Abstract
A simple, fast and sensitive instrumental neutron activation analysis (INAA) method has been optimized for the determination of trace concentrations of chlorine in five types of samples namely zirconium oxide, zircaloy 2, zircaloy 4, Zr–Nb alloy and Zr–Sn alloy samples. The samples were neutron irradiated for short duration (1 min) using pneumatic carrier facility of Dhruva research reactor at a neutron flux of 5 × 1013 cm−2 s−1 and measurement of 1,642.7 keV gamma-ray of 38Cl was carried out using a 40 % HPGe detector coupled to 8 k MCA. Concentrations of chlorine were found to be in the range of 9–575 mg kg−1. The 3σ detection limits of chlorine were in the range of 0.1–2.7 mg kg−1 for various samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zirconium and its compounds are widely used in various fields of science and technology. Zirconium oxide is used as traditional and advanced ceramics and as a refractory material. It is also used as a raw material for production of different types of zirconium based alloys. Zirconium alloys like zircaloy 2, 4 and Zr–Nb alloys are extensively used as cladding for nuclear fuels and for making core structural material for water cooled nuclear power reactors for generation of electricity. The corrosion resistance of zircaloys may change due to variations of (1) cladding material condition, (2) alloying content of the cladding, (3) impurity content of the cladding, and (4) the coolant chemistry. If there is any excess or less concentration with respect to required specifications, it adversely affects its properties as well as durability of the alloys in the power reactors. The trace impurities which are undesired in zircaloys are O, C, Si, P and Cl [1]. Chlorine is considered a detrimental impurity in the zircaloys, because even at trace level it can cause the local depassivation of the oxide film on the surface of the zircaloy clads leading to corrosion and eventual failing of the cladding [2]. The segregated chlorine acts as centers to produce fissures and enhances the rate of ingression of hydrogen during reactor operation leading to deterioration in the strength of pressure tubes made up of zircaloys. As the concentration of Cl increases, the fracture toughness of zircaloys decreases. The source of chloride in pressure tubes is magnesium chloride formed during the Kroll’s process in manufacturing of zirconium sponge. The residual chlorine in the sponge is carried to alloy component during its manufacturing process. Based on examination of Cl content during melting of ingots, four melting cycles are required to reduce its concentration to acceptable level [3]. Thus, for chemical quality control of zirconium based oxides and alloys, determination of concentration of chlorine by a suitable analytical method is a necessity.
The determination of Cl in a wide variety of solid matrices is a challenging task. The turbidimetric method is one of the classical methods for the determination of chlorine in various matrices. However this method is applicable only in the concentration range of 20–2,000 mg kg−1 of chlorine in the sample. Pyrohydrolysis-ion chromatography (PH-IC) is another most widely used technique for the determination of Cl even at ultra trace levels. However, it needs extensive sample preparation before the analysis. The sample needs to be heated slowly and soaked at 1,100 °C in a mixture of moisture and oxygen for about an hour. It was reported that the accuracy depended on the percentage of release of Cl from sample into solution. Loss of volatile halide may occur due to heat generated during the dissolution by acids. In addition, any reagents used in the dissolution and separation processes are potential sources of contamination. PH-IC has been used for the determination of chlorine in nuclear fuels and various material like aluminum foil, Whatman filter paper, surgical gloves, methanol and tetrachloroethelene that were used during fuel fabrication process [4, 5]. Chlorine was determined in nuclear fuel by pyrohydrolysis coupled with spectrophotometry [6], while glow discharge mass spectrometry has been used to measure chlorine in Zr-2.5Nb alloys [7].
Although many solid sampling techniques are used for the determination of Cl in different matrices, these methods require a matrix matching certified reference material (CRM), which may not be available all the time. However, a method that is matrix independent and nondestructive in nature is advantageous. There are a few such analytical techniques capable of quantifying chlorine in materials such as zircaloys and they include X-ray fluorescence spectroscopy (XRF), proton induced X-ray emission analysis (PIXE), instrumental neutron activation analysis (INAA) and prompt gamma-ray NAA (PGNAA). Compared to PIXE and XRF, PGNAA and INAA (used in the present work) are better choice for bulk sample analysis as they do not require any sample pretreatment which helps in avoiding any possible contamination.
INAA has been used for the determination of chlorine using zirconium itself as the comparator [8, 9]. However, it requires the complex mathematical calculations and often it is difficult to accurately calculate the input parameters, which in turn affects the accuracy of the results. Radiochemical neutron activation analysis (RNAA) has been used for determination of Cl in Zr-2.5 % Nb coolant tubes [10]. Special care needs to be taken during the radiochemical separation of chlorine as its amount is low and half-life of 38Cl is short. In this case, INAA can be employed to determine trace amount of chlorine using short duration irradiation of samples with higher neutron flux obtained from research reactors. This paper reports INAA results on chlorine concentrations in zirconium based alloys and oxide samples utilizing short duration neutron irradiation in pneumatic carrier facility (PCF) of Dhruva research reactor.
Experimental
The samples of zircaloys and zirconium oxide powder were received from Nuclear Fuel Complex, Hyderabad, India. Zirconium oxide replicate samples were chosen randomly from every batch of the storage drums. Samples were homogenized by mixing, while cone and quartering method helped in taking subsamples for analysis. The high purity zirconium oxide powder was spiked with chlorine standard solution (amount in the range of 1–20 µg) for determination of chlorine by standard addition method. The samples of zircaloys and Zr–Sn alloy were sequentially cleaned with dil. HNO3, water and acetone. All the samples in the mass range of 75–125 mg were sealed in a precleaned polythene pouches. Standard solution of NaCl (Merck, India) was prepared in distilled water containing 1,000 mg L−1 of chlorine. An aliquot of 100 µL was pipetted out onto Whatman 542 filter paper, which was air dried and then sealed in polythene. Samples and standards along with blank polythene (of same size and mass to that of sample and standard) were sealed in small polythene pouches of approximately square geometry. These samples were kept inside a polypropylene capsule (called rabbit) and irradiated at a neutron flux of 5 × 1013 cm−2 s−1 for 1 min duration using pneumatic carrier facility (PCF) of Dhruva research reactor.
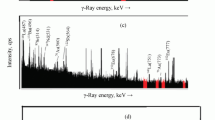
The activity measurement of irradiated sample was carried out using a 40 % relative efficiency HPGe detector coupled to an 8 k MCA having the Pulse Height Analysis SofTware (PHAST) developed at BARC, Mumbai [11]. The resolution of HPGe detector at 1,332 keV photo peak of 60Co was 2.0 keV. Samples and standards were counted after a decay period of 5–30 min for 600–4,000 s to get higher counts with lower counting statistical error. For minimizing the effects of geometry in the detection efficiency, samples and standards were measured at 10 cm from the endcap of the detector. A typical gamma ray spectrum of a neutron activated zircaloy 2 sample is shown in Fig. 1. The relative method of NAA was used for concentration calculations utilizing 1,642.7 keV gamma ray of 38Cl. Blank samples of both Whatman filter paper and polythene (used as sample container) were also analyzed along with the standards and samples, respectively, in a similar way to take care of any contribution of blank to chlorine contents. The 3σ detection limit in mg kg−1 for chlorine in various samples was calculated using background counts under the photopeak of interest of the sample, sensitivity of chlorine and mass of the sample.
Results and discussion
Since no reference material for chlorine in zirconium matrix was available in house, the method was validated by determining concentrations of chlorine in a high purity zirconium oxide matrix spiked with different concentrations of chlorine in the range of 1–20 µg. The results from three independent sample analyses (N = 3) are given in Table 1. The percentage recovery was quantitative (97–100 %). The uncertainties on the results are within ±3 %, arrived from standard deviation of replicate samples.
Determined concentrations of chlorine in zirconium oxide, zircaloy, Zr–Nb and Zr–Sn alloy samples are given in Table 2. Zr–Nb-1, Zr-1 and Zr-3 are the zircaloy samples after first melt, whereas Zr-2, Zr-4 and Zr-5 are finished products of zircaloys. The Cl concentrations in these samples were found to be in the range of 9 to 575 mg kg−1. The results were arrived from replicate (N = 3 or 4) sample analysis. The uncertainties reported in Table 2 are the standard deviations of replicate samples at ±1 s confidence limit. The % relative standard deviations (% RSD) are in the range of ±1–6 %, indicating that the samples analyzed were homogeneous. The samples from two different batches were analyzed and results showed a difference of 6 %. Compared to chlorine concentration values in zirconium oxide, decrease of 20–55 % for the first melt and 80–90 % for the fourth melt (finished product) zircaloys was observed. The expected concentration of the chlorine in first melt zircaloys is ~25 mg kg−1; however the amount detected in the first melt zircaloy samples are between 50 and 100 mg kg−1, while the zirconium oxide powder contains more than 100 mg kg−1 of chlorine. The finished product obtained after the fourth melting contains less than 20 mg kg−1 of chlorine, which is the maximum limit for zircaloy 2 and zircaloy 4 as per the specifications. The results showed that the purification procedure employed for preparing the zircaloys from zirconium oxide maintains the chlorine concentration level well below the specification limit. In the case of Zr–Sn alloy samples, the concentration of chlorine varied from 83 to 575 mg kg−1. Though the method is capable of determining composition of samples including other trace elements, we have focused on determination of chlorine concentrations only. The 3σ detection limits (LD) of chlorine in these samples were in the range of 0.1–2.7 mg kg−1. The variation of detection limit values is due to varying background under the photo peak of sample spectrum arising from experimental conditions in terms of (i) variation in composition of the sample matrix, (ii) mass of sample and (iii) decay period before counting.
Conclusions
INAA using high neutron flux, short duration irradiation and assaying the sample at the site with minimum delay was optimized for the determination of minor to trace concentrations of chlorine in zirconium based oxide and alloys. The method is fast, non-destructive (direct solid sample analysis), simple and sensitive. The detection limit was quite variable for the different samples, ranging from 0.1 to 2.7 mg kg−1. There was no gamma ray interference in the determination of low concentration level of chlorine in the presence of major matrix element Zr and other minor and trace elements, as the higher energy gamma-ray 1,642.7 keV of 38Cl was used for assessing chlorine content.
References
Sell HJ, Pritsching ST, Garzarolli F (2006) J ASTM Int 3:1–14
Theaker JR, Choubey R, Moan G. D, Aldridge SA, Davis L, Graham RA, Coleman CE (1994) Proceedings of the X International Symposium on ASTM STP 1245: 221–242
Rao YB, Rao GP, Prahlad B, Saibaba N (2012) DAE-BRNS Theme meeting on ion chromatography separations: state of art and perspectives (ICSSAP-2012) 39–63
Shah DJ, Verma P, Thakur UK, Mahajan MA, Sawant RM (2009) Nuclear and radiochemistry symposium(NUCAR-2009) 459–460
Jeyakumar S, Raut VV, Ramakumar KL (2008) Talanta 76:1246–1251
Mahajan MA, Prasad MVR, Matre HR, Sawant RM, Rastogi RK, Razvi GH, Chaudhury NK (1991) J Radioanal Nucl Chem 148:93–100
Shekhar R, Arunachalam J, Ravindra HR, Gopalan B (2003) J Anal At Spectrom 18:381–384
Cohen IM, Gomez CD, Mila ML (1981) J Radioanal Nucl Chem 67:393–402
Naik H, Sant VL, Suryanarayana SV, Prajapati PM, Nathaniel TN (2011) Radiochim Acta 99:751–754
Verma R, Parthasarthy R (1996) J Radioanal Nucl Chem 214:391–397
Mukhopadhyay PK (2001) In: Proceedings of symposium on intelligent nuclear instrumentation (INIT-2001) Mumbai, India. 307–310
Acknowledgments
Authors thank Dr. Y. Balaji Rao, Control Lab, NFC, Hyderabad and Mr. B. C. Maji, GAMD, BARC for providing samples and valuable suggestions. Authors thank Dr. A. Goswami, Head, RCD; Dr. P. K. Pujari, Head, NCS, RCD and Dr. R. Verma, Head, NAMS, ACD, BARC for their support and encouragement. Authors thank operation personnel of PCF Dhruva reactor, BARC for their help during irradiation of samples. Authors thank Mr. N. S. Bhamra, ROD Engineer (D), Mr. K. Chakrabarty, ARS (D), Mr. C. G. Karahadkar, RS (D) and Mr. D. K. Shukla, Head, ROD, BARC and PCF Dhruva crew members for their constant support for NAA work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinde, A.D., Acharya, R. & Reddy, A.V.R. Optimization of a fast INAA method for determination of trace concentrations of chlorine in zirconium based alloy and oxide samples. J Radioanal Nucl Chem 304, 693–696 (2015). https://doi.org/10.1007/s10967-014-3854-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3854-y