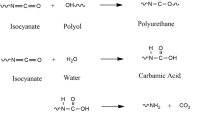
Abstract
Polyurethanes are renowned for their incredible versatility. They are available in various forms, such as foams, thermoplastics, elastomers, and paints. These polymers are synthesized from two chemical substances: isocyanates and polyols. The increasing interest in the utilization of materials from renewable sources has spurred significant research in search of replacements for these two substances. For instance, castor oil can be used as a substitute for polyol, in addition to being an abundant renewable source. In this context, the objective of this study is to investigate how the variables temperature (2 °C, 24 °C, and 70 °C) and mass ratio (2:1, 1:1, and 1:2) influence the final properties of polyurethane foams. The foams were prepared using the one-shot method under controlled agitation. The results indicated that replacing polyol with vegetable oil was successful, as evidenced by the appearance of reference bands in the FTIR spectra. XRD and DSC analyses confirmed the amorphous nature of the polyurethanes, and TG tests demonstrated that a temperature of 70 °C significantly reduced the thermal stability of the foams, especially those based on vegetable sources. However, synthesis temperatures of 2 °C and 24 °C did not show considerable alteration in thermal stability. And the analysis of the effect of the sol fraction variables demonstrated that the polyol/isocyanate ratio has a significant effect, especially in vegetable-based foams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethanes (PUs) are versatile polymeric materials available in various forms, including foams, adhesives, paints, and tapes. Among these variants, PU foams stand out, currently accounting for more than 50% of the market consumption [1]. Due to their remarkable versatility, flexible foams can find applications in the automotive industry, such as in seats, as well as in furniture and mattresses. Rigid foam can be utilized for thermal and acoustic insulation, as well as in packaging and footwear [2,3,4].
PU foams are synthesized through a two-step process known as gelling and blowing reactions [2, 5]. To facilitate this synthesis, two primary reagents are required: isocyanates and polyols [6, 7]. However, these reagents are derived from non-renewable sources, leading to the emission of substantial amounts of carbon dioxide (CO2) into the atmosphere. This contributes to global warming and environmental pollution due to their low degradability [1, 8]. Furthermore, there is a growing dependence on fossil resources, driven by the increasing price of a barrel of oil. Therefore, it is of utmost importance to replace these materials with renewable resources to mitigate their environmental impact [1].
Hence, vegetable oils, such as castor oil extracted from the seeds of the Ricinus communis plant in the Euphorbiaceae family, emerge as sustainable and economically viable alternatives to petrochemical polyols, due to their degradability and low impacts on the environment [2, 9, 10]. Furthermore, it is a renewable resource present in several countries with a tropical climate, which ends up boosting its use as a raw material [2, 11], as it is considered a natural polyol, which does not require chemical modifications to react with isocyanate to form PUs, in addition to being a non-edible oil, which does not affect food safety [12].
Several works in the literature use castor oil as a raw material, along with other materials, such as glycerol and lignin for the production of PUs, however they do not mention the influence of the variables temperature and mass ratio on the synthesis, as well as the final properties arising from the union between only castor oil and isocyanate in the presence of these variables [13, 14].
Therefore, the present study aims to investigate the variables of temperature and mass ratio in the synthesis of PU foams based on castor oil, as well as to evaluate which of these variables influence the final properties of the material, thus being able to assist in a better understanding of the synthesis conditions between crude castor oil and isocyanate.
Materials and methods
Methodology
In order to evaluate the variables that influence the properties of PU foams, syntheses of 18 PU samples were carried out using the one shot method [15], which consists of vigorous agitation of the reagents inside a container, for approximately 60 s until foam formation. Samples were obtained using commercial diphenylmethane-2,4'-diisocyanate (Redelease, Brazil), commercial polyol (Redelease, Brazil) and castor oil (QuimisulSC, Brazil). The samples were synthesized at 2 °C, 24 °C and 70 °C, with the aid of a water bath and a cooling chamber, in which the reagents were arranged in this equipment until they reached stability at the desired temperature and, subsequently, were synthesized under stirring around 300 RPM under the same temperature conditions. The synthesis temperatures of 2 °C, 24 °C and 70 °C correspond to levels (-1, 0 and + 1) according to Table 1.
Thus, a 32 factorial design was performed for castor oil-based foams using castor oil with the diphenylmethane-2,4'-diisocyanate and another 32 design under the same conditions for the control group for comparison purposes with polyol and diphenylmethane-2,4'-diisocyanate. The exponent is the number of variables, in this case the temperature and the reagents. The base refers to the lower, intermediate and higher levels, as demonstrated in Table 1. After the syntheses, the samples were reserved in the laboratory for 24 h and later characterized.
Characterization of polyurethane foams
Fourier-transform infrared spectroscopy (FTIR)
The PU foams were characterized by FTIR using the PerkinElmer Spectrum 65 spectrophotometer. To generate the spectra, a resolution of 4 cm−1 was used with a 64-scan sweep.
X-ray powder diffraction (XRD)
In order to evaluate the crystalline structure of the foams, diffractogram measurements were made with the LabX XRD-6100 equipment, Shimadzu. The analysis parameters were: V = 40 kV, i = 30 mA, v = 2°/min, using the range of 5 to 37 degrees with Cu Kα radiation (λ = 0.15406 nm).
Thermogravimetric analysis (TG)
To evaluate the thermal properties of foams, thermogravimetry tests were carried out with the TGA/DTA equipment model 6200 from the brand SII Nanotechnology. The analysis parameters were the following: 5 mg of sample, heating rate: 10 °C/min, temperature range from 25 to 900°C and nitrogen atmosphere with a flow rate of 100mL/min.
Differrential scanning calorimetry (DSC)
With the purpose of analyzing the thermal transitions of the foams, DSC curves were carried out, the 1st stage being the heating and cooling process with an isotherm of 3 min at 110 °C and subsequent cooling to -90 °C to erase the thermal history. The 2nd stage of heating and cooling were considered for data collection using the DSC 25 equipment from the TA Instruments brand. The analysis parameters were the following: 5 mg of sample, heating rate of 10 °C/min, temperature range from -90 to 110 °C and nitrogen atmosphere with a flow of 50mL/min. Data were collected using the Trios software.
Sol fraction
To determine the sol fraction of the prepared foams, approximately 0.2 g of dry sample was weighed and conditioned in xylene for approximately 72 h at room temperature. Then, the samples were removed and dried for 72 h at 60 °C in an oven. The sol fraction values were determined using Eq. 1, where m1 represents the initial mass of the sample and m2 represents the mass after extraction and drying.
Results and discussions
Foams based on castor oil and conventional foams obtained through factorial design
Figure 1 shows the foams obtained, and it is possible to observe that there was variation in both color and expansion. Castor oil-based foams, synthesized at 2 °C, showed a darker color, close to the color of castor oil, which was not observed for conventional foams. Regarding the expansion, sample 7 was the one that presented the highest volume synthesized with excess isocyanate and at a temperature of 70 °C, both for conventional foams and for foams based on vegetable oil. According to Wang et al. (2022) [16] temperature drastically affects foam formation, since a higher temperature favors an increase in the reactivity of the polymer molecules, which in turn end up colliding more and ends up accelerating polymerization and expansion responses. The result of this is a higher polymerization rate that ends up generating foams faster and with larger volumes [17].
Characterization by Fourier transform infrared spectroscopy of conventional foams (FTIR)
Figure 2 presents the spectra of the 9 samples synthesized with polyol and isocyanate. It can be observed, at approximately 3400 cm−1, the OH stretching present in the samples, which refers to the residual hydroxyl present in the polyol. The peaks in the regions between 2932 cm−1 and 2852 cm−1 indicate the presence of asymmetric and symmetric methyls. The carbonyl bonds [6, 18, 19] are at approximately 1717 cm−1 present in the PU. In the region of 1592 cm−1 there is the C–C stretching from the aromatic ring of the isocyanate, while at 1524 cm−1 there is the coupling of the vibrations of the C-N group to the N–H of the group (-CO–NH-) [18]. In the region of 1415 cm−1, there is the presence of symmetric angular deformation in the plane of the CH2 group, in the region of 1222 cm−1, vibrations of the C-O group are observed linked to the stretching of the carbonyl of the urethane group and finally, in 1078 cm−1 we have the out-of-plane deformation of the C-H of the oxygen united with the out-of-plane deformation of the O = C-O present in the urethane group [18]. Only in samples 7, 8 and 9 was the appearance of the NCO group observed, at approximately 2270 cm−1, attributed to the remaining isocyanate groups that were not consumed during the reaction due to a higher ratio of isocyanate compared to polyol for these samples [20].
Characterization by Fourier transform infrared spectroscopy of castor oil-based foams (FTIR)
In (Fig. 3), in relation to the FTIR spectra of the PU samples with CO, firstly, the characteristic band of the hydroxyl groups, observed in the region of 3350 cm−1, with great intensity, which suggests that the castor oil used contains high amount of OH groups [18]. Then there is the presence of two bands attributed to CH3 and CH2 deformation at 2923 cm−1 and 2853 cm−1, respectively [21]. A double peak referring to the stretching of the C=O carboxyl group [22] was identified at 1732 cm−1 and 1730 cm−1. Next, the band at 1595 cm−1 is observed, which is related to the vibration of the benzene ring of the isocyanate [20]. At 1510 cm−1, vibration of the N–H group of urethane is observed [21]. It is important to highlight that there was no formation of urea-type bonds in any of the spectra, being generally visualized in the region between 1680 cm−1 and 1650 cm−1. In the region 1226 cm−1 there are vibrations of the C-O bond, related to the stretching of the carbonyl present in the urethane group [23]. As observed in the spectra of Fig. 2, of the conventional foams, the PU foam with castor oil also showed peaks at 2260 cm−1 of the isocyanate groups that ended up not reacting due to the higher ratio of this substance in the reaction in samples 7, 8 and 9 [24].
Another interesting characteristic observed in samples 7, 8 and 9 is that sample 7, synthesized at a temperature of 70 °C, presented a peak at 2260 cm−1 referring to the isocyanate groups with lower intensity when compared to samples 8 and 9 the sample that presented the greatest expansion. These same behaviors can be observed in the spectra of conventional foams in (Fig. 2). As pointed out by Trovati et al. (2010) [25]. a large amount of free OH groups contributes to a more flexible polymer structure, since the excess of hydroxyl group ends up decreasing the degree of crosslinking of the PU chain, therefore, it can be stated that all 9 samples of PU with castor oil have flexible characteristics. This same classification can be applied to the conventional foams in (Fig. 3).
Sol fraction values
Table 2 presents the sol fraction values for conventional foams and castor oil-based foams. It is possible to note that the conventional foams PU 1, PU 2 and PU 3, prepared with a 2:1 mass ratio of polyol to isocyanate exhibited higher values of sol fraction. On the other hand, the other samples demonstrated a decrease in soluble fraction values as the amount of isocyanate in the formulation increased. This trend suggests that increasing the amount of isocyanate in the formulation results in a greater crosslinking density of the material [26].
For the castor oil-based foams, it is noteworthy that they exhibited a significantly higher sol fraction compared to conventional foams, especially for the PU CO 1, PU CO 2, and PU CO 3 samples, which were prepared with a 2:1 mass ratio of castor oil to isocyanate. The data obtained corroborates the FTIR discussions, as a higher sol fraction indicates a smaller extent of crosslinking of the polymer molecules, thereby reducing the degree of crosslinking of the polymeric structure and favoring a more flexible structure [27].
Effect of experimental variables
Using each of the experimental levels described in Table 1, it is possible to calculate the effect of each variable by comparing the levels of sample smoothness at high, medium, and low levels (-1, 0, and + 1), corresponding to the isocyanate/polyol ratio of 2:1, 1:1, and 1:2, respectively. To do so, the value obtained from the response at the highest level of comparison, sol fraction, was added and subtracted from the sum of the others. These studies are described in Table 3.
The Table 3 illustrates the differences between PU and PU CO samples. The increase in ambient temperature proved to be of limited significance, as evidenced by the proximity of the obtained values for the temperature variable at the 1 and 0 intervals in both cases. However, notable negative values were observed at the 1 and -1, as well as 0 and -1 intervals, indicating that a decrease in temperature leads to a reduction in the soluble fraction. Thus, within these intervals, an increase in temperature favors a decrease in the soluble fraction, albeit significantly only up to ambient temperature. Furthermore, upon comparing the magnitudes of this effect, it is apparent that PU CO samples exhibited lesser impact compared to PU samples, attributed to the modulus of the effect of PU CO samples being nearly three times smaller than that of PU samples.
Regarding the polyol-to-isocyanate ratio, it was observed that in all intervals, the effect is positive; that is, an increase in polyol in the sample tends to increase the soluble fraction of the material. This influence was more pronounced in PU CO samples, as evidenced by the larger magnitudes of the effects compared to PU samples, with the exception of the analysis at the 0 and -1 interval.
Characterization by X-ray Diffraction of conventional foams (XRD)
Analyzing the XRD spectra of the conventional foam samples in (Fig. 4), it can be observed that all the samples presented a very intense peak close to 2θ = 20°, which is generally observed for pure PU. Furthermore, it is a characteristic peak of materials that contain an amorphous structure [28]. All samples of conventional foams can be classified as soft, since the amount of hydroxyls present in the polyol it controls the degree of crosslinking of the polymeric chains, therefore, a large proportion of polyol leads to a decrease in the degree of crosslinking present in the urethane, which ends up making the foam less rigid and also less crystalline, thus being classified as soft. The results found corroborate the FTIR spectra from the foam, which also classify foams as soft [25].
Characterization by X-ray Diffraction of castor oil-based foams (DRX)
Examining (Fig. 5), which deals with the XRD spectra of foams with castor oil, a great similarity with conventional foams can be noted. Only one peak was observed in all samples, intense and wide close to 2θ = 20° [29], also observed in conventional samples, with a slight displacement for PU CO 6, resulting in a value of 20.3°. This peak found indicates the amorphous nature of PU, which is observed in other works in the literature [30]. Samples 1, 2 and 3 could not be characterized as they were too viscous. As identified for conventional foams, foams with castor oil can also be classified as soft, which is in accordance with the spectra obtained by FTIR [31].
Thermogravimetry and derivative analysis of conventional PU samples (TG-DTG)
Figure 6 demonstrates the TG and DTG curves of conventional PU foams. The thermal decomposition process of PU is divided into three stages [25, 32]: the first stage of thermal decomposition of the rigid PU segments occurs due to the disappearance of the diphenylmethane that is part of the isocyanate, as well as the breaking of the urethane bonds, C-O, which present low binding forces, which when decomposed form volatiles such as CO2, amines, aldehydes, among other compounds [33,34,35].
The second and third stages deal with the thermal decomposition of the soft segments through the breaking of the ester groups, which have greater binding strength [25, 32, 35]. It can be noticed that sample PU 1, which was synthesized at a temperature of 70 °C, presented lower thermal stability than samples PU 5, synthesized at room temperature, and sample PU 9 prepared at 2 °C, with isocyanate indices of 54.21, 111.05 and 223.28, respectively. Therefore, it can be seen that by increasing the isocyanate index, the foam becomes more thermally stable [36].
At the beginning for the DTG curves, there is a discreet thermal event around 50 °C to 100 °C due to the amount of water present in the sample. It is possible to characterize the three foams as being flexible, which is corroborated by the characterizations by FTIR and XRD, since they present three characteristic peaks of thermal degradation as demonstrated by Trovati et al. (2010) [25], disregarding the first peak due to the presence of water.
According to Table 4, the three foams showed very similar thermal behavior, with the exception of PU 1, which was synthesized at a temperature of 70 °C, with a polyol/isocyanate ratio of 2:1, which showed the beginning of decomposition at 177.92 °C.
A possible explanation for the PU 1 sample presenting lower thermal stability than the others may be related to the content of aromatic fractions of the isocyanate, as well as the amount of urethane bonds. This sample showed a lower ratio of isocyanate to polyol, in addition to the influence of temperature, which may contribute to a low thermal property. Therefore, the low thermal stability of the urethane bonds is related to the weak dissociation energies of the chemical bonds that make up the urethane structure, resulting in a smaller amount of crosslinks between the PU chains. This, in turn, may lead to a lower thermal behavior of PU 1 compared to other samples [37].
Thermogravimetry and derivative analysis of castor oil-based PU foams (TG-DTG)
Figure 7 illustrates the TG curves for castor oil-based foams, which show two main stages of thermal degradation [38]. The shape of the curves were quite similar, except for the PU CO 1 sample, which presented a much lower thermal stability than the others. When comparing the samples with the conventional foams, it is noted that the PU 1 sample presented similar thermal behavior, being the sample with the lowest thermal property in relation to the others. It is important to highlight that both PU CO 1 and PU 1 presented isocyanate indices of 41.25 and 54.21, respectively. Low isocyanate index values can significantly reduce the thermal stability of PU, as the isocyanurate content is decreased [39]. Thus, both foams based on castor oil and petrochemical polyol synthesized with low isocyanate indices at 70 °C showed lower thermal stability.
It is possible noticed that for the DTG all samples presented thermal events at approximately 50 °C due to the presence of water, as well as two main thermal degradation stages, as stated by Javni et al. (2000) [38].
Table 5 shows the values of the thermal degradation stages of the samples. The first step is related to the decomposition of urethane bonds, which vary from 150 to 200 °C [38]. The second stage is associated with the decomposition of castor oil, which contains ricinoleic acid, which may result in the formation of 10-undecanoic acid and heptanal [32]. As observed in the conventional foam PU 1, the PU CO 1 showed low thermal stability, with the onset of degradation at approximately 151.03 °C. This may be due to the fact that a higher temperature strongly influences the breaking of crosslinks, which are related to low activation energies. In this way, only the strongest bonds between the chains remain intact, which results in a low thermal stability of the material [40].
Thermal stability between castor oil-based foams and conventional PU foams
It is possible to note that there was a significant difference in thermal stability between castor oil-based foams and conventional foams, which may be directly related to the temperature variables and, mainly, to the mass ratio between the reactants. The PU 1 and PU CO 1 foams, synthesized at 70 °C with a mass ratio of 2:1 (polyol/isocyanate), presented lower thermal stability than the PU 5, PU CO 5, PU 9 and PU CO 9 foams prepared with ratios of 1:1 and 1:2 at temperatures of 24 °C and 2 °C, respectively. Furthermore, it is worth highlighting that castor oil-based foams had low levels of isocyanate compared to conventional foams, which reduces the cross-linking of the polymer chains and, consequently, reduces their final thermal stability [41]. However, it is possible to find PUs derived from renewable sources that present degradation stages between 125°C and 215°C [42]. Thus, castor oil-based foams, despite being thermally inferior due to the synthesis temperature and low isocyanate content, demonstrated to be within the degradation temperature range for PUs from renewable sources.
Differential scanning calorimetry of conventional foams (DSC)
Figure 8 shows the DSC curves of conventional and castor oil-based foams. It can be seen that only PU 1 exhibited an apparent glass transition temperature (Tg), at 56 °C, which is the hard segments of the PU [43]. No β (Tβ) transition was noted at negative temperature for any of the samples, which indicates that conventional foams PU 1, PU 5 and PU 9 did not demonstrate cold crystallization [44].
The Tg, with positive temperature, found for PU 1 is very close to the values found in the literature for conventional foams, which range from 52 to 66 °C [44, 45]. Therefore, it is possible to state that the PU 1 synthesized at a temperature of 70 °C did not present a significant difference in Tg displacement, despite being the foam with the lowest thermal stability, as previously discussed in Section "Thermogravimetry and derivative analysis of conventional PU samples (TG-DTG)" of the TG graph. It is important to highlight that the absence of endothermic peaks related to the melting of the crystalline or rigid segments indicates the amorphous structure of the PUs, which is in agreement with the data obtained by XRD [43].
In contrast to the conventional PUs, the castor oil based samples exhibited different Tg values, especially the PU CO 1, which presented a Tg of -31.79 °C, coming from the flexible segment, since castor oil has C-O bonds [38]. This change in Tg, compared to the other samples, can be explained by the 2:1 ratio between castor oil and isocyanate, resulting in an excess of oil that significantly contributed to the sharp decrease in the Tg value of the material. This effect can lead to a reduction in crosslinking and, consequently, to greater mobility of the material chains [46].
Unlike the conventional PU 5 which did not exhibit any apparent Tg value, the PU CO 5 presented a Tg of 49.98 °C close to the values reported in the literature for the rigid segment, while for the PU CO 9 sample the Tg was not observed [44, 46, 47].
Conclusions
With this research, it was possible to identify the variables that influence PU synthesis through factorial planning. Castor oil successfully replaced petrochemical-source polyol in the preparation of foams, as evidenced by the characteristic PU bands found in the FTIR spectrum. Furthermore, the castor oil-based PU foams showed lower crosslinking compared to conventional foams.
The XRD diffractograms revealed that none of the variables included in the factorial design affected the crystalline structure of the PU foams. However, in TG tests, conventional foams showed greater thermal stability compared to castor oil-based foams, but lower thermal stability synthesized at 70 °C, while samples synthesized at 2 and 24 °C did not show significant differences in thermal stability. The DSC tests only indicated the glass transition of the samples related to the rigid phase, without any other associated thermal event. This confirms that the materials obtained have an amorphous structure, with the exception of PU CO 1, which presented a negative glass transition due to the greater amount of castor oil in relation to isocyanate. Finally, the evaluation of the effects of the experimental variables demonstrated that temperature did not have a strong influence on the soluble fraction of the foams; however, the polyol/isocyanate ratio presented a positive effect, with this effect being more pronounced for foams based on castor oil.
References
Singh I, Samal SK, Mohanty S, Nayak SK (2020) Recent advancement in plant oil derived polyol-based polyurethane foam for future perspective: A review. Eur J Lipid Sci Technol 122(3):1900225. https://doi.org/10.1002/ejlt.201900225
Alves LR, Carriello GM, Pegoraro GM, Filho JF (2021) A utilização de óleos vegetais como fonte de polióis para a síntese de poliuretano: Uma revisão. Disciplinarum Scientia Naturais e Tecnológicas 22(1):99. https://doi.org/10.37779/nt.v22i1.3711
Guo A, Javni I, Petrovic Z (2000) Rigid polyurethane foams based on soybean oil. J Appl Polym Sci 77(2):467–473. https://doi.org/10.1002/(SICI)1097-4628(20000711)77:2%3c467::AID-APP25%3e3.0.CO;2-F
Reghunadhan A, Thomas S (2017) Chapter 1: polyurethanes: structure, properties, synthesis, characterization, and applications. In: Thomas S, Datta J, Haponiuk JT, Reghunadhan A (eds) Polyurethane Polymers. Elsevier, pp 1–16. https://doi.org/10.1016/B978-0-12-804039-3.00001-4
Pegoraro GM, Alves LR, Carriello GM, de Janolla TA, Mambrini GP, de Rezende ML, de Menezes AJ (2023) Polyurethane and rare-earth materials: A review. J Eng Exact Sci 9(3):15627. https://doi.org/10.18540/jcecvl9iss3pp15627-01e
Alves LR, Carriello GM, Pegoraro GM, Lopes HSM, de Janolla TA, Dias ANC, Mambrini GP, de Rezende ML, de Menezes AJ (2023) Synthesis and characterization of polyurethane and samarium(III) oxide and holmium(III) oxide compositess. Polímeros 33:e20230039. https://doi.org/10.1590/0104-1428.20230023
Alves LR, Carriello GM, Pegoraro GM, de Rezende ML, de Menezes AJ (2022) Aplicações de Enzimas em Poliuretano Uma revisão das Dissertações e Teses brasileiras. Disciplinarum Scientia | Naturais e Tecnológicas 23(2):99. https://doi.org/10.37779/nt.v23i2.4313
Zhang S, Xiang A, Tian H, Rajulu AV (2018) Water-blown castor oil-based polyurethane foams with soy protein as a reactive reinforcing filler. J Polym Environ 26(1):15–22. https://doi.org/10.1007/s10924-016-0914-0
Kaikade DS, Sabnis AS (2023) Polyurethane foams from vegetable oil-based polyols: A review. Polym Bull 80(3):2239–2261. https://doi.org/10.1007/s00289-022-04155-9
Wang HJ, Rong MZ, Zhang MQ, Hu J, Chen HW, Czigány T (2008) Biodegradable foam plastics based on castor oil. Biomacromol 9(2):615–623. https://doi.org/10.1021/bm7009152
Mosiewicki MA, Dell’Arciprete GA, Aranguren MI, Marcovich NE (2009) Polyurethane foams obtained from castor oil-based polyol and filled with wood flour. J Compos Mater 43(25):3057–3072. https://doi.org/10.1177/0021998309345342
Sharma C, Kumar S, Unni AR, Aswal VK, Rath SK, Harikrishnan G (2014) Foam stability and polymer phase morphology of flexible polyurethane foams synthesized from castor oil. J Appl Polym Sci 131(17). https://doi.org/10.1002/app.40668
Zhang M, Pan H, Zhang L, Hu L, Zhou Y (2014) Study of the mechanical, thermal properties and flame retardancy of rigid polyurethane foams prepared from modified castor-oil-based polyols. Ind Crops Prod 59:135–143. https://doi.org/10.1016/j.indcrop.2014.05.016
Carriço CS, Fraga T, Pasa VMD (2016) Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur Polymer J 85:53–61. https://doi.org/10.1016/j.eurpolymj.2016.10.012
Santin CK, Petró F (2022) Desenvolvimento e caracterização de espuma poliuretânica à base de Difenilmetano diisocianato (MDI) e óleo de linhaça (Linum usitatissimun L.). Revista Liberato 23(39):39
Wang J, Zhang C, Deng Y, Zhang P (2022) A review of research on the effect of temperature on the properties of polyurethane foams. Polymers 14(21):4586. https://doi.org/10.3390/polym14214586
Harikrishnan G, Khakhar DV (2007) Effect of monomer temperature on foaming and properties of flexible polyurethane foams. J Appl Polym Sci 105(6):3439–3443. https://doi.org/10.1002/app.26391
Jiao L, Xiao H, Wang Q, Sun J (2013) Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym Degrad Stab 98(12):2687–2696. https://doi.org/10.1016/j.polymdegradstab.2013.09.032
Gomes DR, Alves LR, Carriello GM, Lopes HSM, Pegoraro GM, de Barros MFJ, Camargo FB, Corrêa CE, Komatsu D (2024) Addition of calcium carbonate in the synthesis of flexible polyurethane foams. Scientia Plena 20(4). https://doi.org/10.14808/sci.plena.2024.043401
Chen X, Huo L, Jiao C, Li S (2013) TG–FTIR characterization of volatile compounds from flame retardant polyurethane foams materials. J Anal Appl Pyrol 100:186–191. https://doi.org/10.1016/j.jaap.2012.12.017
Chen Y-C, Tai W (2018) Castor oil-based polyurethane resin for low-density composites with bamboo charcoal. Polymers 10(10):1100. https://doi.org/10.3390/polym10101100
Wang C, Zheng Y, Xie Y, Qiao K, Sun Y, Yue L (2015) Synthesis of bio-castor oil polyurethane flexible foams and the influence of biotic component on their performance. J Polym Res 22(8):145. https://doi.org/10.1007/s10965-015-0782-7
Jaganathan SK, Mani MP, Ayyar M, Supriyanto E (2017) Engineered electrospun polyurethane and castor oil nanocomposite scaffolds for cardiovascular applications. J Mater Sci 52(18):10673–10685. https://doi.org/10.1007/s10853-017-1286-0
Pattanayak A, Jana SC (2005) Synthesis of thermoplastic polyurethane nanocomposites of reactive nanoclay by bulk polymerization methods. Polymer 46(10):3275–3288. https://doi.org/10.1016/j.polymer.2005.02.081
Trovati G, Sanches EA, Neto SC, Mascarenhas YP, Chierice GO (2010) Characterization of polyurethane resins by FTIR, TGA, and XRD. J Appl Polym Sci 115(1):263–268. https://doi.org/10.1002/app.31096
Kosmela P, Hejna A, Suchorzewski J, Piszczyk Ł, Haponiuk JT (2020) Study on the structure-property dependences of rigid PUR-PIR foams obtained from marine biomass-based biopolyol. Materials 13(5):1257. https://doi.org/10.3390/ma13051257
Imran M, Rahaman A, Shaik AH, Chandan MR (2020) Stability enhancement of highly loaded nano-clay-based flexible polyurethane foams using hollow glass microspheres. J Cell Plast 56(5):547–557. https://doi.org/10.1177/0021955X20912203
Abu-Jdayil B, Al Abdallah H, Mlhem A, Alkhatib S, El Sayah A, Hussein H, Althabahi A, AlAydaroos A (2022) Utilization of polyurethane foam dust in development of thermal insulation composite. Buildings 12(2):126. https://doi.org/10.3390/buildings12020126
Singh KP, Mishra A, Kumar N, Tripathi DN, Shami TC (2018) Evaluation of thermal, morphological and flame-retardant properties of thermoplastic polyurethane/polyphosphazene blends. Polym Bull 75(6):2415–2430. https://doi.org/10.1007/s00289-017-2156-2
Wang J, Wu C, Liu R, Li S (2014) A polyurethane-based elastomeric nanocomposite with a high dielectric constant. Polym Bull 71(5):1263–1276. https://doi.org/10.1007/s00289-014-1127-0
Wang J, Wu C, Liu R, Li S (2014) All-organic nanocomposites of functionalized polyurethane with enhanced dielectric and electroactive strain behavior. Polym Adv Technol 25(6):657–664. https://doi.org/10.1002/pat.3289
Somani KP, Kansara SS, Patel NK, Rakshit AK (2003) Castor oil based polyurethane adhesives for wood-to-wood bonding. Int J Adhes Adhes 23(4):269–275. https://doi.org/10.1016/S0143-7496(03)00044-7
Carvalho JED, Claro Neto S, Chierice GO (2014) Caracterização térmica do poliuretano derivado de óleo vegetal utilizado para confecção de dispositivo de assistência ventricular. Braz J Therm Anal 3(1–2):16. https://doi.org/10.18362/bjta.v3i1-2.21
Cervantes-Uc JM, Espinosa JIM, Cauich-Rodríguez JV, Ávila-Ortega A, Vázquez-Torres H, Marcos-Fernández A, San Román J (2009) TGA/FTIR studies of segmented aliphatic polyurethanes and their nanocomposites prepared with commercial montmorillonites. Polym Degrad Stab 94(10):1666–1677. https://doi.org/10.1016/j.polymdegradstab.2009.06.022
Wang C, Chen X, Chen J, Liu C, Xie H, Cheng R (2011) Synthesis and characterization of novel polyurethane acrylates based on soy polyols. J Appl Polym Sci 122(4):2449–2455. https://doi.org/10.1002/app.34364
Kamińska K, Barczewski M, Kurańska M, Malewska E, Polaczek K, Prociak A (2022) The effect of a chemical foaming agent and the isocyanate index on the properties of open-cell polyurethane foams. Materials 15(17):6087. https://doi.org/10.3390/ma15176087
Fridrihsone A, Stirna U, Lazdiņa B, Misāne M, Vilsone D (2013) Characterization of polyurethane networks structure and properties based on rapeseed oil derived polyol. Eur Polymer J 49(6):1204–1214. https://doi.org/10.1016/j.eurpolymj.2013.03.012
Javni I, Petrović ZS, Guo A, Fuller R (2000) Thermal stability of polyurethanes based on vegetable oils. J Appl Polym Sci 77(8):1723–1734. https://doi.org/10.1002/1097-4628(20000822)77:8%3c1723::AID-APP9%3e3.0.CO;2-K
Park DH, Park GP, Kim SH, Kim WN (2013) Effects of isocyanate index and environmentally-friendly blowing agents on the morphological, mechanical, and thermal insulating properties of polyisocyanurate-polyurethane foams. Macromol Res 21(8):852–859. https://doi.org/10.1007/s13233-013-1106-6
Narine SS, Kong X, Bouzidi L, Sporns P (2007) Physical properties of polyurethanes produced from polyols from seed oils: II. Foams. J Am Oil Chemists’ Soc 84(1):65–72. https://doi.org/10.1007/s11746-006-1008-2
Abdel Hakim AA, Nassar M, Emam A, Sultan M (2011) Preparation and characterization of rigid polyurethane foam prepared from sugar-cane bagasse polyol. Mater Chem Phys 129(1):301–307. https://doi.org/10.1016/j.matchemphys.2011.04.008
Gama NV, Soares B, Freire CSR, Silva R, Neto CP, Barros-Timmons A, Ferreira A (2015) Bio-based polyurethane foams toward applications beyond thermal insulation. Mater Des 76:77–85. https://doi.org/10.1016/j.matdes.2015.03.032
Lei W, Zhou X, Fang C, Song Y, Li Y (2019) Eco-friendly waterborne polyurethane reinforced with cellulose nanocrystal from office waste paper by two different methods. Carbohyd Polym 209:299–309. https://doi.org/10.1016/j.carbpol.2019.01.013
Gu R, Sain MM, Konar SK (2013) A feasibility study of polyurethane composite foam with added hardwood pulp. Ind Crops Prod 42:273–279. https://doi.org/10.1016/j.indcrop.2012.06.006
Banik I, Sain MM (2008) Role of Refined Paper Fiber on Structure of Water Blown Soy Polyol Based Polyurethane Foams. J Reinf Plast Compos 27(14):1515–1524. https://doi.org/10.1177/0731684407086317
Shen Y, He J, Xie Z, Zhou X, Fang C, Zhang C (2019) Synthesis and characterization of vegetable oil based polyurethanes with tunable thermomechanical performance. Ind Crops Prod 140:111711. https://doi.org/10.1016/j.indcrop.2019.111711
Yang L-T, Zhao C-S, Dai C-L, Fu L-Y, Lin S-Q (2012) Thermal and Mechanical Properties of Polyurethane Rigid Foam Based on Epoxidized Soybean Oil. J Polym Environ 20(1):230–236. https://doi.org/10.1007/s10924-011-0381-6
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lucas Repecka Alves, Giovanni Miraveti Carriello, Guilherme Manassés Pegoraro, Carlos Eduardo Moraes, Maira de Lourdes Rezende and Aparecido Junior de Menezes. The first draft of the manuscript was written by Lucas Repecka Alves, Giovanni Miraveti Carriello, Guilherme Manassés Pegoraro and Carlos Eduardo Moraes and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Repecka Alves, L., Miraveti Carriello, G., Manassés Pegoraro, G. et al. Green polyurethane foams: Replacing petrochemical polyol with castor oil through factorial design. J Polym Res 31, 227 (2024). https://doi.org/10.1007/s10965-024-04077-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-024-04077-2