm NO}_{3}^{-}\) < \({\rm ClO}_{4}^{-},\) the effect of Cl−, \({\rm NO}_{3}^{-}\) and \({\rm ClO}_{4}^{-}\) on U(VI) sorption to GO is weak. The results are similar to the sorption of 60Co(II) on γ-Al2O3 [43] and radiocobalt sorption onto montmorillonite [44], but different to that of 109Cd(II) sorption to MnO2 [45] and Pb(II) sorption onto attapulgite [46].
On the other hand, as can be seen from Fig. 11, the sorption percentage of uranium is the highest in KCl and lowest in LiCl solution under the same pH values at pH < 4.5, and the influence is not very significant as well. Wang et al. [47] investigated the effects of Na+ and K+ on Zn(II) sorption to GO and also found a similar result. The sorption of U(VI) on GO can be considered as a competition of U(VI) with foreign cations (Li+, Na+ and K+) at GO surface. The results might due to the fact that the radius of Li+ is smaller than those of Na+ and K+, hence Li+ has the highest affinity to the surface of GO and the highest tendency for counter-ion exchange with the surface groups of GO, which will reduce the ion interaction sites on the surface of GO with uranium [48, 49].
Particularly, compared to the work conducted by Cheng et al. [22] and Li et al. [24], we have expanded the sorption data and some explanation to the sorption phenomenon, and also proved that the sorption of U(VI) on GO is universal to some extent, which can be used in many different background electrolyte systems (such as HClO4, HCl and HNO3) and can hardly be influenced by foreign cations such as Na+ and K+. From this point of view, GO might be considered as a suitable sorbent for the removal of U(VI) in polluted solution in the near future.
Conclusion
In summary, GO was prepared from natural graphite using a modified Hummers method and characterized by a series of methods. The sorption of U(VI) on GO was strongly pH-dependant, which suggested that the surface complexation contributed mainly to the sorption of U(VI). The sorption process can be described by a pseudo-second-order rate model. Langmuir model fitted the sorption data better than the Freundlich model and the maximum sorption capacity of U(VI) on GO at pH 3.45 and T = 298 K was approximately 3.96 × 10−4 mol g−1. The sorption percentage of U(VI) on GO decreased with increasing of ionic strength and decreasing of solid-to-liquid ratio. The negligible effects of foreign ions on U(VI) sorption to GO indicated that GO could be widely used in different background electrolyte systems (such as HClO4, HCl and HNO3). The rapid sorption kinetics and high sorption capacity of U(VI) on GO suggests that the sorbent has potential applications.
References
Huang ZH, Zheng X, Lv W, Wang M, Yang QH, Kang F (2011) Adsorption of lead(II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 27:7558–7562
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217–224
Si Y, Samulski ET (2008) Synthesis of water soluble graphene. Nano Lett 8:1679–1682
Paredes JI, Villar-Rodil S, Martinez-Alonso A, Tascon JMD (2008) Graphene oxide dispersions in organic solvents. Langmuir 24:10560–10564
Zhao GX, Ren XM, Gao X, Tan XL, Li JX, Chen CL, Huang YY, Wang XK (2011) Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans 40:10945–10952
Balog R, Jørgensen B, Wells J, Lægsgaard E, Hofmann P, Besenbacher F, Hornekær L (2009) Atomic hydrogen adsorbate structures on graphene. J Am Chem Soc 131:8744–8745
Kim G, Jhi SH (2009) Ca-decorated graphene-based three-dimensional structures for high-capacity hydrogen storage. J Phys Chem C 113:20499–20503
Leenaerts O, Partoens B, Peeters FM (2008) Adsorption of H2O, NH3, CO, NO2, and NO on graphene: a first-principles study. Phys Rev B 77:125416–125421
Romero HE, Joshi P, Gupta AK, Gutierrez HR, Cole MW, Tadigadapa SA, Eklund PC (2009) Adsorption of ammonia on graphene. Nanotechnology 20:245501
Zhao GX, Li JX, Ren XM, Chen CL, Wang XK (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462
Yang ST, Chang YL, Wang HF, Liu GB, Chen S, Wang YW, Liu YF, Cao AN (2010) Folding/aggregation of graphene oxide and its application in Cu2+ removal. J Colloid Interface Sci 351:122–127
Sun YB, Wang Q, Chen CL, Tan XL, Wang XK (2012) Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol 46:6020–6027
Sun YB, Shao DD, Chen CL, Yang SB, Wang XK (2013) Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ Sci Technol 47:9904–9910
Romanchuk AY, Slesarev AS, Kalmykov SN, Kosynkin DV, Tour JM (2013) Graphene oxide for effective radionuclide removal. Phys Chem Chem Phys 15:2321–2327
Yusan SD, Akyil S (2008) Sorption of uranium (VI) from aqueous solutions by akaganeite. J Hazard Mater 160:388–395
Yusan SD, Erenturk SA (2011) Sorption behaviors of uranium (VI) ions on α-FeOOH. Desalination 269:58–66
Davis JA, Meece DE, Kohler M, Curtis GP (2004) Approaches to surface complexation modeling of uranium (VI) adsorption on aquifer sediments. Geochim Cosmochim Acta 68:3621–3641
Drot R, Roques J, Simoni É (2007) Molecular approach of the uranyl/mineral interfacial phenomena. C R Chim 10:1078–1091
Guo ZJ, Li Y, Wu WS (2009) Sorption of U(VI) on goethite: effects of pH, ionic strength, phosphate, carbonate and fulvic acid. Appl Radiat Isot 67:996–1000
Guo ZJ, Yan C, Xu J, Wu WS (2009) Sorption of U(VI) and phosphate on γ-alumina: binary and ternary sorption systems. Colloid Surf A 336:123–129
Gao L, Yang ZQ, Shi KL, Wang XF, Guo ZJ, Wu WS (2010) U(VI) sorption on kaolinite: effects of pH, U(VI) concentration and oxyanions. J Radioanal Nucl Chem 284:519–526
Cheng HX, Zeng KF, Yu JT (2013) Adsorption of uranium from aqueous solution by graphene oxide nanosheets supported on sepiolite. J Radioanal Nucl Chem 298:599–603
Zhao GX, Wen T, Yang X, Yang SB, Liao JL, Hu J, Shao DD, Wang XK (2012) Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions. Dalton Trans 41:6182–6188
Li ZJ, Chen F, Yuan LY, Liu YL, Zhao YL, Chai ZF, Shi WQ (2012) Uranium(VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chem Eng J 210:539–546
William S, Hummers J, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Hirata M, Gotou T, Horiuchi S, Fujiwara M, Ohba M (2004) Thin-film particles of graphite oxide 1: high-yield synthesis and flexibility of the particles. Carbon 42:2929–2937
Li Y, Wang CL, Guo ZJ, Liu CL, Wu WS (2014) Sorption of thorium(IV) from aqueous solutions by graphene oxide. J Radioanal Nucl Chem 299:1683–1691
Yang JT, Wu MJ, Chen F, Fei ZD, Zhong MQ (2011) Preparation, characterization, and supercritical carbon dioxide foaming of polystyrene/graphene oxide composites. J Supercrit Fluid 56:201–207
Deng XJ, Lü LL, Li HW, Luo F (2010) The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method. J Hazard Mater 183:923–930
Pimenta MA, Dresselhaus G, Dresselhaus MS, Cancado LG, Jorio A, Saito R (2007) Studying disorder in graphite-based systems by Raman spectroscopy. Phys Chem Chem Phys 9:1276–1290
Wang J, Chen ZM, Chen BL (2014) Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ Sci Technol 48:4817–4825
Shen JF, Hu YZ, Shi M, Lu X, Qin C, Li C, Ye MX (2009) Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem Mater 21:3514–3520
Matsuo Y, Fukunaga T, Fukutsuka T, Sugie Y (2004) Silylation of graphite oxide. Carbon 42:2117–2119
Sharma P, Tomar R (2008) Synthesis and application of an analogue of mesolite for the removal of uranium(VI), thorium(IV), and europium(III) from aqueous waste. Microporous Mesoporous Mater 116:641–652
Zhu JB, Wang XY, Chen T, Liu CL (2012) Chemical species analysis software CHEMSPEC(C++) and its applications. Sci China-Chem 42:856–864
Hu BW, Cheng W, Zhang H, Yang ST (2010) Solution chemistry effects on sorption behavior of radionuclide 63Ni(II) in illite–water suspensions. J Nucl Mater 406:263–270
Ding CC, Cheng WC, Sun YB, Wang XK (2014) Determination of chemical affinity of graphene oxide nanosheets with radionuclides investigated by macroscopic, spectroscopic and modeling techniques. Dalton Trans 43:3888–3896
Bhattacharyya KG, Gupta SS (2006) Adsorption of Fe(III) from water by natural and acid activated clays: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Adsorption 12:185–204
Monash P, Pugazhenthi G (2009) Adsorption of crystal violet dye from aqueous solution using mesoporous materials synthesized at room temperature. Adsorption 15:390–405
Tahir SS, Rauf N (2003) Thermodynamic studies of Ni(II) adsorption onto bentonite from aqueous solution. J Chem Thermodyn 35:2003–2009
Yee N, Fein JB, Daughney CJ (2000) Experimental study of the pH, ionic strength, and reversibility behavior of bacteria–mineral adsorption. Geochim Cosmochim Acta 64:609–617
Sahu M, Suttiponparnit K, Suvachittanont S, Charinpanitkul T, Biswas P (2011) Characterization of doped TiO2 nanoparticle dispersions. Chem Eng Sci 66:3482–3490
Zhang CC, Liu ZJ, Chen L, Dong YH (2012) Influence of pH, humic acid, ionic strength, foreign ions and temperature on 60Co(II) sorption onto γ-Al2O3. J Radioanal Nucl Chem 292:411–419
Wen T, Chen YT, Cai LS (2011) Impact of environmental conditions on the sorption behavior of radiocobalt onto montmorillonite. J Radioanal Nucl Chem 290:437–446
Liu Y, Xu JZ (2013) Effect of environmental conditions on 109Cd(II) sorption to MnO2. J Radioanal Nucl Chem 295:1001–1008
Chi YL, Chen YT, Liu X, Guo ZJ, Xiao J, Xu JB, Zhao WH (2013) Impact of environmental conditions on the sorption behavior of Pb(II) onto attapulgite. J Radioanal Nucl Chem 295:1589–1596
Wang H, Yuan XZ, Wu Y, Huang HJ, Zeng GM, Liu Y, Wang XL, Lin NB, Qi Y (2013) Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl Surf Sci 279:432–440
Fan QH, Shao DD, Hu J, Wu WS, Wang XK (2008) Comparison of Ni2+ sorption to bare and ACT-graft attapulgites: effect of pH, temperature and foreign ions. Surf Sci 602:778–785
Yang ST, Li JX, Shao DD, Hu J, Wang XK (2009) Adsorption of Ni(II) on oxidized multi-walled carbon nanotubes: effect of contact time, pH, foreign ions and PAA. J Hazard Mater 166:109–116
Acknowledgments
We thank the National Natural Science Foundation of China (Grant Nos. 11075006 and 91026010), the Ministry of Education of the People’s Republic of China (Grant No. 20120001110082), and the State Administration of Science Technology and Industry for National Defence (Grant No. 2007-840, 2012-851) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C.L., Li, Y. & Liu, C.L. Sorption of uranium from aqueous solutions with graphene oxide. J Radioanal Nucl Chem 304, 1017–1025 (2015). https://doi.org/10.1007/s10967-014-3855-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3855-x