Abstract
New amphiphilic block copolymers on based benzylmethacrylates or 2,3,4,5,6-pentafluorobenzyl methacrylate and N-isopropylacrylamide with high yields (89–94%) and MM 1.2∙105 and 1.63∙105 were synthesized. The relative constants of chain transfer to tris-(pentafluorophenyl)germanium during the radical polymerization of 2,3,4,5,6-pentafluorobenzyl methacrylate and benzylmethacrylate were 1.26 and 0.45, respectively. The structure of the copolymers was confirmed by IR and NMR spectroscopy. By the method of TGA analysis, the temperature of the beginning of decomposition of polymers was established, which amounted to 228.2 ºC and 150 ºC for the fluorinated and non-fluorinated block copolymer, respectively. The properties of polymers in solutions were studied by GPC, molecular hydrodynamics and optics. For both fluorinated and non-fluorinated samples, only macromolecules were observed in dilute solutions. The behavior of a non-fluorinated sample was well described as a coil in a good solvent, while fluorinated macromolecules were characterized by a compressed coil conformation, due to the presence of fluorine in the polymer structure. It was shown that the block copolymers formed stable Langmuir monolayers with high surface pressures (50–58 mN/m), and the transferred Langmuir–Blodgett films on silicon wafers were hydrophilic (water contact angles are 68–73 ºC). A large hysteresis loop on the surface pressure isotherm obtained under compression-expansion conditions for a fluorinated block copolymer indicates significant inter- and intramolecular interactions at the water–air interface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Block copolymers (BCP) form the basis of the most ubiquitous materials such as thermoplastic elastomers, bridge interfaces in polymer blends, and are fundamental for the development of high-performance materials. Important advance of these materials is the accessibility of block copolymers, their wide variety in composition and functional group content, and ability to tune their properties [1,2,3].
Fluorinated polymers are well known for their thermal, chemical, and physical stability. For this reason, they can be used for sensors and cable insulation, membranes, packaging, sealing materials or chemical resistant components [4]. Moreover, fluorinated polymers belong to the category of low surface energy materials, due to their relatively low attraction to other molecules. Therefore, their interfacial energy is low, which causes their easy segregation [5]. Due to the advantages of fluorinated polymers, they are widely used to synthesis block copolymers.
Bosson et al. have reported on the successful synthesis of poly(pentafluorostyrene)-block-poly(butylacrylate) (PPFS-block-PBuA) block copolymers. Regulated radical polymerization of BCPs using poly(pentafluorostyrene) have already been reported, however, generally by ATRP. Click-chemistry for para-fluorine substitutions with azide and thiol have already been proposed for PPFS. Additionally, the self-assembly of the prepared BCPs before and after modification with 1-hexanethiol was studied by AFM and SAXS [6].
Polyvinyl ethers have attracted significant interest due to their applications in gas-selective permeable membranes, transparent materials for optical lenses [7], bioinert interfaces and hydrogen generation [8]. Block copolymers composed of vinyl ethers were prepared by living cationic polymerization technique [9, 10], for application as polymer surfactants [11], stimuli-responsive gels [12], and thermoplastic elastomers [13].
Block polymers prepared via cationic polymerization still show some possible applications despite the recent scarcity of researches respectively other branches of fundamental science. Since the breakthrough in 1980s, the conventional living cationic polymerization (LCP) has made remarkable progress and has become one of the most important pathways to synthesize well-defined polymers. Investigations and expansions of Lewis acid/base pairs have greatly promoted the development of LCP, and have inspired lots of subsequent developments. Tailored polymers with desirable chemical structures, molar masses, and functional groups have benefitted numerous studies and applications [14].
The study by Blagodatskikh et al. exploited several methods and was aimed to investigate the influence of the lengths of both fluorinated and anchor blocks of amphiphilic fluorinated diblock copolymers (DBCP) based on 2,3,4,5,6- pentafluorostyrene (PFS) and 2-hydroxyethyl methacrylate (HEMA) on repellent properties of the DBCP coatings applied on cotton/polyester fabrics. PFS was chosen as comonomer because it is commercially-available and its homopolymer is more soluble in most organic solvents than other fluorinated polymers [15]. Hydroxyl groups of hydroxyethyl methacrylate (HEMA) units were used to covalently attach DBCPs to hydroxyl groups of cotton/polyester fibers using hexamethylene diisocyanate (HMDI) as a crosslinking agent [16] and to form intradomain crosslinking between poly(2-hydroxyethyl methacrylate) (PHEMA) blocks. The amphiphilic fluorinated DBCPs were synthesized by two-stage radical polymerization with reversible addition–fragmentation chain transfer (RAFT) mechanism of PFS and HEMA. The resulting DBCP were characterized by GPC and FTIR. The repellent properties of cotton/polyester fabric DBCP coatings were estimated by water and diiodomethane (DI) contact angles (WCA and DICA) measurements. The chemical composition and morphology of the DBCP coatings on smooth (silicon slides) and rough (cotton/polyester fabric) surfaces were investigated using FTIR, energy dispersive spectroscopy (EDS) and SEM [16].
Fedorczyk, Krzywicka et al. have reported a new synthetic approach for the block copolymers of polyN-isopropylacrylamide–block–polystyrene (PNIPAM-b-PS) via the ATRP technique. The proposed method relies on application of 2-chloro-N-(2-hydroxyethyl)propanamide (NCPAE) acting as bifunctional initiator. Functional groups in NCPAE opens up the opportunity to perform ATRP of two monomers differing in activity in a sequential manner. Based on this protocol, copolymers with molecules containing two well-defined polymer chains linked by the proposed initiator were synthesized. To the best of our knowledge, NCPAE has not been used as an initiator in ATRP; and the synthesis of block copolymers via the ATRP technique with a sequence wherein PNIPAM is the first block and PS is the second one has not been reported yet. Importantly, the developed synthetic way allows to build macromolecules with required lengths of their hydrophobic blocks. Due to the sequence of the blocks, the synthesized molecules have an active end group on the hydrophobic side. Therefore, a hydrophobic block can be easily extended, modified and attached to the surface. The last possibility makes the synthesized materials especially promising for the preparation of modified surfaces with hydrophobic inside layer and hydrophilic as well as thermosensitive outside layer. Such surfaces are highly desirable in tissue engineering and construction of smart membranes [17, 18]. Consequently, it is proved that physical properties of block copolymers provide and even guarantee their future application in both industry and science despite of the difficulty of their synthesis [19,20,21].
A thermo- and pH-responsive copolymer of N-isopropylacrylamide (NIPAM) with maleic acid was studied by the light scattering and turbidimetry [22, 23]. Aqueous solutions with different pH and concentration were investigated. The temperatures of the onset of the phase separation and the width of this interval increased with decrease in copolymer concentration and increase in pH. Copolymers of NIPAM with fluorine-containing comonomers are of particular interest. The need to create methods for the synthesis of block copolymers based on fluorine-containing compounds is primarily due to the surface properties of these materials, which are characterized by low values of surface energy [24,25,26].
Moreover, the presence of fluorinated groups with a strong cohesive potential in the composition of block copolymers causes increased propensity of macromolecules for self-organization in solutions [27,28,29].
In previous study we investigated linear-dendritic block copolymers based on NIPAM and perfluorinated polyphenylengermane with different molar masses of hydrophilic blocks. These copolymers were studied in solutions and in Langmuir–Blodgett (LB) films. It was found that the macromolecules are capable of forming stable monolayer films at the water–air interface, regardless of the subphase acidity. For solutions of PNIPAM containing terminal a bis-pentafluorophenyl germanium group at the end of the chain, no low critical solution temperature behavior was found. In addition, the introduction of pentafluorophenylgermanium groups into PNIPAM significantly changes its properties at various interface boundaries [30]. The amphiphilic block copolymer PNIPAM–Ge(C6F5)2–poly(2,2,3,3-tetrafluoropropyl methacrylate) was prepared by the reaction of chain transfer to bis-(pentafluorophenyl)germane during the polymerization of N-isopropylacrylamide and the subsequent postpolymerization of isolated functional polymers in 2,2,3,3–tetrafluoropropyl methacrylate. The colloidal chemical properties of Langmuir monolayers and LB films of synthesized block copolymer have been studied. It was shown that regardless of the acidity of the subphase, high pressure of fracture of films are characteristic of monolayers of collapse pressures πcoll = (48–61) mN/m. The morphology of the LB films of functional polymer exhibit isolated elongated micelles with high densities in the “octopus” shape on the periphery of which there are terminal hydrophobic groups. For the LB film of block copolymer, a comb-like structure is observed with characteristic protrusions.
The technology for producing LB films has recently become a tool for studying various intermolecular interactions under conditions where the distance between molecules and their mutual orientation are strictly fixed. LB films are three-dimensional assemblies with predetermined and controllable properties at the molecular level. Analysis of the behavior of macromolecules in LB films will make it possible to determine the nature of intramolecular interactions and predict the properties of polymer materials in order to determine their potential area of use.
Amphiphilic fluorinated block copolymer films are promising materials for the creation of biosensors, artificial endoprostheses, heart valves and other implants for which the adhesion of blood clots is unacceptable. The thromboresistance of their surface is based on the formation of a thin organized layer of blood components due to the irreversible adsorption of proteins on the surface of the implant or biosensor, with the formation of a uniform hydrophilic layer with increased biocompatibility.
The aims of this work are synthesis, characterization analysis of possibility of formation and thin and stable monolayers and LB films of the new amphiphilic block-copolymers on based benzylmethacrylates и N-isopropylacrylamide.
Experimental
Materials
Tris-(pentafluorophenyl)germanium had been recrystallized from hexane and dried in vacuum. Bis-(pentafluorophenyl)germanium was used without any additional purification. The monomers, 2,3,4,5,6-pentafluorobenzylmethacrylate (BMA(F)) (boil temperature Tboil = 205 ºC) and benzylmethacrylate (BMA) (Tboil = 250 ºC) were distilled in vacuum with 4-methoxyphenol as their inhibitor. The initiator, AIBN, was recrystallized twice from ethanol, and then it was dried in vacuum at room temperature. Structure of the studied monomers and organic compounds is shown in Scheme 1.
Before the polymerization, NIPAM was recrystallized twice from hexane, the crystals were dried in vacuum at room temperature (melt temperature Tmelt = 62–63 ºC). Chain transfer constants Cs were determined by Mayo equation [31]:
where \(\overline{p }\)—degree of polymerization, V—polymerization rate, kp—chain growth constant, ko – open circuit constant, [S] – chain transfer concentration, [M] – monomer concentration, Cs—chain transfer constants.
The radical polymerization of NIPAM was carried out with AIBN qua the initiator in the mixture of solvents benzene-acetone (volume ratio 1:1) with an addition of [bis-(pentafluorophenyl)germane] = 2 10–2 mol/L in dilatometer ampoules. The ampoule was degassed in vacuum thrice, after that it was sealed off and polymerization was carried out at 60 ºC for 24 h.
PNIPAM synthesis conditions: [AIBN] = 9.1 10–3 mol/L, benzene-acetone solvent, t = 24 h, T = 60 ºC, [NIPAA] = 1.3 mol/L [32].
The obtained PNIPAM samples were purified from the monomer by threefold reprecipitation using the solvent, the acetone-hexane precipitant system, and dried in vacuum at room temperature until their stable weights. Thus, a functional polymer containing bis-(pentafluorophenyl)germanium groups was obtained.
Postpolymerization of PNIPAM-GeH(C6F5)2 in BMA(F) and BMA
Conditions of polymerization PNIPAM-GeH(C6F5)2 in BMA(F) were: (PNIPAM + BMA(F)) = 4% + 96%, [AIBN] = 5·10–3 mol/L, conversion 12%, acetone: BMA(F) = 2:1, [BMA(F)] = 2.08 mol/L. The same conditions were applied for the polymerization in BMA.
During the postpolymerization of functional PNIPAM-GeH(C6F5)2 in BMA(F) and BMA, not all macromolecules having active bis-pentafluorophenylgermanium groups participate in the chain transfer reaction. Thus, the postpolymerization product contained, in addition to the block copolymers PNIPAM-Ge(C6F5)2-PBMA(F) and PNIPAM-Ge(C6F5)2-PBMA, also PNIPAM-GeH(C6H5)2H, as well as homopolymers PBMA(F) and PBMA. For their separation, the hot extraction method was used in a Soxhlet apparatus in specially selected solvents: ethyl alcohol and benzene. In the case of the PNIPAM-Ge(C6F5)2-PBMA(F), the yield was 94 wt % of block copolymer and 1.2 wt % of PNIPAM-HGe(C6F5)2 homopolymer. For PNIPAM-Ge(C6F5)2-PBMA, the yield 89 wt % of copolymer and 2.0 wt % of PNIPAM-HGe(C6F5)2 homopolymer.
Experimental methods
IR spectroscopy
The IR spectra of polymers were recorded on an Infralum-FT801 IR-Fourier spectrometer (Russia, Novosibirsk) in the wavelength range from 400 to 4000 cm−1. For this purpose, tablets of polymers with KBr were prepared by pressing. The obtained absorption ranges were assigned to the respective groups using the table of characteristic frequencies.
NMR spectroscopy
Analysis of the structure of polymers was performed with aid of 1H NMR by spectrometer Agilent DD2 400 NB (USA, United Kingdom). NMR spectra were recorded using a solution in CDCl3. The reference combination was tetramethylsilane. The spectra were processed and interpreted by program MestReNova.
Gel-penetration chromatography (GPC)
The molar mass characteristics of the polymers were determined by GPC in high performance liquid chromatograph LC-20AD (Shimadzu, Japan). The analysis conditions: THF as an eluent, flow rate 1.0 cm3/min; the temperature level of the column oven and the refractometric detector cells equaled 40 ºC. Polymethyl methacrylate was used as standads.
Differential scanning Calorimetry (DSC)
Thermal analysis of the studied copolymers was performed using DSC 204 F1 Phoenix (NETZSCH-Geratebau, Germany). The measurement instrumentation and technique allow to measure phase transformations temperatures with an error of ± 1 ºC enthalpy of transformation ± 2%. During the differential scanning calorimetry, the samples were held at constant temperature (25 ºC) for 30 min under argon, followed with cooling to -70 ºC and then heating with a rate of 5 ºC/min up to 300 ºC.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis was performed with NETZSCH TG 209 F1 Phoenix (NETZSCH-Geratebau, Germany). During the thermogravimetric analysis, the samples were heating with a rate of 10 ºC/min from 25 ºC up to 300 ºC.
CHN(S)—analysis
The fraction of the hydrophilic block in the block copolymers was determined using a Elementar Vario EL cube for simultaneous CHN(S) determination (Elementar Analysensysteme GmbH, Germany).
Methods molecular hydrodynamics and optics
The absolute molar masses (MM) and the hydrodynamic radii Rh-D of macromolecules are determined by static (SLS) and dynamic light scattering (DLS) methods in solutions in chloroform (density ρ0 = 1.486 g/cm3, dynamic viscosity η0 = 0.57 cP, and refractive index n0 = 1.4443). The experiments were performed using Photocor Complex instrument (Photocor Instruments Inc., Moscow Russia). For investigated solutions in chloroform, the distribution of the light scattering intensity I over the hydrodynamic radii Rh-D(c) of scattering objects was unimodal. The values of translation diffusion coefficient D(c) were determined in the wide concentration range and extrapolated to zero concentration to obtain the diffusion constant D0. As is well known, the constant D0 are related to value of hydrodynamic radius Rh-D of macromolecules, which is defined using Stokes–Einstein equations [33, 34]:
where kB is Boltzmann’s constant and T is the absolute temperature. SLS measurements were performed at the angle of 90º since no angular dependence of the scattered light was observed [35, 36]. The obtained results were analyzed according to the Debye method, and the values of the weight-average molar masses Mw and the second virial coefficient A2 were calculated using the following formula [36]:
where H is the optical constant [36].
Here, I90 is the excessive intensity of light scattered at an angle of 90º, NA is Avogadro’s number, and dn/dc is the refractive index increment, which was determined using an RA-620 refractometer (Shimadzu, Kyoto, Japan) with a wavelength λ0 = 589.3 nm. The dn/dc values were calculated from the slope of concentration dependences of the difference Δn = n – n0 between refractive indexes of the solution n and the solvent n0. Note that the second virial coefficient A2 for solutions for both copolymers was positive and high enough: A2∙104 = 2.4 and 4.1 cm3 mole/g2 PNIPAM-Ge(C6F5)2-PBMA(F) and PNIPAM-Ge(C6F5)2-PBMA. Therefore chloroform is thermodynamically good for investigated samples.
The viscometry experiments were performed using an Ostwald-type Cannon–Manning capillary viscometer (Cannon Instrument Company Inc., PA, USA). The dependencies of the reduced viscosity ηsp/c on the concentration were analyzed using the Huggins equation [35]:
where [η] is the intrinsic viscosity, and kH is the Huggins constant. The Huggins constant kH characterizes the polymer–solvent hydrodynamic interaction and the hydrodynamic behavior of macromolecules copolymers in solutions. For the highest molar mass samples, the Huggins constants for linear polymers are close in magnitude and lie in the range of values typical for polymers in thermodynamically good solvents (0.3 < kH < 0.5).
Light scattering, viscometry, and refractometry experiments were carried out at 21 ºC. Millipore filters (Millipore Corp., Billerica, MA, USA) with a PTFE membrane with a pore size of 0.20 nm were used.
Receiving isotherms of surface pressures
Surface pressure isotherms were obtained by an installation KSVMini (Finland, Helsinki). Registration of π-A compression isotherms of monolayers was carried out using an automated Langmuir balance using the Wilhelmy plate method. Solutions of polymers in chloroform were being added to the surface of the aqueous subphase with a microsyringe for uniform distribution of the substance (volume of spreading solution Vs.sol. = 30 µL). The time for dissolving and evaporation of the solvent was 30 min during every experiment. After evaporation of the solvent from the surface of the subphase, the monolayer compressed with velocity 10 mm/min. The instrument was operated with deionized water (deionizer Chromatech (Russia, Dzerzhinsk) calculated electroconductivity less than 0.1 μΩ/cm).
The effective molecular area A0 of polymers in a monolayer was determined graphically by extrapolating the descending part of the isotherm π = f(A) to the abscissa axis (π = 0).
Receiving surface pressure isotherms under compression-expansion conditions was carried out under compression rate was 10 mm/min, expansion rate was 2 mm/min until surface pressure reached 45 mN/m.
Preparation of langmuir–blodgett films
The transfer of monomolecular films from the water–air interface was carried out by LB method. Silicon plates were used as transfer substrates (JSC "Telecom-STV", Russia, Moscow). Substrate parameters: width 1 cm, length 2.5 cm, thickness 0.014 cm. Before testing, the substrates were degreased with ethyl alcohol.
The purity of the substrates was controlled by the contact angle of deionized water on a silicon plate (θ = 56º). The levels of the surface pressure during the transfer of LB films equaled 45 mN/m, the volume of spreading solution was 30 μL, the transfer coefficients varied from 0.9 to 1.0.
Wetting contact angles
The wetting contact angles of the LB films were determined under leakage conditions by the “sitting drop” method with aid of a setup consisting of a microscope with a light source (Digital Microscope Model 500X, China), a lift table for a plate, and the CoolingTech program. To do this, a drop of deionized water (3 µL) was applied to the films with a microsyringe. The value of the contact angle θ was calculated from the size of the liquid droplets deposited on the solid surface, i.e., the height h and the base diameter d. Wetting kinetics was studied for all the copolymer films. It was found that there is no change for the contact angle while any timespan.
The value of the cosine of the wetting angle was calculated by the formula [33]:
AFM
The surface topography of the LB films was studied in the semi-contact mode by such an AFM as Solver P47 (NT-MDT) (Zelenograd, Russia) at room temperature.
Results and discussion
Synthesis and description of the block copolymers
The relative chain transfer constants Cs of radical polymerization of 2,3,4,5,6-pentafluorobenzyl methacrylate and benzyl methacrylate on tris-(pentafluorophenyl)germanium were equaled 1.26 and 0.45, respectively. Identically with the case of ST, NIPAM, and FMA, tris-(pentafluorophenyl)germanium proved to be an active chain transmitter [27, 30]. At the initial stage, N-isopropylacrylamide was radically polymerized in presence of bis(pentafluorophenyl)germanium to synthesized the PNIPAM-GeH(C6F5)2 functional polymer.
As a result of further postpolymerization of the functional polymer with 2,3,4,5,6-petafluorobenzyl methacrylate and benzyl methacrylate, after separation of homopolymers by hot extraction, PNIPAM-Ge(C6F5)2-PBMA(F) and PNIPAM-Ge(C6F5)2-PBMA were prepared (Scheme 2). Scheme 3 shows the structure formulas of the studied copolymers.
In Fig. 1 (a, b), IR ranges of BCP(F) (2) and BCP (3) are shown. Evidence of the formation of polymers is the presence bands characteristic of units of the functional polymer PNIPAM-GeH(C6F5)2 and PBMA(F) or PBMA homopolymers in the IR spectra of absorption. Typical stretching vibrations of the C–H bonds of the PBMA benzene ring appears in the range from 2953 to 3077 cm−1 (a). The bending vibrations of the C–H bonds and the stretching vibrations of the –C6H5 group of the aromatic ring are observed in the ranges of 696–750 cm–1 and 1454 cm–1, respectively (Fig. 1b). There are spectra related to vibrations of the carbonyl of the functional polymer PNIPAM-GeH(C6F5)2, PBMA(F) 1650 cm−1 (a), –NH group of chains PNIPAM 1644 cm−1 (a,b) and the –NH group (a wide band 3304 cm−1 and 3306 cm−1). There is also some growth in the intensity of the absorption band corresponding to the –C6F5 group (963 cm–1) [37] in Fig. 1a, representing the presence of PBMA(F) blocks in the copolymer.
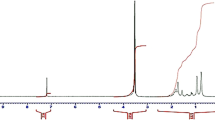
Another confirmation of the block copolymer formation are the results obtained by NMR spectroscopy. Figure 2 shows 1H NMR spectra of 3 and 2 BCPs. The block copolymer spectra present chemical shifts characteristic of both units (Table 1). The chemical shifts characteristic of a functional polymer in the block copolymers (Fig. 2a) shifted to a lower area: -CH3 (0.96 ppm) and > CH- (2.00 ppm). In fluorinated BCP (Fig. 2b), compared to PNIPAM-Ge(C6F5)2H, the chemical shifts of the -CH3 group to a lower area (0.86 ppm), the > CH-NH- group to a higher area (4.03 ppm).
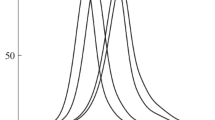
In Fig. 3 presents data from DSC analysis of the obtained polymers. The melting point of the PNIPAM homopolymer depends on the production conditions and can vary over a wide temperature range. In the case of the functional polymer PNIPAM-Ge(C6F5)2H, which has a bispentafluorophenylgermanium group at the end of the chain, the melting point is 76.6 ºC. The thermodynamic characteristics of fluorinated and non-fluorinated block copolymers differ significantly; in the case of BCP(F), a completely amorphous polymer with Tg = 76 ºC is obtained, while for block copolymer 3, in addition to the amorphous phase, there is also an ordered phase, as evidenced by the DSC curve, where areas of both melting and devitrification are present. Moreover, in the latter case, the presence of the amorphous phase slightly disorderes the system, lowering the melting temperature (Tmelt) by 7 ºC, compared to the original polymer 1, while the glass transition temperature of the amorphous block (Tg) increases compared to BCP(F) by 12.8 ºC.
One can be seen that transfer from the functional polymer to the block copolymer induces by growth of molar mass not breaking the single mode of molar mass distribution (Fig. 4), which means formation of BCPs, while which Mw of the block copolymers overcomes Mw of the functional polymer in many times (Table 2).
Molar masses and hydrodynamic characteristics of amphiphilic copolymers on based N-isopropylacrylamide and benzylmethacrylate
Analyzing experimental data obtained by SLS for multicomponent polymer systems, it is necessary to take into account the possible effect of compositional inhomogeneity on the determined values of MM. The compositional inhomogeneity of the polymer, that is, the inhomogeneity of its molecules in chemical composition, manifests itself in the fact that the components of the copolymer, scattering light independently, make different contributions to the total measured light scattering intensity. Therefore, the scattering intensity can be intensive even when the refractive index increment dn/dc of the block copolymer is close to zero. Accordingly, the measured molar mass will be apparent Ma, which may differ from the real Mw [38].
As shown in theoretical and experimental studies [39, 40], the compositional inhomogeneity leads to strongly overestimated values of the determined MM in cases where the refractive index increment dn/dc of the copolymer is low (generally dn/dc ≤ 0.03), while the refractive index increments components (dn/dc)1 and (dn/dc)2 are large and differ in meanings. For such systems, the apparent MM is described by the ratio [39, 40]:
where φ12 = [(dn/dc)1 – (dn/dc)2]/(dn/dc), Mw-1 and Mw-2 are weight-average molar mass of components 1 and 2, respectively, x is the mass fraction of component 1 in the sample. It can be seen from the above ratio that the magnitude of the apparent molar mass Ma varies from solvent to solvent. It can be shown that if the value of the parameter φ12 is in the range from –1 to 1 then the value of the measured Ma differs from the real molar mass by no more than 10%. In the case of a non-fluorinated polymer, where dn/dc = 0.11 cm3/g, this information is redundant, because the refractive index increment is high. For a linear copolymer, dn/dc was determined, but for its fluorinated copolymer, it was less, which is explained by the presence of fluorine in the units of the polymer chain, namely, by the fact that fluorine reduces the refractive index. Molar mass and hydrodynamic parameters are presented in Table 3.
Molar masses determined by SLS are higher than MM determined by GPC (Table 2). When comparing these MM values, the following circumstances must be taken into account. As is known, GPC is not an absolute method, and in this case, the polystyrene standard was used to determine the Mw. This single fact can lead to a systematic experimental error in the measurement of MM. In addition, the studied block copolymers, which contain components that differ greatly in chemical nature. Consequently, the hydrodynamic volumes of block copolymers can differ significantly from those of homopolymers of the same Mw, which can lead to an incorrect value of Mw according to GPC data.
The first value is obtained using the molar masses determined by the SLS method; the second value is the value determined using the molar masses determined by the SEC method.
The correctness of the MM values determined by SLS and GPC can be judged by the values of the hydrodynamic invariant A0-hd, which is calculated by the formula [41,42,43]:
with using the experimental values of molar mass M, intrinsic viscosity [η], and translation diffusion coefficient D0. The use of MM determined by SLS leads to A0-hd typical for flexible chain polymers (A0-hd = (3.2 ± 0.2) 10–10 erg/K mol1/3 [38, 43, 44]. The values A0-hd, obtained by MM from GPC data, much less than the given value. These facts indicate that the MM measured using the GPC is most likely underestimated. Accordingly, in further discussion we will use MM determined by the SLS method.
The difference in MM determined by different methods is also observed for the functionalized polymer PNIPAM-GeH(C6F5)2. In the same way, the values of the hydrodynamic invariant calculated using SLS and GPC differ for this sample (Table 3). The low MM value determined by GPC may be due to the conformation of its macromolecules. Actually functional group is high enough; its MM is eight percent of MM of functionalized polymer.
As is known, in the case of amphiphilic polymers, a linear chain surrounds the functional group, shielding it from the selective solvent [24, 28, 41]. Accordingly, such polymers are characterized by high intramolecular density in solutions. This may lead to underestimated MM values when determined by GPC.
As it can be seen from Table 3, the MM of BCP(F) is approximately 30% lower than the MM of PNIPAM-Ge(C6F5)2-PBMA. Taking into account molar masses M0 of the monomer units of the compared copolymers, one can expect that the difference in the chain lengths of PBMA(F) and PBMA will be larger. Actually, the polymerization degree m of PBMA(F) and PBMA components are related to the molar mass Mw of the block copolymers and MPNIPAM the functionalized polymer PNIPAM-Ge(C6F5)2 by the following relationship
Calculations using formula (9) lead to m = 153 and 236 for PBMA(F) and PBMA, respectively. Thus, the length of the PBMA(F) and PBMA chains differs by a factor of 1.5.
Apparently, this is due to the different nature of the units in the resulting block copolymers. With similar degrees of conversion (12%) and almost the same rate of postpolymerization of the functional polymer in PBMA(F) and PBMA, the formation of a lower molar mass polymer in the case of PBMA(F) is associated with pronounced hydrophobic (in the case of a fluorinated unit) and hydrophilic interactions (for the unit N-isopropylacrylamide) in macromolecules, which leads to compression of the polymer coil during chain growth and restricts the access of growth radicals to the monomer, resulting in the formation of a polymer with a lower Mw than for PBMA.
It is interesting to compare the results obtained for block copolymers with the literature data for the poly(benzylmethacrylate) homopolymer. Figure 5 and Table 4 shows Kuhn-Mark-Houwink dependences ([η] = K·Ma) for poly(benzylmethacrylate) in MEK and toluene according to the data of R. W. Richards [42].
The points for the block copolymers studied in this work are also presented there. It is seen that experimental point for 3 lie on the dependences polybenzylmethacrylate in MEK and toluene, while point for 2 is lower than ones dependencies. It indicates an increase in the intermolecular density at passage from of the BCP to BCP(F) and which is explained by the presence of fluorine in the polymer structure [43].
Isotherms of surface pressure of the copolymers
Surface pressure isotherms were received for the 2 and 3 BCPs (Fig. 6) while compression. Functional polymer molecules (1) do not form Langmuir monolayers, which is evidenced by the low surface pressure (10 mN/m) and the isotherm type, which indicates micelle formation at the water–air interface.
The surface pressure isotherm for the 2 (Fig. 6a) demonstrates that the compressible monolayers undergo several phase transitions. The segment of the obtained isotherm at a pressure close to zero, describes the area of a so-called two-dimensional gas, in which the molecules interact little with each other and are quite far from each other. With further compression of the film, the molecules begin to contact in the area of a two-dimensional liquid; the first increase in surface pressure is observed with a sufficiently large fall in the film area. At the interphase, there is a short linear segment on the isotherm i.d. a plateau. In this region the macromolecules begin to rearrange: the hydrophilic groups associate in water, while the hydrophobic ones remain on the surface. With further compression of the barrier, a sharp jump in surface pressure occurs with a small increase in the area of the film. Further compression of the film leads to its destruction (πcoll ~ 58 mN/m), since in this area of the isotherm the film is in the most compressed state.
Figure 6b shows an isotherm, which has no “liquid area”, but demonstrates intensive transfer from the area of the two-dimensional gas to the area of a “two-dimensional solid”, which shows that the molecules form a dense ordered monolayer on the water surface, in which each molecule adjoins the adjacent and contacts with the aqueous subphase Table 5.
To determine the stability of monolayers, isotherms were obtained for regime of compression and expansion (Fig. 7). The hysteresis of the isotherm curves takes place.
The value rigidity of the monolayer (β) was calculated of the tangent of the linear section of the isotherm with the maximum steepness. β describes the phase state with the stiffest packing of the condensed films [44]:
For the non-fluorinated polymers, the pressure on the isotherms also drops, but not as much as for fluorinated ones. The hysteresis loop is smaller in magnitude in the case of 3, which indicates minor inter- and intramolecular interactions in the polymer chains at the water–air interface (Fig. 7).
Surface properties of LB films researched by AFM and wetting
The surface topography of the LB films was investigated by the AFM. Figure 8 shows that the surface of the films is close to a flat with a large number of irregularities. One can see many protrusions oriented vertically with respect to the substrate surface.
Contact wetting angles of the LB films for block copolymers 2 and 3, installed into silicon plates, were determined. The value of the equilibrial contact angle of water on a pure silicon substrate was 56º. For BCP 2 and 3 theses angles were 73º and 68º, respectively, which meant the surfaces of those films were hydrophilic (Scheme 4).
Conclusion
New amphiphilic block copolymers based on N-isopropylacrylamide and 2,3,4,5,6-pentafluorobenzyl methacrylate and benzyl methacrylate were synthesized in high yields (89–94 wt. %) due to the double sequential chain transfer reaction with bis-(pentafluorophenyl)groups germanium. The relative constants of chain transfer to tris-(pentafluorophenyl)germanium during the radical polymerization of 2,3,4,5,6-pentafluorobenzyl methacrylate and benzylmethacrylate were 1.26 and 0.45, respectively.
The samples of block copolymers were dissolved molecularly in chloroform. It makes it possible to determined molar mass and hydrodynamic characteristics of block copolymers. Molar masses of block copolymers and functionalized polymers differed thirty times. Analysis of the values of molar mass of intrinsic viscosities and hydrodynamic radii showed that the synthesized copolymers are the typical flexible-chain polymers. The molecules of the non-fluorinated sample had a swollen coil conformation. In the case of PNIPAM-Ge(C6F5)2-PBMA(F), the presence of a fluorinated block leads to compaction of molecules and, accordingly, an increase in intramolecular density. It was shown that the amphiphilic block copolymers PNIPAM-Ge(C6F5)2-PBMA and PNIPAM-Ge(C6F5)2-PBMA(F) formed stable Langmuir monolayers (π = 50–58 mN/m). The AFM and wetting methods demonstrated the Langmuir–Blodgett films received of the block copolymers are hydrophilic. The study of surface pressure isotherms in Langmuir monolayers is necessary to determine the potential area of application of these objects, since if polymers are able to orient themselves in monomolecular films, this opens up wide opportunities in various fields from medicine to nanotechnology, since it is possible to control the self-organization of macromolecules at various interfaces.
Thus, the obtained we have proposed a non-labor-intensive two-step synthesis of new amphiphilic block copolymers with different structures containing a hydrophobic fragment of polybenzylmethacrylate and poly(2,3,4,5,6-pentafluorobenzyl methacrylate) with low surface energy and a hydrophilic poly(N-isopropylacrylamide) block may be one of the promising materials suitable for biomedical applications. This comprehensive approach to studying the physicochemical properties of polymers makes it possible to determine their potential area of use, for example, in the formation of polymerosomes during the delivery of drugs.
Data availability
The datasets generated during the study are available from the corresponding author upon reasonable request.
References
Dau H, Jones G, Tsogtgerel E, Nguyen D, Keyes A, Liu Y, Rauf H, Ordonez E, Puchelle V, Alhan H, Zhao C, Harth E (2022) Linear block copolymer synthesis. Chem Rev 122:14471. https://doi.org/10.1021/acs.chemrev.2c00189
Aoshima S, Kanaoka SA (2009) Renaissance in living cationic polymerization. Chem Rev 109:5245. https://doi.org/10.1021/cr900225g
Dey A, Haldar U, De P (2017) Block copolymer synthesis by the combination of living cationic polymerization and other polymerization methods. Front Chem 9:644547. https://doi.org/10.3389/fchem.2021.644547
Rösler A, Vandermeulen GWM, Klok H-A (2012) Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev 3:95. https://doi.org/10.1016/s0169-409x(01)00222-8
Agrahari V, Agrahari V (2018) Facilitating the translation of nanomedicines to a clinical product: challenges and opportunities. Drug Discov Today 23:974. https://doi.org/10.1016/j.drudis.2018.01.047
Bosson K, Marcasuzaa P, Bousquet A, Tovar GEM, Atanasov V, Billon L (2022) Pentafluorostyrene-based block copolymers controlled self-assembly pattern: A platform paving the way to functional block copolymers. Europ Polym J 179:111560. https://doi.org/10.1016/j.eurpolymj.2022.111560
Sakaguchi T, Yamazaki S, Hashimoto T (2017) Crosslinked membranes of poly(vinyl ether)s having oxyethylene side chains: The effects of the side chain length and the crosslinkable group on CO2 permeability. Polymer 112:278. https://doi.org/10.1016/j.polymer.2017.02.027
Yagci Y, Attila TM (2006) Mechanistic transformations involving living and controlled/living polymerization methods. Prog Polym Sci 31:1133. https://doi.org/10.1016/j.progpolymsci.2006.07.003
Corrigan N, Jung K, Moad G, Hawker CJ, Matyjaszewski K, Boyer C (2020) Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog Polym Sci 111:101311. https://doi.org/10.1016/j.progpolymsci.2020.101311
Jones GR, Whitfield R, Anastasaki A, Risangud N, Simula A, Keddie DJ, Haddleton DM (2018) Cu(0)-RDRP of methacrylates in DMSO: importance of the initiator. Polym Chem 9:2382. https://doi.org/10.1039/C8PY00814K
Kubisa P (2009) Ionic liquids as solvents for polymerization processes. Prog Challenges Prog Polym Sci 34:1333. https://doi.org/10.1016/j.progpolymsci.2009.09.001
Wu YB, Han L, Zhang XQ, Mao J, Gong LF, Guo WL, Gu K, Li SX (2015) Cationic polymerization of isobutyl vinyl ether in an imidazole-based ionic liquid: characteristics and mechanism. Polym Chem 6:2560. https://doi.org/10.1039/c4py01784f
Hirano T, Kizu R, Hashimoto J, Munekane J, Miwa Y, Oshimura M, Ute K (2018) Thermally induced cationic polymerization of isobutyl vinyl ether in toluene in the presence of solvate ionic liquid. Polym Chem 9:1421. https://doi.org/10.1039/C8PY00164B
Ayat M, Belbachir M, Rahmouni A (2017) Synthesis of block copolymers consists on vinylidene chloride and α- Methylstyrene by cationic polymerization using an acid exchanged motmorillonite clay as heterogeneous catalyst. J Molec Struct 1139:381. https://doi.org/10.1016/j.molstruc.2017.03.056
Ayat M, Belbachir M, Rahmouni A (2018) Cationic polymerization of poly(α-methylstyrene-block-isobutyl vinyl ether) using Maghnite-H+ (Algerian MMT) clay as catalyst. Polym Bull 75:5355. https://doi.org/10.1007/s00289-018-2328-8
Florin RE (1949) Note on Friedel-Crafts Copolymerization. J Am Chem Soc 71:1867. https://doi.org/10.1021/JA01173A507
Timofeeva MN, Panchenko VN, Gil A, Zakusin SV, Krupskaya VV, Volcho KP, Vicente MA (2015) Effect of structure and acidity of acid modified clay materials on synthesis of octahydro-2H-chromen-4-ol from vanillin and isopulegol. Catal Commun 69:234. https://doi.org/10.1016/J.CATCOM.2015.07.005
Tarabukina EB, Simonova MA, Bucatariu S, Fundueanu G, Filippov AP (2016) Behavior of thermo- and pH-responsive copolymer of N-isopropylacrylamide and maleic acid in aqueous solutions. Int J Polym Anal Charact 21: 11. https://doi.org/10.1080/1023666X.2015.1089459
Simonova MA, Tarabukina EB, Filippov AP, Constantin M, Popescu I (2015) Effect of Concentration on the properties of heat- and PH-sensitive copolymers of poly(N-isopropylacrylamide) with maleic acid in aqueous solutions. Fiber Chem 47:152. https://doi.org/10.1007/s10692-015-9656-3
Zamyshlyayeva OG, Semchikov YD, Kiryanov KV, Gasilova ER, Simonova MA, Filippov AP, Kozlov AV, Shandryuk GA, Bochkarev MN (2011) Synthesis and properties of hyperbranched copolymers based on perfluorinated germanium hydrides. Polym Sci Ser B 53:1453. https://doi.org/10.1134/S1560090411080057
Pitois C, Vestberg R, Rodlert M (2002) Fluorinated dendritic polymers and dendrimers for waveguide applications. Opt Mater 21:499. https://doi.org/10.1016/S0925-3467(02)00190-8
Zamyshlyayeva OG, Ionychev BN, Frolova AI, Kopylova NA, Zaytsev SD, Semchikov YD, Batenkin MA, Simonova MA (2019) Controlled synthesis of methacrylic acid-methyl acrylate copolymers and their properties at various interfaces. Rus J Appl Chem 92:775. https://doi.org/10.1134/S1070427219060077
Zakharova OG, Tarasova YV, Simonova MA, Semchikov YD, Filippov AP (2009) Synthesis and structural and conformational properties of hybrid polymers of styrene with perfluorinated compounds of germanium. Polym Sci Ser A 51:512. https://doi.org/10.1016/j.molliq.2019.111355
Tarabukina E, Kozlov A, Simonova M, Zamyshlyayeva O, Semchikov Y (2011) Hydrodynamic and molecular properties of hyperbranched copolymers formed by pentafluorophenylgermane hydrides. Int J Polym Anal Charact 16:369. https://doi.org/10.1080/1023666X.2011.595960
Simonova MA, Zamyshlyayeva OG, Simonova AA, Tarasova YV, Filippov AP (2009) Model and hybrid polystyrenes containing trispentafluorophenylgermanium and groups. Int J Polym Anal Charact 14:454. https://doi.org/10.1080/10236660903031330
Zamyshlyayeva O, Shaliagina Z, Simonova M, Filippov A, Baten′kin M (2023) Properties in Langmuir monolayers and Langmuir-Blodgett films of a block copolymer based on N-Isopropylacrylamide and 2,2,3,3-tetrafluropropyl methacrylate. Polymers 14:5193. https://doi.org/10.3390/polym14235193
Polymer Handbook, ed. by Brandrup J, Immercut EH, Grulke EA. John Wiley & Sons, INC. 1999. https://doi.org/10.1002/1097-0126(200007)49:7%3C807::AID-PI436%3E3.0.CO;2-1
Fujishige S (1987) Intrinsic viscosity-molecular weight relationships for poly(N-isopropilacrylamide) solutions. Polym J 19:297. https://doi.org/10.1295/POLYMJ.19.297
Tsvetkov VN (1989) Rigid-Chain Polymers, 1st ed, Plenum Press, New York. https://doi.org/10.1126/science.246.4927.272.b
Bushuk W, Benoit H (1958) Light scattering studies of copolymers: I. effect of heterogeneity of chain composition on the molecular weight. Canad J Chem 36:1616. https://doi.org/10.1139/v58-235
Kratochvil P (1987) Classical Light Scattering from Polymer Solution. 1st ed, Elsevier, Amsterdam, 346. https://doi.org/10.1016/0166-6622(88)80098-2
Schärtl, W. (2007) Light Scattering from Polymer Solutions and Nanoparticle Dispersions. 1st ed, Springer, Berlin. 187. https://doi.org/10.1007/978-3-540-71951-9
Summ BD, Goryunov TuV (1976) Physicochemical basis of wetting and spreading. Chemistry, Moscow ((in Russ.))
Zamyshlyayeva OG, Smirnov EA, Zakharycheva NA (2017) Linear-dendritic block copolymers based on N-isopropylacrylamide and perfluorinated polyphenylengermane: synthesis and properties at various interfacial boundaries. Polym Sci 59:708. https://doi.org/10.1134/S1560090417060112
Eskin V (1986) Light distraction by polymer solutions and macromolecular properties. Nauka, Leningrad. 288 (Russian)
Stockmayer W, Moore L, Fixman M (1955) Copolymers in dilute solution. i. preliminary results for styrene-methyl methacrylate. J Polym Sci 16:517. https://doi.org/10.1002/pol.1955.120168236
Filippov AP, Zamyshlyaeva OG, Tarabukina EB, Simonova MA, Kozlov AV, Semchikov YD (2012) Structural and conformational properties of hyperbranched copolymers based on perfluorinated germanium hydrides. Polym Sci Ser A 54:319. https://doi.org/10.1134/S0965545X12050033
Tsvetkov VN, Lavrenko PN, Bushin SV (1984) Hydrodynamic invariant of polymer molecules. J Polym Sci Part A 22:3447. https://doi.org/10.1002/pol.1984.170221160
Tsvetkov VN, Lavrenko PN, Bushin SV (1982) A Hydrodynamic invariant of polymeric molecules. Russ Chem Rev 51:975. https://doi.org/10.1070/RC1982V051N10ABEH002935
Simonova MA, Tarasova EV, Dudkina MM, Tenkovtsev AV, Filippov AP (2019) Synthesis and hydrodynamic and conformation properties of star-shaped polystyrene with calix[8]arene core. Int J Polym Anal Charact 24:87. https://doi.org/10.1080/1023666X.2018.1555894
Tarabukina E, Amirova A, Belyaeva E, Krasova A, Simonova M, Filippov A, Meleshko T, Ilgach D, Bogorad N, Yakimansky A (2013) Conformational characteristics of polyimide initiator for the synthesis of poly(methylmethacrylate) grafted block-copolymers. J Macromol Sci B 52:1545. https://doi.org/10.1080/00222348.2013.810018
Richards RW (1977) Dilute solution properties of poly(benzylmethacrylate). Polymer 18:114. https://doi.org/10.1016/0032-3861(77)90024-6
Wurm F, Frey H (2011) Linear-dendritic block-copolymers: The state of the art and exciting perspectives. Prog Polym Sci 36:1. https://doi.org/10.1016/j.progpolymsci.2010.07.009
Adamson AW (1976) Physical chemistry of surfaces, 3rd edn. Wiley, New York
Author information
Authors and Affiliations
Contributions
Investigation, visualization, writing-original draft preparation, methodology O.Z. and M.S; conceptualization, project administration O.Z. and A.P.; synthesis, analysis of polymers and obtaining surface pressure isotherms O.Z. and E.O.; research by the method of AFM M.B.; wettability E.O.; DSC and TGA analysis A.M; molar masses and hydrodynamic characteristics M.S. and M.Z. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zamyshlyayeva, O., Simonova, M., Zelentsov, M. et al. Synthesis and properties of N-isopropylacrylamide and benzylmethacrylate-based amphiphilic block copolymers on different interphase surfaces. J Polym Res 31, 232 (2024). https://doi.org/10.1007/s10965-024-04073-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-024-04073-6