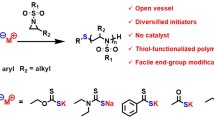
Abstract
This work outlines the synthesis of Schiff base derivatives through the reaction of O-acyl salicylaldehyde with diverse amines, facilitated by ethanol as a solvent. The derivatives were structurally analyzed using 1H and 13C-NMR, FT-IR, and LC–MS techniques. The Schiff base derivative, bearing a phenol moiety, subsequently underwent oxidative polycondensation reaction in water, employing NaOCl as the mild and safe oxidant, presenting a sustainable alternative to conventional methods. The polymer was then subjected to investigations using 1H-NMR, FT-IR, DSC, TGA, SEM, and EDX spectroscopies, revealing exceptional thermal stability suitable for high-temperature applications. The results not only contribute to the development of poly(azomethine-ester) but also highlight the importance of adopting environmentally friendly methodologies in materials synthesis. The findings highlight the potential biological applications of the imine linkage and emphasize the use of green chemistry principles to promote sustainability in synthetic chemistry practices.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salicylaldehyde, an aromatic compound with applications as a precursor for compounds possessing antiviral [1, 2], antibacterial [3, 4], antioxidant [5, 6], and antifungal[7, 8] properties, underwent acylation using diverse catalysts [9, 10]. Substituted hydroxybenzaldehyde yielded esters with various carboxylic acid chlorides [11, 12]. Oxidative esterification of the hydroxyl group in salicylaldehyde results in the generation of ester derivatives, such as 2-formylphenyl benzoate [13]. Aromatic esters are recognized for their facile synthesis and relative resilience to heat and hydrolysis [14]. The investigation into a bio-based polyester's thermal properties highlighted a need for eco-friendly reagents in its synthesis [15]. Poly(anhydride-ester) based on bioactive salicylic acid in a green one-pot method was studied for biodegradability and cytocompatibility by Uhrich et al.[16]
The widespread use of salicylaldehyde and its derivatives includes their interaction with amine derivatives to create antimicrobial Schiff bases and their complexes [17, 18]. Salicylaldehyde azine has demonstrated noteworthy antibacterial qualities [19]. Investigation into the addition of hydroxybenzaldehyde into the recurring units of polyacrylonitrile revealed potential antimicrobial effects [20, 21]. Polymeric materials that incorporated substituted salicylaldehyde and either chitosan or cellulose were studied for their imine or azomethine linkages [22, 23]. Al-Ameiry investigated the polyimine synthesis via microwave-assisted method [24].
Poly(azomethine-esters) are polymers characterized by the presence of –C = N- and –COO- linkages, showcasing unique properties derived from the combination of polyester and polyazomethine traits [25]. These polymers exhibit features such as thermal stability [26], mechanical strength [27], antimicrobial activity [28, 29], mesomorphic traits [30], as well as optical [31], fibre-forming [32], and semi-conductive[33] characteristics. Many of these polymers fall within the thermotropic class of polymers [34]. Recognizing the significance of these polymers, the current study is dedicated to the design and development of systems based on conjugated poly(azomethine-esters).
Despite its various commendable properties, poly(azomethine-ester) encounters a drawback related to its synthesis requiring specific conditions and its insolubility in any solvent due to strong internal interactions [35]. Therefore, there is a need for enhancing the solubility of this polymer using an environmentally friendly approach to broaden its applications. In this context, the oxidative polycondensation of O-acyl salicylaldehyde with amines using a green oxidant NaOCl has been investigated to examine the thermal behavior and solubility of the polymer.
We present here the procedures employed to synthesize Schiff base derivatives. This involves the reaction of an O-acyl salicylaldehyde with various amines, followed by the polymerization of one of the derivatives with a phenol moiety via an oxidative polycondensation reaction. As far as we are aware based on existing literature, there are limited documentation on environmentally friendly methods used for the oxidative polycondensation of O-acyl salicylaldehyde schiff base derivative. This preliminary report outlines the synthesis and characterization of a poly(azomethine-ester) based on salicylaldehyde, contributing to the development of organic polymers suitable for biomedical applications.
Experimental section
Materials
Salicylaldehyde and sodium sulphate (anhydrous) were received from Sisco Research Laboratories Pvt. Ltd. 1,3,5-benzenetricarbonyl trichloride was obtained from Sigma-Aldrich, Co. (St Louis, USA). Ethyl acetate was purchased from Finar Chemicals Ltd. (Ahmedabad, India). Triethylamine (Et3N), ethanolamine and sodium hypochlorite solution (NaOCl, 4% w/v active chlorine) were supplied by Merck Specialties Pvt. Ltd. (Mumbai, India). 3-Aminophenol was purchased from S. D. Fine Chemicals (Mumbai, India). Ethylenediamine and potassium hydroxide pellets (KOH) was acquired by Thermo Fisher Scientific India Pvt. Ltd. (Mumbai, India).1,6-Diaminohexane was supplied by Alfa Aesar (Massachusetts, USA).
Synthesis of Schiff base derivatives (Ia-Id)
0.02 mol of the previously synthesized purified derivative (I) were dissolved in 20.00 ml ethanol at 60 °C with continuous stirring. To this homogeneous solution, 0.02 mol of the amine was gradually added, and stirring was maintained at the same temperature for 3 h. The appearance of a yellow color in the mixture over time indicated the formation of imine linkages. This reaction progress was monitored using TLC, revealing a distinct yellow spot characteristic of the Schiff base derivative. The resulting mixture was then extracted with ethyl acetate, followed by washing with cold ethanol and hexane to remove any unreacted substrates and yield the desired products (Fig. 1).
Tris(2-(2-hydroxyethyliminomethyl)phenyl) benzene-1,3,5-tricarboxylate (Ia): Yellow oily liquid, yield 86.50%. IR, ν (cm−1): 3398.60 (O–H); 2926.17 (C-H); 1738.08 (C = O); 1632.05 (CH = N). 1H-NMR (Chloroform-D, 400 MHz): δ 8.35 (s, 5H), 7.31 (dd, 2H), 7.27 (d, 1H), 7.24 (ddd, 4H), 6.92 (dd, 3H), 6.87 (td, 3H), 3.88 (m, 7H), 3.71 (m, 8H). 13C-NMR (Chloroform-D, 400 MHz): δ 167.04, 161.41, 132.63, 132.60, 131.57, 118.76, 118.74, 118.71, 117.22, 117.20, 62.18, 61.69. ESI–MS for C36H33N3O9, calculated [M + H]+: 652.68, observed 652.68.
Tris(2-(3-hydroxyphenyliminomethyl)phenyl) benzene-1,3,5-tricarboxylate (Ib): Yellowish powder, yield 90.10%, m. p. 130–131 °C. IR, ν (cm−1): 3386.69 (O–H); 2924.37 (C-H); 1738.08 (C = O); 1616.82 (CH = N). 1H-NMR (DMSO-d6, 400 MHz): δ 8.85 (s, 3H), 8.71 (s, 3H), 8.32 (s, 3H), 7.42 (dd, 3H), 7.24 (ddd, 3H), 7.17 (dd, 3H), 6.97 (m, 6H), 6.77 (m, 6H), 6.31 (t, 3H). 13C-NMR (DMSO-d6, 400 MHz): δ 160.79, 158.53, 150.30, 129.89, 120.01, 115.21, 109.44, 105.88, 103.74, 101.41. ESI–MS for C48H33N3O9, calculated [M + H]+: 796.81, observed 796.81.
Tris(2-(2-aminoethyliminomethyl)phenyl) benzene-1,3,5-tricarboxylate (Ic): Yellow solid, yield 92.30%, m. p. 111–112 °C. IR, ν (cm−1): 3476.12, 3307.32 (N–H); 2919.89 (C-H); 1729.25 (C = O); 1626.74 (CH = N). 1H-NMR (Chloroform-D, 400 MHz): δ 8.34 (s, 5H), 7.28 (ddd, 4H), 7.22 (dd, 3H), 6.94 (dd, 3H), 6.84 (td, 3H), 3.92 (m, 10H), 3.66 (m, 4H), 3.06 (m, 4H). 13C-NMR (Chloroform-D, 400 MHz): δ 166.60, 161.08, 132.49, 131.59, 118.79, 118.72, 117.04, 59.82, 57.83. ESI–MS for C36H36N6O6, calculated [M + H]+: 649.73, observed 649.72.
Tris(2-(6-aminohexyliminomethyl)phenyl) benzene-1,3,5-tricarboxylate (Id): Yellow powder, yield 89.40%, m. p. 58–59 °C. IR, ν (cm−1): 3435.05, 3210.54 (N–H); 2925.21 (C-H); 1729.25 (C = O); 1626.74 (CH = N). 1H-NMR (Chloroform-D, 400 MHz): δ 8.31 (s, 5H), 7.29 (ddd, 4H), 7.21 (dd, 3H), 6.95 (dd, 3H), 6.85 (td, 3H), 3.58 (m, 12H), 1.70 (t, 12H), 1.43 (m, 14H), 1.25 (m, 4H). 13C-NMR (Chloroform-D, 400 MHz): δ 164.70, 161.46, 132.17, 131.21, 118.89, 118.51, 117.12, 59.44, 30.79, 29.81, 26.93. ESI–MS for C48H60N6O6, calculated [M + H]+: 818.05, observed: 818.06.
Oxidative polycondensation of Schiff base derivative (Ib)
0.02 mol of Schiff base derivative (Ib) was dissolved in a 0.02 mol aqueous 10% KOH solution at 60 °C. Subsequently, a 30% NaOCl aqueous solution was slowly added dropwise to the reaction mixture, causing the color to deepen to a dark brown. The temperature of the reaction mixture was then raised to 90 °C and stirred continuously for 6 h (Fig. 2). To neutralize the reaction, HCl was added at room temperature, resulting in the formation of a precipitate. The crude product was washed with hot water and then dried in an oven at 100°C, yielding the final solid black-colored polymer (IbP). Polymerization attempts were made by varying the NaOCl concentration, revealing a lower precipitated crude product at lower concentrations, with little effect observed upon increasing the concentration.
Characterization
1H-NMR and 13C-NMR (400 MHz) spectroscopy were employed using a Jeol JNM-ECZL400G spectrometer (Jeol Asia PTE Ltd., Singapore) to characterize the purified compounds. FT-IR spectroscopy was performed with a Perkin Elmer Spectrum Two spectrometer (USA) in KBr mode, covering a spectral range from 400–4000 cm−1. Mass spectrometric data were acquired using an Acquity TQD LC–MS spectrometer (Waters, USA) operating in electrospray ionization (ESI) mode. TGA of the polymer was conducted on a SHIMADZU DTG-60S spectrometer (Japan) with a heating rate of 20 °C/min in a nitrogen atmosphere, covering a range of 20 °C to 500 °C. DSC of the polymer was carried out using a Setaram LABSYS DSC 131 spectrometer (France) from 30 °C to 300 °C with a heating rate of 10 °C/min in an N2 atmosphere. Morphological characteristics of the polymer were investigated through field emission scanning electron microscopy (FE-SEM) and energy-dispersive x-ray analysis (EDX) using a ZEISS EVO 18 spectrometer (Germany).
Results and discussion
The detailed procedure for producing derivative (I) from salicylaldehyde, employing triacyl chloride and Et3N in ethyl acetate, is outlined in our prior research [36]. In this study, we conducted reactions of derivative (I) with diverse amines in ethanol to generate Schiff base derivatives (Ia-Id), consistent with established methodologies from earlier studies [37, 38]. To confirm the outcomes, we performed the preliminary 2,4-DNP test. The 2,4-DNP test involves an aqueous ethanolic solution of 2,4-dinitrophenylhydrazine, which forms an orange-yellow precipitate with aldehydes and ketones. Derivative (I) contains free aldehyde groups that react with the 2,4-DNP reagent, resulting in an orange-yellow precipitate. However, Schiff base derivatives, which feature imine and ester linkages, give negative results in the 2,4-DNP test.
A Schiff base derivative featuring a phenol moiety undergoes polymerization through oxidative polycondensation reactions at its ortho and para positions, as documented in related literature [39, 40]. The repeating units of the polymer are connected via the ortho and para positions of the phenol group, as illustrated in similar work by Demetgül et al.[41] NaOCl was preferred due to its easily accessible conditions, such as room temperature and ambient pressure, as highlighted by Kaya et al.[42, 43] It is also low-cost and environmentally friendly. Polymers obtained using NaOCl as the oxidant have higher molecular weights and increased yields, with a PDI close to 1.
To facilitate a comparative analysis of the stability and morphology of polymer (IbP), polymer (II) was synthesized. This involved the one-pot synthesis of a known Schiff base by combining salicylaldehyde and 3-aminophenol, followed by subjecting it to oxidative polycondensation reactions, using the methods employed for the synthesis of polymer (IbP). The polymer (IbP) readily dissolved in DMSO and DMF solvents but exhibited limited solubility in acetone and no solubility in methanol, ethanol and chloroform.
FT-IR spectroscopy
In the supplementary materials, the FT-IR spectrum of derivative (I) indicated C = O peaks at 1779.52 cm−1 for the ester and at 1700.23 cm−1 for the aldehyde. Additionally, C-H peaks were observed at 3075.23 cm−1 and 2751.56 cm−1, while C-O peaks were found at 1224.45 cm−1 and 1104.65 cm−1. The Schiff base derivatives are illustrated in Fig. 3 through the FT-IR spectra. It is evident that imine linkages form between the aldehyde group of derivative (I) and various amines, leading to the emergence of the –CH = N- absorption at 1632.05 cm−1 for derivative (Ia), 1616.82 cm−1 for derivative (Ib), and 1626.74 cm−1 for derivatives (Ic) and (Id). The ester C = O peak is consistently observed at 1729.25 cm−1 or 1738.08 cm−1 while the C-H stretching is noticeable within the range of 2853.27–2931.78 cm−1 across all derivatives. A broad peak is evident at 3398.60 cm−1 for the O–H group in derivative (Ia) and at 3386.69 cm−1 for derivative (Ib). Similarly, the N–H stretching of the primary amine is observed at 3476.12 cm−1 and 3307.32 cm−1 for derivative (Ic), while derivative (Id) exhibits peaks at 3435.05 cm−1 and 3210.54 cm−1.
In Fig. 4, the FT-IR spectrum reveals the distinctive features of polymers (II) and (IbP). Both polymers exhibit an imine linkage, appearing at around 1629.55 cm−1. Polymer (IbP) displays a distinct C = O peak associated with the ester group at 1746.10 cm−1, a characteristic absent in polymer (II). The C-H stretching in polymer (IbP) are clearly evident at 2922.30 cm−1 and 2856.38 cm−1. The O–H stretching for both polymers is observed at 3432.83 cm−1.
Mass spectrometry
The mass spectra of the Schiff base derivatives indicated that the observed mass values matched the calculated masses of the amide derivatives as detailed in the supplementary materials.
NMR spectroscopy
The derivative (I) was confirmed using 1H-NMR spectroscopy, as shown in the supplementary materials. The 1H-NMR spectra of Schiff base derivatives (Ia-Id) depicted in Fig. 5 were acquired using Chloroform-D and DMSO-d6 solvents. In derivative (Ib), the most deshielded singlet peak, observed at 8.85 ppm, belongs to the three –OH protons. A strong singlet peak at 8.35 ppm in (Ia), 8.34 ppm in (Ic), and 8.31 ppm in (Id) indicates the presence of three central aromatic ring protons and two imine protons (–CH = N-). In (Ib), the 3 central aromatic ring protons and 3 imine protons (–CH = N-) are observed at 8.71 and 8.32 ppm, respectively. For derivatives (Ia), (Ic), and (Id), 12 aromatic protons and 1 imine proton are evident within the 6.83–7.31 ppm range, while derivative (Ib) exhibits 24 aromatic protons in the 6.31–7.42 ppm range. In derivative (Ia), the 12 –CH2 protons and 3 –OH protons were observed between 3.70–3.90 ppm. Similarly, for (Ic), the 12 –CH2 protons and 6 –NH2 protons were observed in the 3.03–3.92 ppm range, while in (Id), the 36 –CH2 protons and 6 –NH2 protons were found within the 1.25–3.59 ppm range.
Figure 6 illustrates the 13C-NMR spectra of Schiff base derivatives. The most downfield signals indicative of the ester C = O bond and imine -C = N- linkage were recorded at 167.04 ppm in derivative (Ia), 160.79 ppm in derivative (Ib), 166.60 ppm in derivative (Ic), and 164.70 ppm in derivative (Id). Meanwhile, the C-O peak was found at 161.41 ppm in (Ia), 158.53 ppm in (Ib), 161.08 ppm in (Ic), and 161.46 ppm in (Id). A distinct C-N peak appeared at 150.30 ppm for derivative (Ib), while for the other derivatives, it fell within the aromatic region. The aromatic region was consistently observed for all derivatives within the range of 101.41–132.63 ppm. Aliphatic peaks were observed at 61.69–62.18 ppm for (Ia), 57.83–59.82 ppm for (Ic), and 26.93–59.44 ppm for (Id). These aliphatic peaks, combined with additional signals, serve as confirmation for the formation of the Schiff bases from derivative (I).
The 1H-NMR spectrum of polymer (IbP) was recorded in DMSO-d6 solvent (Fig. 7). It exhibits peaks at 6.44–7.60 ppm for aromatic protons and 8.34–8.95 ppm for –OH, imine, and central aromatic protons. These peaks closely resemble those in the 1H-NMR spectrum of the derivative (Ib), showing slight shifts and broadening. This similarity confirms the successful polymerization of the derivative (Ib).
Thermal analysis
The thermograms of polymers (IbP) and (II) are illustrated in Fig. 8 (a). The initial degradation of both polymers occurred at < 83 °C, resulting in a 3% weight loss for polymer (IbP) and a 10% weight loss for polymer (II) due to entrapped moisture. Polymer (IbP) exhibited second and third stage weight losses at 208 and 413 °C, likely due to release of small groups. In contrast, polymer (II) displayed a significant degradation weight loss at 312 °C. At 480 °C, polymer (IbP) exhibited a weight reduction of only 20%, whereas polymer (II) experienced a more substantial 45% decrease in weight. The improved resistance to degradation in polymer (IbP) was potentially due to its bulky rings and conjugated structure, enhancing its thermal stability compared to the simpler polymer (II).
DSC results for polymers (IbP) and (II) are shown in Fig. 8 (b). The Schiff base polymer (IbP) exhibits a Tg value of approximately 125 °C, slightly higher than that of polymer (II) at 112 °C. This difference is attributed to the presence of imine linkages and increased bulky aromatic rings in polymer (IbP).
Morphological analysis
The micrographs in Fig. 9 depict the surface characteristics of polymers (II) and (IbP). Polymer (II) displays structured cubic aggregations on its surface, devoid of any cavities. In contrast, polymer (IbP) reveals prominent cavities on its densely packed smooth and uniform top surface. The observed changes in the surface morphology of the polymer are attributed to the presence of an increased number of aromatic rings, coupled with Schiff base functionality and reinforced by intermolecular forces.
The elemental mapping (Fig. 10) using EDX revealed that solely C and O elements were identified in the polymer (IbP) and no contaminants were observed.
Conclusions
This study successfully demonstrated the synthesis of Schiff base derivatives and their subsequent transformation into poly(azomethine-ester) through a green and sustainable approach. The use of salicylaldehyde and various amines in ethanol as a solvent facilitated the efficient production of the Schiff base derivatives. The structures of the derivatives were well-established. The polymerization was carried out via oxidative polycondensation reactions in water using a green oxidant like NaOCl, which forms a heat-resistant polymer at substantial temperatures. This work advocates for the integration of green chemistry principles in the pursuit of sustainable and eco-friendly materials, thereby encouraging a shift towards more responsible practices in the field of synthetic chemistry. The examination of the biological characteristics of the Schiff base derivatives and the obtained polymer is currently underway, and the results are scheduled for future publication.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonablerequest.
References
Bhandarkar SE, Pathare PP, Khobragade BP (2023) New Nickel (II), Copper (II) and Cobalt (II) Complexes Based Salicyaldehyde Schiff Base: Synthesis, Characterisation, and Antiviral Activity. Mater Today Proc 92:807–816. https://doi.org/10.1016/j.matpr.2023.04.381
Kratky M, Vinsova J (2011) Antiviral Activity of Substituted Salicylanilides - A Review. Mini-Rev Med Chem 11:956–967. https://doi.org/10.2174/138955711797068382
Joseph J, Mary NL, Sidambaram R (2010) Synthesis, Characterization, and Antibacterial Activity of the Schiff Bases Derived from Thiosemicarbazide, Salicylaldehyde, 5-bromosalicylaldehyde and their Copper(II) and Nickel(II) Complexes. Synth React Inorganic, Met Nano-Metal Chem 40:930–933. https://doi.org/10.1080/15533174.2010.522661
Belay Y, Muller A, Ndinteh DT et al (2023) Synthesis, antibacterial activities, cytotoxicity, and molecular docking studies of Salicyledene derivatives. J Mol Struct 1275:134623. https://doi.org/10.1016/j.molstruc.2022.134623
Bountagkidou OG, Ordoudi SA, Tsimidou MZ (2010) Structure–antioxidant activity relationship study of natural hydroxybenzaldehydes using in vitro assays. Food Res Int 43:2014–2019. https://doi.org/10.1016/j.foodres.2010.05.021
Rahmawati NR, Ngadiwiyana N, Prasetya NB, Sarjono PR, Andriani Y, Syamsumir DF, Ismiyarto I (2020) Synthesis of hydroxylated azomethine compounds and the antioxidant activity. AIP Conf Proc 2237:020023. https://doi.org/10.1063/5.0005806
Huseynzada A, Jelsch C, Akhundzada HV et al (2023) Crystal structure, Hirshfeld surface analysis, computational and antifungal studies of dihydropyrimidines on the basis of salicylaldehyde derivatives. J Iran Chem Soc 20:109–123. https://doi.org/10.1007/s13738-022-02659-9
Santos Oliveira I, Marrote Manzano C, Hideki Nakahata D et al (2022) Antibacterial and antifungal activities in vitro of a novel silver(I) complex with sulfadoxine-salicylaldehyde Schiff base. Polyhedron 225:116073. https://doi.org/10.1016/j.poly.2022.116073
Elizalde LE, delos Santos G (2008) Preparation of 6-benzyloxo-spirobenzopyran-indoline compounds and the evaluation of their optical activities. Dye Pigment 78:111–116. https://doi.org/10.1016/j.dyepig.2007.10.011
Gao W, Li Q, Chen J et al (2013) Total synthesis of six 3,4-unsubstituted coumarins. Molecules 18:15613–15623. https://doi.org/10.3390/molecules181215613
Moghaddam FM, Kiamehr M, Khodabakhshi MR et al (2010) A new domino Knoevenagel-hetero-Diels-Alder reaction: An efficient catalyst-free synthesis of novel thiochromone-annulated thiopyranocoumarin derivatives in aqueous medium. Tetrahedron 66:8615–8622. https://doi.org/10.1016/j.tet.2010.09.048
Zakhari JS, Kinoyama I, Hixon MS et al (2011) Formulating a new basis for the treatment against botulinum neurotoxin intoxication: 3,4-Diaminopyridine prodrug design and characterization. Bioorganic Med Chem 19:6203–6209. https://doi.org/10.1016/j.bmc.2011.09.019
Zheng Y, Bin SW, Xuan LJ (2015) Copper-catalyzed oxidative esterification of ortho-formyl phenols without affecting labile formyl group. Tetrahedron Lett 56:4569–4573. https://doi.org/10.1016/j.tetlet.2015.06.024
Sandhya D (2014) Novel Thermotropic Liquid Crystals with Lateral Aryl Substituent 4:22–26
Lavilla C, De Ilarduya AM, Alla A et al (2012) Bio-based aromatic polyesters from a novel bicyclic diol derived from d-mannitol. Macromolecules 45:8257–8266. https://doi.org/10.1021/ma3013288
Faig JJ, Smith K, Moretti A et al (2016) One-Pot Polymerization Syntheses: Incorporating Bioactives into Poly(anhydride-esters). Macromol Chem Phys 217:1842–1850. https://doi.org/10.1002/macp.201600115
Iftikhar B, Javed K, Khan MSU et al (2018) Synthesis, characterization and biological assay of Salicylaldehyde Schiff base Cu(II) complexes and their precursors. J Mol Struct 1155:337–348. https://doi.org/10.1016/j.molstruc.2017.11.022
Devi J, Yadav J, Singh N (2019) Synthesis, characterisation, in vitro antimicrobial, antioxidant and anti-inflammatory activities of diorganotin(IV) complexes derived from salicylaldehyde Schiff bases. Res Chem Intermed 45:3943–3968. https://doi.org/10.1007/s11164-019-03830-3
Li DQ, Tan MX, Jie L (2012) Synthesis, antioxidant and antibacterial activities of salicylaldehyde azine. Adv Mater Res 396–398:2366–2369. https://doi.org/10.4028/www.scientific.net/AMR.396-398.2366
Alamri A, El-Newehy MH, Al-Deyab SS (2012) Biocidal polymers: Synthesis and antimicrobial properties of benzaldehyde derivatives immobilized onto amine-terminated polyacrylonitrile. Chem Cent J 6:1–13. https://doi.org/10.1186/1752-153X-6-111
El-Khouly AS, Kenawy E, Safaan AA et al (2011) Synthesis, characterization and antimicrobial activity of modified cellulose-graft-polyacrylonitrile with some aromatic aldehyde derivatives. Carbohydr Polym 83:346–353. https://doi.org/10.1016/j.carbpol.2010.07.047
Negi H, Singh RK (2021) Study of azomethine functionalized cellulose with salicylaldehyde as novel demetallization agent for metalloporphyrins in crude oil. Cellulose 28:2635–2648. https://doi.org/10.1007/s10570-021-03718-9
Dos Santos JE, Dockal ER, Cavalheiro ÉTG (2005) Synthesis and characterization of Schiff bases from chitosan and salicylaldehyde derivatives. Carbohydr Polym 60:277–282. https://doi.org/10.1016/j.carbpol.2004.12.008
Kamil F, Abid Hubeatir K, Shamel M, Al-Amiery AA (2015) Microwave-assisted solvent-free synthesis of new polyimine. Cogent Chem 1:1075853. https://doi.org/10.1080/23312009.2015.1075853
Şenol D, Kaya İ (2017) Synthesis and characterization of azomethine polymers containing ether and ester groups. J Saudi Chem Soc 21:505–516. https://doi.org/10.1016/j.jscs.2015.05.006
Kausar A, Hussain ST (2013) New generation of thermally stable and conducting poly(azomethine-ester)s: nano-blend formation with polyaniline. Polym Int 62:1442–1450. https://doi.org/10.1002/pi.4438
Vasanthi BJ, Ravikumar L (2013) Synthesis and Characterization of Poly(azomethine ester)s with a Pendent Dimethoxy Benzylidene Group. Open J Polym Chem 03:70–77. https://doi.org/10.4236/ojpchem.2013.33013
Qureshi F, Khuhawar MY, Jahangir TM, Channar AH (2021) Synthesis and characterization of new thermally stable, antimicrobial and red-light-emitting poly(azomethine-ester)s. Polym Bull 78:5055–5074. https://doi.org/10.1007/s00289-020-03357-3
Gul A, Akhter Z, Siddiq M et al (2013) Ferrocene-Based Aliphatic and Aromatic Poly(azomethine)esters: Synthesis, Physicochemical Studies, and Biological Evaluation. Macromolecules 46:2800–2807. https://doi.org/10.1021/ma400192u
Hamad WM, Azeez HJ, Al-Dujaili AH (2017) Synthesis and mesomorphic properties of 2,4-bis(4′-n-pentyloxybenzoyloxy)- benzylidine-4″- n-alkoxyaniline. Mol Cryst Liq Cryst 652:67–75. https://doi.org/10.1080/15421406.2017.1357426
Iwan A, Palewicz M, Sikora A et al (2010) Aliphatic–aromatic poly(azomethine)s with ester groups as thermotropic materials for opto(electronic) applications. Synth Met 160:1856–1867. https://doi.org/10.1016/j.synthmet.2010.06.029
Temizkan K, Kaya İ (2019) Synthesis, characterization, optical and electrochemical band gaps of green poly(azomethine-ester)s containing oxalyl and succinyl units. Bull Mater Sci 42:106. https://doi.org/10.1007/s12034-019-1763-y
Temizkan K, Kaya İ (2017) Synthesis, characterization, electrochemical and surface morphology properties of poly(azomethine-ester)s. Polym Bull 74:2575–2592. https://doi.org/10.1007/s00289-016-1851-8
Shukla U, Rao KV, Rakshit AK (2002) Thermotropic liquid-crystalline polymers: synthesis, characterization, and properties of poly (azomethine esters). J Appl Polym Sci 88:153–160. https://doi.org/10.1002/app.11618
Gul A, Akhter Z, Qureshi R, Bhatti AS (2014) Conducting poly(azomethine)esters: synthesis, characterization and insight into the electronic properties using DFT calculations. RSC Adv 4:22094–22100. https://doi.org/10.1039/C4RA02443E
Perwin A, Mazumdar N (2024) Synthesis of O-acyl salicylaldehyde derivatives and copolymerization of bis-(2-formylphenyl) fumarate with methyl methacrylate. J Mol Struct 1304:137690. https://doi.org/10.1016/j.molstruc.2024.137690
Kaya I, Kolcu F, Demiral G et al (2015) Synthesis and characterization of imine polymers of aromatic aldehydes with 4-amino-2-methylquinoline via oxidative polycondensation. Des Monomers Polym 18:89–104. https://doi.org/10.1080/15685551.2014.971395
Sıdır YG, Pirbudak G, Berber H, Sıdır İ (2017) Study on the electronic and photophysical properties of the substitute-((2-phenoxybenzylidene)amino)phenol derivatives: Synthesis, solvatochromism, electric dipole moments and DFT calculations. J Mol Liq 242:1096–1110. https://doi.org/10.1016/j.molliq.2017.07.070
Kaya I (2004) Synthesis, characterization, and optimum reaction conditions of oligo-benzylidene-3′-hydroxyaniline. Int J Polym Anal Charact 9:137–151. https://doi.org/10.1080/1023660490890529
Kaya İ, Koça S, Karaer Yağmur H (2022) The green synthesis of oligo(azomethine)s based on p-anisidine and o-anisidine: reaction conditions, electrochemical and thermal properties. J Macromol Sci Part A Pure Appl Chem 59:849–862. https://doi.org/10.1080/10601325.2022.2140675
Demetgül C, Delikanlı A, Sarıbıyık OY et al (2012) Schiff Base Polymers Obtained by Oxidative Polycondensation and Their Co(II), Mn(II) and Ru(III) Complexes: Synthesis, Characterization and Catalytic Activity in Epoxidation of Styrene. Des Monomers Polym 15:75–91. https://doi.org/10.1163/156855511X606164
Kaya İ, Bora E, Aydın A (2014) Synthesis and characterization of Schiff base derivative with pyrrole ring and electrochromic applications of its oligomer. Prog Org Coatings 77:463–472. https://doi.org/10.1016/j.porgcoat.2013.11.008
Kirihara M, Osugi R, Saito K et al (2019) Sodium Hypochlorite Pentahydrate as a Reagent for the Cleavage of trans -Cyclic Glycols. J Org Chem 84:8330–8336. https://doi.org/10.1021/acs.joc.9b01132
Acknowledgements
The author, Aashna Perwin expresses gratitude to the UGC for granting a fellowship and acknowledges the assistance received from IIT Delhi, MNIT-Jaipur, and CIF-JMI for conducting instrumental analysis on the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perwin, A., Mazumdar, N. Exploring the synthesis of poly(azomethine-ester) through oxidative polycondensation of salicylaldehyde schiff bases. J Polym Res 31, 214 (2024). https://doi.org/10.1007/s10965-024-04070-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-024-04070-9