Abstract
In this study PLA and PHB were melt-blended with/without maleic anhydride (MA) as a compatibilizer and melt-spun into a monofilament. The aquatic biodegradation of PLA/PHB fibers was tracked regarding the PLA and PHB components and the total carbon in the blend. Pure PLA fiber was recalcitrant to aquatic biodegradation in the present conditions, whereas PHB was readily degradable, as expected. The 75/25% and 50/50% PLA/PHB fibers showed zero biodegradation. The 25/75% PLA/PHB blend showed only an 11% final biodegradation extent and a 13% PHB biodegradation extent. It is shown that an unintended consequence of blending PLA with PHB is that after biodegradation, micro/nano plastic pollution is evolved. MA increased the miscibility, which further decreased the PHB biodegradation. The lower biodegradation extent with MA indicates that increased miscibility and more intimate coverage of the biodegradable polymer with the unbiodegradable polymer may have a negative effect on the biodegradation of biodegradable polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polylactic acid (PLA) is a versatile bio-based polymer and has been recognized as biodegradable plastic [1]. However, its biodegradability is affected by environmental factors, such as temperature, pH and pressure [2,3,4,5,6,7,8,9,10]. PLA biodegrades typically in industrial composting conditions [11,12,13]. In industrial composting facilities, polymeric materials are treated at elevated temperatures (50–60 °C) with higher moisture levels [14, 15]. There have been reports of biodegradation of PLA [16,17,18]. However, most studies were done at a temperature higher than 30 °C or pH higher than 7. Also, in our previous study [19, 20], the biodegradation extent of PLA was close to 0% for 56 days of the biodegradation experiment. Thus, it is obvious that PLA can biodegrade in relatively harsh conditions, different from our natural environment.
Poly(β-hydroxybutyrate) (PHB) also has been known as a bio-based and biodegradable polymer [21,22,23,24], as confirmed in our previous study (the PHB powder showed 65% of biodegradation for 56 days, and the PHB fiber showed 63% biodegradation for 42 days) [19, 20, 25]. As well as biodegradability, PHB has many desirable properties, such as biocompatibility [26,27,28,29], barrier property [24], and mechanical properties [22, 24]. However, its brittleness has been pointed out as a limitation for the application of PHB [22, 30].
Polymer blending is a cost-effective and convenient method to improve the polymer performance and compensate for the shortcomings of the polymers [1, 23, 31]. Since PLA and PHB are both polyester types of bio-based polymers and have a closed melting temperature with each other (160–180°C) [32,33,34], there have been plenty of studies on the biodegradation of PLA/PHB blends [35,36,37,38,39,40,41,42,43,44,45,46,47,48]. However, most of the biodegradation experiment in the previous studies was done in the composting [35,36,37,38, 40,41,42,43, 45,46,47,48,49], or soil condition [39, 44]. There is a study that tested the biodegradation of PLA/PHB blend in aquatic conditions [50]; however, they only tested one blending condition (PLA80/PHB20), and the blend was not biodegradable in marine and freshwater. Since the biodegradability of polymeric materials can be dependent on the environment, it can be expected that the biodegradation of PLA/PHB blends can be different in aerobic aquatic environments at room temperature. Most importantly, in none of these studies was the biodegradation of the PLA and PHB polymers tracked separately, all of the studies simply reported the overall biodegradation. It is, however, critical to understand the biodegradation of the individual components in the blends in order to interpret properly the biodegradation process. Therefore, in the present study, PLA/PHB blended fibers were investigated with several different blending ratios (25/75, 50/50, 75/50) in aquatic aerobic conditions and the PLA and PHB components tracked separately before and after biodegradation.
Fiber materials are a significant source of microfibers, and microfiber is the dominant microplastic in the oceans [51, 52]. Characteristics of polymeric materials can be different depending on the production process; for example, fiber materials have different morphologies since polymer chains are aligned in the direction of spinning. Therefore, it is important to investigate the biodegradation of polymer blends with a fiber form in the aquatic conditions. However, most studies on biodegradation of the PLA/PHB blend were done with films [35,36,37,38, 40, 40, 41, 44, 47, 48] or extruded pellets [43] of the polymer blends, which have different structural properties from fibers. There are a few studies on the PLA/PHB blended fibers [40, 42, 45, 46]. Arrieta et al. [40, 42, 45, 46] electrospun the PLA/PHB blends and make them to a fiber mat. They tested the electrospun mat in the composting condition, reporting the electrospun fiber composites were entirely disintegrated. However, in their research, the degradation was analyzed by the visual appearance, and the biodegradation extent was not quantified. There is a study of the PLA/PHB blended nonwovens [39] that reported the biodegradation of PLA/PHB blended nonwovens (10% biodegradation for 15 days), but this research was done with only one blending ratio (80:20 of PLA to PHB) in the soil condition and the isolated bacterial suspension from the soil.
As a polymer blend of bio-based polyesters, PLA/PHB blend has been intensely researched. However, there are lack of studies on aquatic biodegradation of PLA/PHB fibers. This study has significance as the first study of aquatic aerobic biodegradation of PLA/PHB blended fibers versus blend ratio that tracks PLA and PHB separately. Furthermore, in this study, the effect of compatibilizer on the biodegradation of the polymer blended fibers was investigated. Compatibilizers have been considered as a solution to improve the performance and miscibility of polymer blends, but its effect on the aquatic biodegradation of PLA/PHB blends has not been reported yet. Considering that the compatibilizer can affect the interactions between the polymers in the blend system, it can also affect the biodegradation of the polymer blends. Therefore, in the present study, the aquatic biodegradability of PLA/PHB blends, with and without maleic anhydride (MA) as a compatibilizer, was investigated. Further, the biodegradation residuals were collected and analyzed to examine the effect of blending and biodegradation on the fiber morphology. It was found in this study that adding PHB to PLA did not increase the biodegradation of the PLA and in some blends also decreased the biodegradation of the PHB. Further, increased miscibility of PHB and PLA with a compatibilizer was accompanied by lower biodegradation of the PHB than the same system without the compatibilizer.
Materials and methods
Materials
Table 1 shows the information of the polymers and compatibilizer used in this study. The PLA (PLA 6400D, NatureWorks, MN, USA) and PHB (Ningbo Tianan Biologic Material Co., Ltd) were the polymers used for the melt-blending, and MA (Sigma Aldrich, USA) was used as a compatibilizer. Benzoyl peroxide (BPO; Sigma Aldrich, USA) was used as an initiator for the reactive compatibilization. Inorganic salts for aquatic biodegradation experiments, including potassium dihydrogen phosphate (KH2PO4), dipotassium hydrogen phosphate (K2HPO4), disodium hydrogen phosphate dihydrate (Na2HPO4·2H2O), ammonium chloride (NH4Cl), magnesium sulfate heptahydrate (MgSO4·7H2O), calcium chloride dihydrate (CaCl2·2H2O), and iron III chloride hexahydrate (FeCl3·6H2O) were purchased from Sigma-Aldrich (USA) and used as received. The thermal properties measured by differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA) are shown in Table 1. Based on the melting and decomposition temperature, the extrusion process temperature used was 185 °C; the detailed process is described in the next section.
Polymer blending and fiber production
PLA, PHB, MA, and BPO were pre-mixed before the melt-blending process, and the mixed material was added to a twin-screw extruder (DSM micro 15 cc twin-screw compounder, Xplore). The 12 ml of mixed material was fed at 100 rpm and 185 °C. After feeding, the polymers were blended for five minutes at 10 rpm at 185 °C. The extruded polymer blends were wound by a high-speed winding unit (Xplore) with 10 torque setting of winding force and 10 m/min of winding speed), formed into a monofilament. The polymer blending and fiber production was conducted as per our previous study [19, 25].
Two kinds of blended fibers were produced: PLA/PHB with and without MA. For PLA/PHB blended fibers, a total of five kinds of fibers were produced, including pure PLA and PHB fibers, PLA75/PHB 25, PLA50/ PHB 50, and PLA25/PHB 75 fibers where the numbers indicate the mass % fed to the extruder. For PLA/PHB/MA blended fibers, PLA25/PHB75 condition was selected. MA was added at 7% based on the PLA and PHB polymers’ weight. This concentration was determined based on the previous research [53]. In the previous research, PLA/PHB blends with several concentrations of MA were investigated, and 7% loading by mass showed an optimal value of elongation at break. All blended fibers were extruded and wound under the same conditions. The width of produced fibers was 180 – 300 μm (c.f. Table S2).
Aerobic aquatic biodegradation
Aquatic biodegradability in aerobic conditions was evaluated according to the procedures of previous studies of our research group [19, 20, 25, 54,55,56] and an ISO standard method [57]. Activated sludge from the Neuse River Wastewater Treatment Plant (WWTP) was used as an inoculum; thus, the microorganisms in the sludge are expected to be responsible for the biodegradation of the test materials.
The test medium was composed of deionized water and inorganic salts (Table S3). The inoculum was added to the test medium with a suspended solids content of 120 ppm. Subsequently, 400 mL of this medium was added to each flask. In each test flask, 100 mg of polymer fibers were added. The fibers were cut into 1 cm lengths with a sharp guillotine trimmer to prevent agglomeration/entangling during the biodegradation experiments. To compare the effect of blending, two non-blended conditions were used: 1) PLA50/PHB50 (NB), where pure PLA fibers and pure PHB fibers were added to the flask in a 50:50 ratio, and 2) PLA50/MCC50 (NB), in which pure PLA fibers and pure MCC powder were added in a 50:50 ratio. The aquatic biodegradation experiment was conducted under controlled dark conditions at a temperature of 25 °C. The pH was monitored and adjusted to 7 once a week.
Closed respirometers (RSA PF-7000, Respirometer Systems and Applications, LLC., USA) measured the oxygen uptake by the biodegradation activities of the microorganisms, and the % biodegradation was calculated based on the theoretical oxygen demands (ThOD) of test materials (Eq. 1). The oxygen consumption was calculated by subtracting the oxygen uptake of the samples from the oxygen uptake of the blank samples that do not contain any test materials. The reference material for the biodegradation experiment was microcrystalline cellulose (MCC, 50-micron diameter, Acros organics™, USA).
The ThOD of each test material was calculated with the elemental composition of each test material (Eq. 2).
The Gompertz model, reported as an adequate model to analyze the biodegradation kinetics in our previous research [56], was used to investigate the biodegradation kinetics (Eq. 3) [58, 59]. By model fitting the dynamic biodegradation results, the initial biodegradation rate, ultimate biodegradation extent, and the lag phase were determined.
(B: the biodegradation extent (%), \({B}_{0}\): the ultimate biodegradation (%), r: the biodegradation rate (%/day), \(\lambda\): lag-phase period (day)).
Characterization of polymer blended fibers before and after biodegradation
The fiber morphology and structure of PLA/PHB blended fibers were investigated before and after the biodegradation experiment. The residuals were collected after 66 days duration of the biodegradation experiment by filtering on glass microfiber filter paper (pore size 1.6 μm, Whatman® Grade GF/A Glass Microfiber Filters, Whatman, UK).
The fiber morphology of PLA/PHB blends was characterized using Scanning Electron Microscopic (SEM, JCM-6000Plus Versatile Benchtop SEM, JEOL, Japan). For obtaining fiber cross-sections, the fibers were embedded in an adhesive (Norland Electronic Adhesive 121 Red, Norland Products Incorporated, NJ, USA) that was cured for three hours with UV light (3,600 microwatts/cm2 of average UV light intensity; Splice Lamp, Norland Products, NJ, USA). The embedded samples were sectioned using a microtome (Leica RM2145 Microtome, Leica Biosystems Inc, IL, USA). The top exposed surfaces of the embedded samples were used for the SEM images. The embedded fibers for SEM images were sputter-coated with gold (3.5 min with the Smart Coater, JEOL, Japan).
Time-of-flight secondary ion mass spectrometry (ToF–SIMS, TOF. IMS 5, IONTOF GmbH, Germany) was used to investigate the phase morphology of PLA/PHB/MA blended fibers. By analyzing the ejected secondary ions from the fiber surface, PLA and PHB phases on the surface were identified.
Thermal properties of PLA/PHB/MA fibers were investigated using DSC (2 °C/min, ± 0.64 °C with 120 s modulation, DSC 250, TA instruments, DE, USA) and TGA (20 °C/min, TGA Q500, TA instruments, DE, USA). Also, X-ray diffraction (XRD, Empyrean, Malvern Panalytical, UK) was used to analyze the crystalline structure of PLA/PHB/MA blended fibers (2-theta range: 5°- 45°, operation voltage: 45 kV, current: 40 mA, radiation source: Cu K-α radiation at a 201 wavelength of 1.54 Å, scan step size: 0.026°, collected for 30 min).
Results
Aerobic aquatic biodegradation
The aquatic biodegradation experiments of PLA/PHB blend fibers were carried out for 66 days with a closed respirometer following an ISO standard (ISO 14851, 2019), with 120 ppm activated sludge as inoculum. The biodegradation extent was calculated based on the theoretical oxygen demand (ThOD) of each test material (cf. Table S1). Additionally, the biodegradation extent based on the PHB carbon was calculated with the ThOD based on PHB carbons. The overall results of biodegradation are shown in Fig. 1 and Table 2. Two non-blended conditions, PLA50/PHB50 (NB) and PLA50/MCC50 (NB), were included to investigate the effect of blending versus the mere presence of PLA on the PHB and MCC biodegradation activities of microorganisms. In these conditions, pure PLA and PHB fibers or MCC powders were added with a 50/50 ratio into the flask.
Aquatic biodegradation of PLA/PHB/MA blended fibers versus time. a: PLA/PHB without MA, b: PLA/PHB with MA, c: final biodegradation extent of PLA/PHB blended fibers, and d: final biodegradation extent of PLA/PHB/MA blended fibers. (Sample PLA50/PHB50 (NB) and PLA50/MCC50 (NB) contained a 50:50 addition of pure PLA fibers and pure PHB fibers or MCC powders as a comparison. Biodegradation extents are the average values of three measurements)
An amount of 79% of the pure PHB fibers biodegraded after 66 days, whereas the PLA fibers did not biodegrade in the present conditions for 66 days (cf. Fig. 1(a, c) and Table 2). Since PLA was not biodegradable in the present condition, the PHB-based biodegradation extent was calculated with the assumption that only PHB biodegraded in the present condition (Fig. 1(d) and Table 2). PLA 25/PHB 75 fibers showed 11% final biodegradation after 66 days (cf. Fig. 1(a, c) and Table 2). However, all PLA/PHB blend fibers, except for PLA25/ PHB75 fiber, showed zero biodegradation. The final PHB biodegradation in PLA25/PHB75 blended fibers was 14%, generally confirming the significant hindrance of PLA on the PHB biodegradation.
The non-blended condition of PLA and PHB (PLA50/PHB50 (NB)) showed 22% of final biodegradation, and only 40% of PHB in the non-blended condition biodegraded. In the condition, the pure PLA and pure PHB fibers were suspended in the same water medium independently. This result suggests that the presence of PLA fibers also hindered the biodegradation of pure PHB fibers. However, in the PLA50/MCC50 (NB) condition, the PLA hindrance was not significant as PLA was in the PLA50/PHB50 (NB) condition (showing 74% of MCC biodegradation in the PLA50/MCC50 (NB) condition). This suggests that PLA did not have a fatal effect, such as toxicity, on the activity of microorganisms.
The initial biodegradation rate of PLA50/PHB50 (NB) condition was also significantly lower than pure PHB conditions, while the lag phase of PLA50/PHB50 (NB) condition was very similar to the pure PHB condition (cf. Table 2). It may be said that the microorganisms still biodegrade PHB in the non-blended condition, but the biodegradation process was not as fast as in the pure PHB condition.
The effect of a compatibilizer on the biodegradation of PLA/PHB blended fibers was investigated with maleic anhydride (MA) as a compatibilizer. The PLA25/PHB75 condition was selected since only this blending condition showed biodegradation extent higher than 10% after 66 days. The PLA/MA showed a higher final biodegradation extent (8%) than pure PLA fibers (Fig. 1(c)), even though it is still lower than 10% after 66 days. However, PHB/MA showed a very similar final PHB biodegradation extent (78%) to the pure PHB fibers (76%) (Fig. 1(d)), as well as similar lag phase (11 days for PHB and 10 days for PHB/MA) and initial biodegradation rate (4%/days for both PHB and PHB/MA fibers) (Table 2). This result confirms that MA did not have a significant effect on the PHB biodegradation and did not exhibit toxicity that could inhibit the microbial biodegradation activity. The final biodegradation extent of PLA25/PHB75/MA blended fiber was 5%, and the final PHB biodegradation was 7%, which was lower than the final biodegradation extent of PLA25/PHB75 blended fibers.
Characterization of polymer blended fibers before and after biodegradation
The biodegradation residues of PLA/PHB/MA blended fibers were further investigated to explore how the blended fibers biodegraded under aqueous conditions. In the case of pure PHB and PHB/MA fibers, since the fibers were completely disintegrated, making the collection of analyzable residuals not possible by filter papers.
The PLA and PHB carbon that remained in the PLA/PHB/MA fibers after the biodegradation experiment was investigated with thermal gravimetric analysis (TGA, summary in Table S4 and raw TGA curves in Figs. S3 and S4). TGA curves were used to determine the PLA and PHB weight percentages in samples since they had different decomposition temperature ranges when heating in nitrogen at 20 °C/min. The PLA and PHB carbon percentages were not significantly decreased or increased in the PLA, PLA75/PHB25, and PLA50/PHB50 samples after biodegradation. This is due to the near zero biodegradation that occurred in these samples. However, it was observed for the PLA25/PHB75 blended fibers, that the PLA carbon percentage increased by 4% (25% → 29%) and the PHB carbon percentage decreased accordingly after the biodegradation. This result is in agreement with the biodegradation results that indicate that the PLA did not biodegrade under these aquatic aerobic conditions. A detailed explanation follows in the discussion section.
Figure 2 shows the SEM images of the PLA/PHB/MA blended fibers before and after the biodegradation experiment. When the PHB concentration was lower than 75%, the blended fibers were intact even after 66 days of the biodegradation experiment. The pure PLA fiber, PLA25/PHB75fiber, and PLA50/PHB50 fiber did not change in their morphology, shape, and size after the biodegradation experiment. This indicates that there was no significant biodegradation of those fibers. However, in the case of PHB 75% fiber, the fibers’ surface was damaged, and granule-like residuals were left in the fiber remnants and are easily observed.
PLA/PHB blended with MA showed a smooth surface before biodegradation. The fiber surface of PLA25/ PHB75/MA condition showed an even smoother surface than the PLA25/PHB75. This may be attributed to the interaction between PLA/PHB, which may be enhanced by the MA. After the biodegradation, the appearance of the blended fibers was not observed to be changed regarding their structure, surface, and morphology, agreeing with the significantly low biodegradation extent. However, PHB/MA fibers were completely disintegrated similar to the PHB fibers. This confirms that MA itself did not significantly affect the biodegradation of PHB in the blended fibers, agreeing with the biodegradation results shown in Fig. 1 and Table 2.
Figure 3 shows the cross-section of PLA/PHB fibers before biodegradation. PLA75/PHB25 and PLA50/PHB50 fibers did not show prominent phase-separated regions in the SEM images. In comparison, PLA25/PHB75 condition showed spherical phase-separated regions in the cross-section, which is similar to the granule-like residual structures observed in Fig. 2. The diameters of the spherical regions were from 0.05 µm to 1.78 µm (average 0.96 µm, standard deviation: 0.34 µm).
The PLA25/PHB75/MA blended fibers did not have phase-separated region observed different from PLA25/PHB75 blended fibers, suggesting improved miscibility by MA. However, in PLA fibers with MA, there are some phase-separated regions observed. It is unknown what caused these separated regions but this could be possibly due to impurities, imperfect mixing, or some new crystalline region.
Since PLA is not biodegradable in the present biodegradation conditions, the granules can be inferred to consist of PLA. Therefore, Time-of-Flight Secondary Ion Mass Spectrometry (ToF–SIMS) images of PLA/PHB/MA blended fibers were analyzed to identify the granules and the surface phase morphologies of the blended fibers. A focused primary ion beam was sputtered on the surface and the secondary ions are ejected from the surface and analyzed.
The ToF–SIMS images of PLA/PHB/MA and PLA/PHB blended fibers are shown in Fig. 4. The C3H3O3- and C3H4O + ions were signature ions from pure PLA fibers, and C4H7O3- and C4H7O2 + ions were PHB signature ions detected from the pure PHB fibers. Those same ions were also dominantly detected from PLA/MA and PHB/MA blended fibers with 7% MA contents. With those negative and positive ions as a reference, the blended fibers of the PLA25/PHB75 condition were further analyzed.
Before biodegradation, PLA is dominant on the surface of the PLA25/PHB75 fibers, making a somewhat homogenous surface. However, after the biodegradation, the PHB signal increased, and the PLA signals were scattered on the surface, confirming that the granule-like structures were mainly composed of PLA components. PLA25/PHB75/MA blended fibers showed both PLA and PHB signals on the fiber surface, in contrast to the PLA25/PHB75 blended fibers which showed a PLA-dominant surface. After the biodegradation, the PHB component was removed from the surface and the PLA component became more dominant on the surface (Fig. 4).
Discussion
Aerobic aquatic biodegradation of PLA and PHB in PLA/PHB/MA fibers
PLA biodegradation is known as highly variable depending on the conditions [2,3,4,5,6,7,8,9,10]. PLA degrading microorganisms are not widely distributed in the natural environment [60, 61]. In most studies that reported the PLA biodegradation, experimental temperature conditions higher than 30 °C have been used. In addition, PLA degrades faster under basic conditions in previous work [7, 16,17,18, 62]. Thus, in the present experimental conditions, the population of microorganisms, the pH and/or temperature did not support the biodegradation of PLA.
PHB biodegradation was calculated with the assumption that only PHB in PLA/PHB blends was degradable under the current conditions, and this assumption was validated through TGA analysis performed on PLA/PHB fibers both before and after biodegradation. With this assumption, the biodegradation of PHB in PLA25/PHB75 fibers was determined to be 14% (c.f. Table 2). In 100 mg of PLA25/PHB75 fibers, the PLA component remained intact at 25 mg, while the PHB component degraded from 75 mg to 64.5 mg, resulting in a total residual amount of 89.5 mg. Calculating the percentages of PLA and PHB based on these values, PLA accounted for 28% and PHB for 72% of the mass in the remaining fibers. This ratio is similar to the PLA/PHB/MA ratio calculated using the TGA results in Table S4. This result confirms that only PHB biodegrades in this system, thereby validating the assumption.
In our previous study with PP/PHB blends, the biodegradation extent and the PHB biodegradation extent increased with the PHB concentration in the blend [19, 25]. Even though the PHB biodegradation was hindered by PP in the blend in the case of higher PP concentration conditions, the PHB biodegradation of the PLA25/PHB75 condition was similar to the biodegradation extent of pure PHB fibers (64%). However, in the present study, even PLA25/ PHB75 fiber showed very low PHB biodegradation, implying that the hindrance of PLA is more significant than the hindrance of PP on the PHB biodegradation.
PLA and PHB are both polyester-type polymers with ester groups in the polymer chain. Thus, more interaction between PLA and PHB can be expected compared to PP and PHB. It is possible that PLA and PHB are partially miscible to each other, and specific interfacial surfaces are greater than in the PP/PHB blends, a less miscible combination. Therefore, the surface of PHB is coated more extensively by the PLA, and this coverage may interfere with the access of microorganisms and enzymes. This will be discussed further with regards to the characteristics of PLA/PHB blended fibers in the next section.
The non-blended condition of PLA and PHB (PLA50/PHB50 (NB)) showed a lower final PHB biodegradation extent and lower initial biodegradation rate compared to the pure PHB condition. This result may be related to differences in the affinity of microorganisms to the polymers: there may be a relatively higher possibility of non-productive adsorption of enzymes/microorganisms to PLA, decreasing the active microorganism population to biodegrade PHB or MCC. Therefore, it can be suggested that PLA hinders the biodegradation of PHB in the PLA/PHB blends due to the partially miscible property, but it also hinders the biodegradation activities of microorganisms by a higher affinity to the microorganisms. However, there has not been studies on the enzyme affinity to the PLA or PHB yet.
The PLA/MA showed a higher final biodegradation extent (8%) than pure PLA fibers. This may be caused by the changes in the crystalline structure of PLA fibers by the reaction with MA. With MA as a compatibilizer, the PLA/MA fibers displayed another melting peak (124 °C) in addition to the melting peak of PLA component (162 °C) (Detailed data is shown in the supplementary section, Table S2 and Fig. S2). Also, the crystallinity of PLA/MA fibers were lower than pure PLA fibers (Table S2). This might make the PLA structure more susceptible to microorganisms, slightly enhancing the biodegradation of the fibers.
The final biodegradation extent of PLA25/PHB75/MA blended fiber was lower than the final biodegradation extent of PLA25/PHB75 blended fibers. When we examined the crystallinity of PLA25/PHB75/MA fibers (c.f. Table S2), it was observed that even with MA, the PLA25/PHB75/MA fiber did not show significant changes in crystallinity. Instead, a single melting peak was detected, differing from PLA25/PHB75 fibers. These results imply that the biodegradation of PHB was hindered significantly by the introduction of compatibilizers even though MA may help the biodegradation of PLA. This result suggests that the improved miscibility may not have a positive effect on the biodegradability of the polymer blends.
Biodegradation residuals and phase morphology of PLA and PHB in PLA/PHB/MA fibers
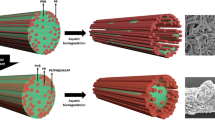
The PHB 75% fiber showed a degraded surface with granule-like residuals left inside after the biodegradation based on the SEM images. This indicates that the blending of two immiscible polymers may have an unintended consequence of releasing smaller particles upon biodegradation of one component, contributing to micro and nano sized plastic pollution. Similar phase-separated regions were also observed for a PP/PHB blend after biodegradation [19, 25]. However, PP/PHB fibers showed fibril-form phase-separated regions, different from the granule-like spherical phase-separated regions of PLA/PHB fibers (cf. Fig. 5). PLA and PHB are polyester-type polymers with an ester functional group in every repeat unit of their main chain. Therefore, the interaction between two polymers are expected to be stronger in PLA/PHB blends than the interactions in PP/PHB blends. There is more possibility in the PLA/PHB blends that the two polymers interrupt each other to make a granule-like phase-separated region during the fiber production. There have been studies suggesting a transesterification reaction occurring in PLA/PHB blends, which may make the polymers less likely to phase separate [35, 63,64,65]; however, there has not been any solid evidence of the transesterification reaction at this point.
It was observed from ToF–SIMS images that PLA was more dominant on the fiber surface in PLA/PHB fibers, but the PLA signal decreased after the biodegradation. It can be suggested that the PLA is highly distributed on the fiber surface before the biodegradation with its relatively hydrophobic property (water contact angle of PLA is 88° and for PHB is 66°). This may block access of microoganisms to the PHB retarding the biodegradation of PHB. Also, given that still relatively low PHB signals were detected on the surface after biodegradation, it can be expected that more PHB was initially distributed near the center of the fiber.
Unlike PLA/PHB fibers without MA, PLA/PHB fibers with MA showed both PLA and PHB signals on the fiber surface in the ToF–SIMS images. This suggests that PLA and PHB had more interactions at the molecular scale so that more PHB components were distributed on the surface. Even though the PHB component was removed from the surface after the biodegradation (Fig. 4), considering that PLA25/PHB75/MA blended fibers showed relatively low final PHB biodegradation extent (7%), it can be expected that still more PHB components were entrapped by PLA inside the fibers. Therefore, based on the SEM and ToF–SIMS residual images, the PLA25/PHB75 fiber model can be suggested as Fig. 5.
The inclusion of MA created more uniform and miscible blends of PLA and PHB polymers, as shown in the cross-section images (Fig. 3) and ToF–SIMS images of fiber surface (Fig. 4). However, the improved miscibility had a negative effect on the PHB biodegradation in the PLA/PHB blended fibers. This research suggests that the biodegradation of a biodegradable polymer can be impeded by melt-blending with highly interactive polymers, and it can be even more hindered when the interaction/miscibility is increased by other additives, such as compatibilizers.
Conclusions
Blending with PLA had a negative effect on the PHB biodegradation in the PLA/PHB blended fibers. Due to the interaction between PLA and PHB, the PLA and PHB phases can reveal granule-like phase-separated regions of PLA after biodegradation. PLA is observed to cover the surface of PHB regions, preventing the access of microorganisms to biodegrade PHB components. After the biodegradation of PHB components, PLA components remained as granule-like micro and nano structures. The compatibilizer MA can increase the interaction between PLA and PHB and improve their miscibility. PLA and PHB components can be more uniformly distributed in the PLA/PHB/MA blended fibers compared to PLA/PHB blended fibers. However, the PHB biodegradation of the blended fibers are hindered by the increased miscibility between PLA and PHB and by the relatively more homogeneously distributed PLA components. These results imply that the increased miscibility and interaction between a more biodegradable and a less biodegradable polymer may decrease the biodegradability of the more biodegradable polymer and result in unintended micro and nano plastic pollution.
Data availability
All of the data supporting underlying findings are included in the manuscript and its supplemental files.
7. References
Ahmed T, Shahid M, Azeem F et al (2018) Biodegradation of plastics: current scenario and future prospects for environmental safety. Environ Sci Pollut Res 25:7287–7298. https://doi.org/10.1007/s11356-018-1234-9
Yagi H, Ninomiya F, Funabashi M, Kunioka M (2009) Anaerobic biodegradation tests of poly(lactic acid) and polycaprolactone using new evaluation system for methane fermentation in anaerobic sludge. Polym Degrad Stab 94:1397–1404. https://doi.org/10.1016/j.polymdegradstab.2009.05.012
Yagi H, Ninomiya F, Funabashi M, Kunioka M (2013) Thermophilic anaerobic biodegradation test and analysis of eubacteria involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym Degrad Stab 98:1182–1187. https://doi.org/10.1016/j.polymdegradstab.2013.03.010
Yagi H, Ninomiya F, Funabashi M, Kunioka M (2014) Mesophilic anaerobic biodegradation test and analysis of eubacteria and archaea involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym Degrad Stab 110:278–283. https://doi.org/10.1016/j.polymdegradstab.2014.08.031
Fukushima K, Abbate C, Tabuani D et al (2009) Biodegradation of poly(lactic acid) and its nanocomposites. Polym Degrad Stab 94:1646–1655. https://doi.org/10.1016/j.polymdegradstab.2009.07.001
Weng Y-X, Jin Y-J, Meng Q-Y et al (2013) Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym Test 32:918–926. https://doi.org/10.1016/j.polymertesting.2013.05.001
Lee SH, Kim IY, Song WS (2014) Biodegradation of polylactic acid (PLA) fibers using different enzymes. Macromol Res 22:657–663. https://doi.org/10.1007/s13233-014-2107-9
Qi X, Ren Y, Wang X (2017) New advances in the biodegradation of poly(lactic) acid. Int Biodeterior Biodegrad 117:215–223. https://doi.org/10.1016/j.ibiod.2017.01.010
Pattanasuttichonlakul W, Sombatsompop N, Prapagdee B (2018) Accelerating biodegradation of PLA using microbial consortium from dairy wastewater sludge combined with PLA-degrading bacterium. Int Biodeterior Biodegrad 132:74–83. https://doi.org/10.1016/j.ibiod.2018.05.014
Nakayama A, Yamano N, Kawasaki N (2019) Biodegradation in seawater of aliphatic polyesters. Polym Degrad Stab 166:290–299. https://doi.org/10.1016/j.polymdegradstab.2019.06.006
Chinga-Carrasco G (2018) Novel Biocomposite Engineering and Bio-applications. Bioengineering 5:80. https://doi.org/10.3390/bioengineering5040080
Ghosh K, Jones BH (2021) Roadmap to Biodegradable Plastics—Current State and Research needs. ACS Sustain Chem Eng 9:6170–6187. https://doi.org/10.1021/acssuschemeng.1c00801
European Bioplastics Background paper: EN 13432 industrial composting
(2019) UK should stop plans to ramp up use of compostable packaging. In: Powersystems UK. https://www.powersystemsuk.co.uk/news/uk-stop-plans-ramp-use-industrially-compostable-packaging/. Accessed 27 Oct 2021
European Bioplastics (2009) FACT SHEET IndustrIal CompostIng
Lucas N, Bienaime C, Belloy C et al (2008) Polymer biodegradation: mechanisms and estimation techniques – A review. Chemosphere 73:429–442. https://doi.org/10.1016/j.chemosphere.2008.06.064
Dopico-García S, Ares-Pernas A, Otero-Canabal J et al (2013) Insight into industrial PLA aging process by complementary use of rheology, HPLC, and MALDI: INSIGHT INTO INDUSTRIAL PLA AGING PROCESS: RHEOLOGY, HPLC AND MALDI. Polym Adv Technol 24:723–731. https://doi.org/10.1002/pat.3136
Larrañaga A, Lizundia E (2019) A review on the thermomechanical properties and biodegradation behaviour of polyesters. Eur Polym J 121:109296. https://doi.org/10.1016/j.eurpolymj.2019.109296
Kwon S (2022) Aquatic biodegradation of fibers/bio-based materials for nonwovens and microfiber generation of nonwovens. North Carolina State University
Kwon Soojin, Zambrano Marielis C., Venditti Richard A., Pawlak Joel J. (2023) Aerobic aquatic biodegradation of bio-based and biodegradable polymers: Kinetic modeling and key factors for biodegradability. Int Biodeterior Biodegradation 185:105671. https://doi.org/10.1016/j.ibiod.2023.105671
Nishida H, Tokiwa Y (1993) Distribution of poly(β-hydroxybutyrate) and poly(ε-caprolactone)aerobic degrading microorganisms in different environments. J Environ Polym Degrad 1:227–233. https://doi.org/10.1007/BF01458031
El-Hadi A, Schnabel R, Straube E et al (2002) Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym Test 21:665–674. https://doi.org/10.1016/S0142-9418(01)00142-8
Tokiwa Y, Calabia B, Ugwu C, Aiba S (2009) Biodegradability of Plastics. Int J Mol Sci 10:3722–3742. https://doi.org/10.3390/ijms10093722
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez V (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Express Polym Lett 8:791–808. https://doi.org/10.3144/expresspolymlett.2014.82
Kwon S, Zambrano MC, Pawlak JJ, Ford E, Venditti RA (2023) Aquatic biodegradation of poly(β-hydroxybutyrate) and polypropylene blends with compatibilizer and the generation of micro- and nano-plastics on biodegradation. J Polym Environ 31(8):3619–3631. https://doi.org/10.1007/s10924-023-02832-y
Li Z, Cheng S, Li S et al (2008) Novel amphiphilic poly(ester-urethane)s based on poly[(R)-3-hydroxyalkanoate]: synthesis, biocompatibility and aggregation in aqueous solution. Polym Int 57:887–894. https://doi.org/10.1002/pi.2424
Wee CY, Liow SS, Li Z et al (2017) New Poly[(R)-3-hydroxybutyrate-co-4-hydroxybutyrate] (P3HB4HB)-Based thermogels. Macromol Chem Phys 218:1700196. https://doi.org/10.1002/macp.201700196
Lim J, You M, Li J, Li Z (2017) Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater Sci Eng C 79:917–929. https://doi.org/10.1016/j.msec.2017.05.132
Wang X, Liow SS, Wu Q et al (2017) Codelivery for Paclitaxel and Bcl-2 Conversion Gene by PHB-PDMAEMA Amphiphilic Cationic Copolymer for Effective Drug Resistant Cancer Therapy. Macromol Biosci 17:1700186. https://doi.org/10.1002/mabi.201700186
Rabnawaz M, Wyman I, Auras R, Cheng S (2017) A roadmap towards green packaging: the current status and future outlook for polyesters in the packaging industry. Green Chem 19:4737–4753. https://doi.org/10.1039/C7GC02521A
Koning C (1998) Strategies for compatibilization of polymer blends. Prog Polym Sci 23:707–757. https://doi.org/10.1016/S0079-6700(97)00054-3
Grassie N, Murray EJ, Holmes PA (1984) The thermal degradation of poly(-(d)-β-hydroxybutyric acid): part 3—The reaction mechanism. Polym Degrad Stab 6:127–134. https://doi.org/10.1016/0141-3910(84)90032-6
Ariffin H, Nishida H, Shirai Y, Hassan MA (2008) Determination of multiple thermal degradation mechanisms of poly(3-hydroxybutyrate). Polym Degrad Stab 93:1433–1439. https://doi.org/10.1016/j.polymdegradstab.2008.05.020
Yeo JCC, Muiruri JK, Thitsartarn W et al (2018) Recent advances in the development of biodegradable PHB-based toughening materials: approaches, advantages and applications. Mater Sci Eng C 92:1092–1116. https://doi.org/10.1016/j.msec.2017.11.006
Zhang M, Thomas NL (2011) Blending polylactic acid with polyhydroxybutyrate: the effect on thermal, mechanical, and biodegradation properties. Adv Polym Technol 30:67–79. https://doi.org/10.1002/adv.20235
Abdelwahab MA, Flynn A, Chiou B-S et al (2012) Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym Degrad Stab 97:1822–1828. https://doi.org/10.1016/j.polymdegradstab.2012.05.036
Arrieta MP, López J, Hernández A, Rayón E (2014) Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur Polym J 50:255–270. https://doi.org/10.1016/j.eurpolymj.2013.11.009
Arrieta MP, López J, Rayón E, Jiménez A (2014) Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym Degrad Stab 108:307–318. https://doi.org/10.1016/j.polymdegradstab.2014.01.034
Liu Y, Zhan Z, Ye H et al (2019) Accelerated biodegradation of PLA/PHB-blended nonwovens by a microbial community. RSC Adv 9:10386–10394. https://doi.org/10.1039/C8RA10591J
Arrieta MP, Perdiguero M, Fiori S et al (2020) Biodegradable electrospun PLA-PHB fibers plasticized with oligomeric lactic acid. Polym Degrad Stab 179:109226. https://doi.org/10.1016/j.polymdegradstab.2020.109226
Arrieta MP, Fortunati E, Dominici F et al (2014) PLA-PHB/cellulose based films: mechanical, barrier and disintegration properties. Polym Degrad Stab 107:139–149. https://doi.org/10.1016/j.polymdegradstab.2014.05.010
Arrieta MP, López J, López D et al (2016) Biodegradable electrospun bionanocomposite fibers based on plasticized PLA–PHB blends reinforced with cellulose nanocrystals. Ind Crops Prod 93:290–301. https://doi.org/10.1016/j.indcrop.2015.12.058
Sedničková M, Pekařová S, Kucharczyk P et al (2018) Changes of physical properties of PLA-based blends during early stage of biodegradation in compost. Int J Biol Macromol 113:434–442. https://doi.org/10.1016/j.ijbiomac.2018.02.078
Jeszeová L, Puškárová A, Bučková M et al (2018) Microbial communities responsible for the degradation of poly(lactic acid)/poly(3-hydroxybutyrate) blend mulches in soil burial respirometric tests. World J Microbiol Biotechnol 34:101. https://doi.org/10.1007/s11274-018-2483-y
Arrieta MP, López J, López D et al (2015) Development of flexible materials based on plasticized electrospun PLA–PHB blends: structural, thermal, mechanical and disintegration properties. Eur Polym J 73:433–446. https://doi.org/10.1016/j.eurpolymj.2015.10.036
Arrieta MP, López J, López D et al (2016) Effect of chitosan and catechin addition on the structural, thermal, mechanical and disintegration properties of plasticized electrospun PLA-PHB biocomposites. Polym Degrad Stab 132:145–156. https://doi.org/10.1016/j.polymdegradstab.2016.02.027
Tabasi RY, Ajji A (2015) Selective degradation of biodegradable blends in simulated laboratory composting. Polym Degrad Stab 120:435–442. https://doi.org/10.1016/j.polymdegradstab.2015.07.020
Iglesias-Montes ML, Soccio M, Luzi F et al (2021) Evaluation of the factors affecting the disintegration under a composting process of poly(lactic acid)/Poly(3-hydroxybutyrate) (PLA/PHB) blends. Polymers 13:3171. https://doi.org/10.3390/polym13183171
Fogašová M, Figalla S, Danišová L et al (2022) PLA/PHB-Based materials fully biodegradable under both Industrial and Home-Composting conditions. Polymers 14:4113. https://doi.org/10.3390/polym14194113
Narancic T, Verstichel S, Reddy Chaganti S et al (2018) Biodegradable plastic blends create new possibilities for end-of-Life Management of plastics but they are not a panacea for Plastic Pollution. Environ Sci Technol 52:10441–10452. https://doi.org/10.1021/acs.est.8b02963
Dubaish F, Liebezeit G (2013) Suspended microplastics and Black Carbon Particles in the Jade System, Southern North Sea. Water Air Soil Pollut. https://doi.org/10.1007/s11270-012-1352-9
Kane IA, Clare MA (2019) Dispersion, Accumulation, and the Ultimate Fate of Microplastics in Deep-Marine environments: a review and future directions. Front Earth Sci 7:80. https://doi.org/10.3389/feart.2019.00080
Jandas PJ, Mohanty S, Nayak SK (2014) Morphology and Thermal properties of renewable resource-based polymer blend nanocomposites influenced by a reactive compatibilizer. ACS Sustain Chem Eng 2:377–386. https://doi.org/10.1021/sc400395s
Zambrano MC, Pawlak JJ, Daystar J et al (2019) Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar Pollut Bull 142:394–407. https://doi.org/10.1016/j.marpolbul.2019.02.062
Zambrano MC, Pawlak JJ, Daystar J et al (2020) Aerobic biodegradation in freshwater and marine environments of textile microfibers generated in clothes laundering: effects of cellulose and polyester-based microfibers on the microbiome. Mar Pollut Bull 151:110826. https://doi.org/10.1016/j.marpolbul.2019.110826
Kwon S, Zambrano MC, Pawlak JJ, Venditti RA (2021) Effect of lignocellulosic fiber composition on the aquatic biodegradation of wood pulps and the isolated cellulose, hemicellulose and lignin components: kinetic modelling of the biodegradation process. Cellulose 28:2863–2877. https://doi.org/10.1007/s10570-021-03680-6
ISO 14851 (2019) Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium -method by measuring the oxygen demand in a closed respirometer
Lay J-J, Li Y-Y, Noike T (1997) Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res 31:1518–1524. https://doi.org/10.1016/S0043-1354(96)00413-7
Cho HS, Moon HS, Kim M et al (2011) Biodegradability and biodegradation rate of poly(caprolactone)-starch blend and poly(butylene succinate) biodegradable polymer under aerobic and anaerobic environment. Waste Manag 31:475–480. https://doi.org/10.1016/j.wasman.2010.10.029
Suyama T, Tokiwa Y, Ouichanpagdee P et al (1998) Phylogenetic Affiliation of soil Bacteria that degrade aliphatic polyesters available commercially as biodegradable plastics. Appl Environ Microbiol 64:5008–5011. https://doi.org/10.1128/AEM.64.12.5008-5011.1998
Tokiwa Y, Calabia BP (2006) Biodegradability and biodegradation of poly(lactide). Appl Microbiol Biotechnol 72:244–251. https://doi.org/10.1007/s00253-006-0488-1
Apinya T, Sombatsompop N, Prapagdee B (2015) Selection of a Pseudonocardia sp. RM423 that accelerates the biodegradation of poly(lactic) acid in submerged cultures and in soil microcosms. Int Biodeterior Biodegrad 99:23–30. https://doi.org/10.1016/j.ibiod.2015.01.001
Zhang L, Xiong C, Deng X (1996) Miscibility, crystallization and morphology of poly(β-hydroxybutyrate)/poly(d,l-lactide) blends. Polymer 37:235–241. https://doi.org/10.1016/0032-3861(96)81093-7
Arrieta M, Samper M, Aldas M, López J (2017) On the Use of PLA-PHB blends for sustainable food packaging applications. Materials 10:1008. https://doi.org/10.3390/ma10091008
Villegas C, Torres A, Bruna J et al (2021) Obtaining active polylactide (PLA) and polyhydroxybutyrate (PHB) blends based bionanocomposites modified with Graphene Oxide and Supercritical Carbon Dioxide (scCO2)-Assisted cinnamaldehyde: Effect on Thermal-Mechanical, Disintegration and Mass Transport properties. Polymers 13:3968. https://doi.org/10.3390/polym13223968
Acknowledgements
We appreciate the contributions of Carl Wust and Christopher Thompson of FiberVisions for their collaboration and efforts to prepare embedded fiber samples.
Funding
This research was supported by the Nonwoven Institute (NWI) at NC State University. Their support is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest or competing interests for any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kwon, S., Zambrano, M.C., Pawlak, J.J. et al. Aquatic biodegradation of poly(β-hydroxybutyrate) in polylactic acid and maleic anhydride blended fibers. J Polym Res 31, 100 (2024). https://doi.org/10.1007/s10965-024-03930-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-024-03930-8