Abstract
A novel flame retardant of polyaromatic ring phosphor-nitrogen imidazole derivative (PNB) was prepared and added to epoxy resin (EP) to develop epoxy resin thermosetting materials (PNB/EP) with excellent comprehensive properties. The peak temperature (Tp) and apparent activation energy (E) of PNB/EP-10 were lower than those of EP-0, indicating that PNB could catalyze the curing process. Moreover, PNB/EP had remarkable mechanical properties in addition to its outstanding fire safety. Concretely, the LOI of PNB/EP-10 was 37.9% and could reach V-0 level in UL-94 test. The total heat release (THR) and peak heat release rate (PHRR) were reduced by 34.6% and 36.6%, in comparison with the EP-0, respectively. Since the PNB structure contained nitrogen and phosphorus elements, which could reduce the fire risk of the polymer in both the gas and condensed phases, further improving the flame retardancy of the composite. Furthermore, PNB/EP had significant mechanical properties because PNB not only contained a polyaromatic ring structure capable of forming π-π bonds, which enhanced the interaction between the epoxy resin matrix and PNB, but the -NH structure also contributed to its dispersibility in the epoxy resin. This was reflected in the fact that the impact strength, tensile strength as well as flexural strength of PNB/EP-10 had increased by 22.5%, 47.1% and 34.5%, respectively. All the results indicated that PNB/EP overcame the inherent flammability and brittleness of epoxy resins and increased their curing activity, which had broadened their industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resin (EP) can be found everywhere in daily life thanks to the prominent properties such as benign heat resistance, high corrosion resistance, outstanding mechanical strength, and low dielectric properties [1]. Regrettably, EP is a flammable material that generates a lot of smoke and heat when burned. As a result, it is somewhat limited in many areas where a high fire safety factor is required. Consequently, it is urgent to develop excellent flame retardants that can dramatically reduce the fire risk of polymers.
Conventionally, the introduction of halogen-based flame retardants into a polymeric material works in achieving superior flame retardancy. However, environmental contamination is a concern when burning or handling products containing these compounds, leading to an extensive ban on halogen-based flame retardants [2]. Therefore, halogen-free flame retardants, especially flame retardants containing phosphorus had attracted people's attention gradually because they can well reduce the risk of fire. Specifically, they not only had an excellent catalytic effect of facilitating carbon formation, forming a physical protection barrier to isolate the heat and oxygen exchange, but also could capture free radicals, further blocking the combustion reaction [3]. In addition, when phosphorus and nitrogen elements are introduced in the polymer, the phosphorus and nitrogen component can act in both phases, which can hinder the combustion reaction and thus greatly enhance the fire safety factor [4, 5]. Yang et al. [6]. synthesized a polyphosph-azene compound (PBFA) containing an active amine group and added it to EP. The fire resistance of EP was increased after the introduction of PBFA, and the generation of smoke was inhibited with HRR and THR reduced by 46.7% and 29.3%, respectively. Yang et al. [7]. synthesized a phosphor-nitrogen flame retardant (DOPO-T) through nucleophilic substitution reaction between DOPO and melamine. When only 0.9 phr of DOPO-T were added, the fire safety performance of EP was significantly improved, the LOI value was 36.2% and the THR and PHRR decreased by 31% and 48%, respectively. Therefore, adding phosphorus-nitrogen elements to suppress the flammability of EP composites is one of the most effective and feasible methods. However, the introduction of many phosphorus-nitrogen elements may damage the mechanical properties of epoxy resins. For example, Qian et al. [8]. reported that piperazine (1, 4-methylene phenyl phosphonic acid (MPPAP), a novel phosphorus-nitrogen flame retardant, could effectively enhance the fire protection factor of EP, but the addition of MPPAP reduced the mechanical properties of epoxy resins (the impact strength of MPPAP/EP was 21.1% lower than EP-0). Therefore, it is of great significance to develop novel high efficiency flame retardants so that the modified EP can overcome their own shortcomings without sacrificing their advantages, and thus broaden their applications.
In order to prepare phosphorus-nitrogen flame retardants with superior flame-retardant properties and outstanding mechanical properties, we designed and synthesized a novel polyaromatic ring phosphor-nitrogen imidazole derivative (PNB) using chloride hypophosphate (PPDC), 2-aminobenzimidazole (NBZ) as raw materials. After then, PNB was used to prepare epoxy resin thermosetting materials (PNB/EP). The structure, curing activity, flame resistance, thermal stability and mechanical performance of the modified EP were investigated by various tests. Furthermore, the flame retardancy and mechanical enhancement mechanism had also been thoroughly studied.
Experimental section
Materials
Hypophosphorous chloride (PPDC, 98%), methylene chloride (CH2Cl2, 99.9%) and 2-aminobenzimidazole (NBZ) were provided by Shanghai Titan Technology Co., Ltd. E-51 was provided by Fujian Yunsen Technology Co., LTD. 4, 4-diamine diphenylmethane (DDM, 98%) and tetrahydrofuran (THF, 99.5%) were provided by Sane Chemical Technology (Shanghai) Co., Ltd. Triethylamine (TEA, 99%) was provided by Sinopharm Chemical Reagents Co., Ltd.
Synthesis of PNB
NBZ (5.4 g, 40 mmol), THF (60 mL) and TEA (5.5 mL, 40 mmol) were added sequentially to a glass flask. After the above reactants were evenly mixed at room temperature, PPDC (7.7 mL) was added at room temperature and reacted at 65 °C for 6 h, respectively. Finally, the precipitate was removed by filtration, followed by washing twice with ether and rotary evaporation to remove the solvent to obtain the product. The corresponding synthesis process was demonstrated in Fig. 1.
Preparation of PNB/EP
PNB/EP was prepared according to the formulation shown in Table 1 and the specific synthesis steps were as follows. Stoichiometric amount of epoxy resin (E-51), curing agent (DDM) and flame retardant (PNB) were added and the mixture was heated to 100 °C and stirred until homogeneous. After then, the mixture was poured into a hot mold for curing according to certain procedures (100 ℃ / 2 h, 150 ℃ / 2 h). PNB/EP containing 0, 5, 10 and 15 phr of PNB were marked as EP-0, PNB/EP-5, PNB/EP-10 and PNB/EP-15, respectively.
Characterization
Fourier transform infrared (FT-IR) and nuclear magnetic resonance (NMR) tests were performed on a Thermo-Nicolet iS50 spectrometer and a Bruker AV400 NMR spectrometer (Bruker, Switzerland), respectively. The FT-IR scan range was 500–4000 cm−1 with a resolution of 4 cm−1 while the NMR uses DMSO as the solvent.
The limiting oxygen index (LOI) was performed on HC-2C with a sample size of 130 × 6.5 × 3 mm3 using ASTM D2863 as the standard. Samples were tested at the vertical combustion (UL-94) rating specified in ASTM D3801 with a sample size of 130 × 13 × 3 mm3.
Thermogravimetric analysis (TGA) was tested at a heating rate of 10 °C/min from 30 °C to 800 °C under nitrogen conditions.
The residual carbon and the brittle fracture surface of the samples after cone calorimetric testing were analyzed using a Hitachi S4800 scanning electron microscope (SEM) with an accelerating voltage of 5 kV.
The combustion behavior of the sample was evaluated using the FTT0007 conical calorimeter with a sample size of 100 × 100 × 3 mm3 according to ISO 5660 and an external current of 50 kW/m2.
Tensile and flexural strength were tested on a CMT4104 universal testing machine (SANS, USA) with five repetitions for each scale of sample. The impact strength was tested by ZBC1251 pendulum impact tester from SANS.
The cracking products of the samples were analyzed by thermogravimetric analysis infrared spectroscopy (TG-IR) on STA 449 F5 (NETZSCH, Germany) and Nicolet is10 (Thermo Fisher, USA), recording the volatile gases produced from 30 to 700 °C at a rate of 10 °C/min under air condition.
The chemical structure of the residual carbon was analyzed by EscaLab Xi + X-ray photoelectron spectrometer (XPS), which was manufactured by Thermo Fisher.
The curing behavior of the samples was analyzed by means of RCS90 DSC 25 produced by TA USA at 30–250 °C and under N2 condition with a heating rate of 5 ℃/min, 10 ℃/min, 15 ℃/min, 20 ℃/min and 25 ℃/min, respectively.
Results and discussion
Characterization and analysis of the structure of PNB
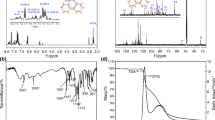
1H NMR, 13C NMR, 31P NMR and FT-IR were exploited to study the structure of PNB. 1H NMR spectrum of PNB was presented in Fig. 2a, proton signals with chemical shifts of 13 ppm and 6.9–7.6 ppm were attributed to -NH (1) [9] on the imidazole ring and the proton on the benzene ring, respectively. The peak at 7.9 ppm belonged to P-NH (2) [10]. Meanwhile, the structure was further analyzed by using 13C NMR spectrum, as depicted in Fig. 2b. To be specific, the peaks appeared at 127 ppm, 129 ppm, 131 ppm and 136 ppm were corresponded to Cc, Cf, Cd and Ce of the benzene ring on phosphorous oxychloride, respectively. The peak at 151 ppm was assigned to C = N (Ch) on imidazole and the peaks Ca and Cb caused by the benzene ring on imidazole appeared at 110 ppm and 122 ppm, respectively. The peak of C-N (Cg) on imidazole occurred at 135 ppm, which proved that PNB had been successfully synthesized. Furthermore, in the 31P NMR spectrum of PNB (Fig. 2c), there was only one absorption peak at 21 ppm corresponded to the structure of PNB, which excluded the existence of impurities containing phosphorus [6]. The FT-IR spectrum was taken to further identify the structure of PNB (Fig. 2d). The characteristic absorption peaks of benzene rings could be observed at 3069 cm−1 and 725 cm−1. The characteristic peak showed at 3265 cm−1 (P-NH stretching) revealed that PPDC had successfully reacted with NBZ. In addition, the absorption peak of P = O appeared at 1263 cm−1, the peak around at 1474 cm−1 corresponds to C = N [11]. Ultimately, it was confirmed that PNB had been successfully synthesized.
Curing process analysis
The curing kinetics of the samples were analyzed using DSC and the results of EP-0 and PNB/EP-10 were shown Fig. 3. The peak temperature (Tp) of EP-0 were higher than EP-0 at the same heating rate, indicating that PNB could promote the reactivity of the curing process. This was because the tertiary amine on the imidazole ring could act as a lewis base to enhance the nucleophilicity of the hydroxyl group and the secondary amine was directly involved in the curing reaction, increasing the number of reactive groups in the curing reaction. These two effects simultaneously promoted the curing of epoxy values [12].
The study of curing kinetics would help to find effective ways to reduce internal stresses and produce high performance thermoset polymers [13]. The curing kinetic of EP-0 and PNB/EP-10 were analyzed by nonisothermal DSC at various heating rates (5, 10, 15, 20 and 25 °C/min) under N2 and the obtained results were shown in Fig. 3. The activation energy (E) was obtained by calculating the slope of linear fit through the Kissinger equation (Eq. (1)) and shown in Fig. 4 [14]. The E of PNB/EP-10 was 51.71 kJ / mol, which was lower than that of EP-0 (60.35 kJ/mol). This further demonstrates that tertiary amines were rich in lone pair electrons, which promoted ring opening reactions, and secondary amines were directly involved in the curing reaction, thus catalyzing the curing process [15].
where β represented the heating rate (K/min), Tp denoted the temperature at which the exothermic peak value appears (K), R was the ideal gas constant of 8.314 (J/ (mol·K)) and E was the apparent activation energy of curing reaction (kJ/mol).
Thermal stability
The thermal stability were analyzed by TGA and the TGA curves were shown in Fig. 5a, the corresponding thermal decomposition characteristic parameters, including initial decomposition temperature (Td) and the char residue at 600 ℃ were shown in Fig. 5b. With the increased of PNB content, the Td value gradually decreased (from 306 °C to 370 °C) due to the early degradation of PNB [16]. Moreover, the char residue of EP-0 at 600 °C was just 16.2%, which was lower than that of PNB/EP-10 (19.6%), indicating that PNB had a strong catalytic charring effect.
Fire behavior of PNB/EP
LOI and UL-94 test
The minimum oxygen concentration required for combustion could be tested with LOI, while the combustion process of the sample could be visualized with UL-94 test. EP-0 had the lowest LOI value of 26.7% and could not pass UL-94 (NR). On the contrary, when PNB was introduced, the LOI showed an upward trend and easily achieved V-0 rating in UL-94 test. When adding only 5 phr of PNB, the LOI value could be increased by 25.8%, reaching 33.6% and easily reaching V-0 level. The maximum LOI was the sample of PNB/EP-15 with a value of 38%.
Cone calorimeter
Cone calorimeter was useful for illustrating the combustion behavior of PNB/EP. The heat release rate (HRR), total heat release rate (THR) and total smoke production (TSP) curves of the samples with time were exhibited in Fig. 6, and the detailed data were shown in Table 2.
Table 2 revealed that the peak heat release rate (PHRR) was 941.8 KW/m2 and THR was 96.9 MJ/kg for EP-0. After adding PNB, HRR, THR and TSR were reduced to different degrees, indicating that PNB had a better flame-retardant effect. It could be observed from Table 2 that time to ignition (TTI) value of PNB/EP decreased from 74 to 71 s with the addition of PNB due to the early decomposition of PNB, which coincided with TGA [17]. The average effective heat of combustion (av-EHC) of pure epoxy resin was 25.9 MJ/kg, displaying more flammable gases were released. In contrast, the av-EHC value was significantly reduced with the addition of PNB, showing PNB had excellent flame retardancy and was effective in reducing the release of combustible gases [18]. The PHRR, THR and av-EHC values were significantly reduced by the addition of PNB, with the largest reductions of 36.6%, 34.6% and 18.6% for PNB/EP-10, respectively. These results proved that PNB could reduce the fire risk.
Flame retardant mechanism analysis
The char residual after cone testing was analyzed using SEM to explore the flame retardant mechanism, and the results were as follows. The burned pure EP produced incomplete char residue with many cracks (Fig. 7a1), which were consistent with the SEM image (Fig. 7a2), resulting in the easy access of the combustible gases and heat sources to polymer matrix through these cracks and then accelerated the combustion. However, with the addition of PNB, the amount of char residual improved significantly and the surface structure became more dense and complete, indicating that the addition of PNB promoted the formation of char residue, which was consistent with the test results of cone calorimeter (Fig. 7b1–d1). Moreover, the tighter and more continuous char residue prevented combustible gases and heat from propagating through the pores, thereby slowing the spread of fire hazards [19]. In brief, the PNB/EP surface was tighter and smoother (Fig. 7b2–d2) compared to the incomplete EP-0 carbon layer surface (Fig. 7a1), which further proved that the addition of PNB could inhibit heat transfer and isolate air to delay the progress of fire.
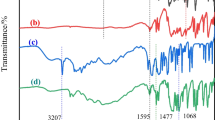
The mechanism by which PNB exerted its flame retardant effect in the condensed phase could be assessed by FT-IR. Several meaningful characteristic peaks could be seen in the FT-IR spectrum of PNB/EP-10. For example, the symmetric vibrational absorption peaks of N–H/O–H and P-O-Ph were appeared at 3384 cm−1 and 1681 cm−1, respectively. 1126 cm−1 and 1295 cm−1 were attributed to the absorption peaks of P = O [20]. By comparing the FT-IR spectra of EP-0 and PNB/EP-10, it was obvious that there was no absorption peak of P = O and P-O-Ph in EP-0. These absorption peaks were attributed to compounds containing phosphorus produced during the thermal decomposition of PNB [21].
XPS spectra (Fig. 8b) showed that the products contained C, N and O elements, while P elements could only be detected in PNB/EP-10. Moreover, in the C1s XPS spectra of PNB/EP-10 (Fig. 8c), 284.8 eV, 286.5 eV and 288.8 eV belonging to C–C/C-H, C-O/C-N and C = O [22, 23], respectively. Among the N1s spectrum (Fig. 8d), PNB/EP-10 had two signals at 398.5 eV and 400.7 eV, which were ascribed to C-N/P-N and N–H [24], respectively. In O1s XPS spectra (Fig. 8e), the binding energies at 531.5 eV and 533.7 eV matched the C = O/P = O and P-O-P/C–O–C/C-O-P signals, respectively [25]. Accordingly, there were peaks of P-O and P = O bonds presented in the P2p XPS spectra [26]. The above results indicated that PNB generated phosphoric acid derivatives, which were involved in protecting the matrix and improved the fire safety [27, 28].
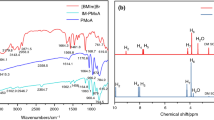
The TG-IR technique was used to analyze the generation of volatile gases in the composite. The evaluation of volatile gases could help investigate the mechanism by which PNB/EP-10 reduced the fire risk of the polymer. Figure 9 was the absorption spectra of different pyrolysis products. Several characteristic signals were detected in EP-0, such as a C-H absorption peak at 3018 cm−1, CO2 characteristic peaks at 2363 cm−1, peaks of P-O-C aromatic hydrocarbons at 1608 cm−1 and peaks of C-H bond on the alkyl chain at 835 cm−1 [29, 30]. In the spectra of PNB/EP-10, characteristic peaks presented at 1507 cm−1, 1180 cm−1 and 743 cm−1 belonged to P-O-Ph and esters, respectively [31]. Compared with pure EP, it was obvious that PNB/EP-10 emitted more CO2, thus diluting combustible gases and toxic gases and reducing the risk of fire from EP and improving the safety of people and property. Noticeably, PNB/EP-10 showed a new signal at 3670 cm−1 at 330 °C (attributed to N–H) [9], which was attributed to the pyrolysis of imidazole in the PNB to produce NH3 and other non-combustible gases. Moreover, PNB/EP-10 showed an absorption peak at 1180 cm−1, belonging to P-O-C, which did not appear in EP-0 [32]. The results demonstrated that PNB decomposed in advance to generate volatiles of nitrogen and phosphorus as non-combustible gases, which could dilute combustible gases, further reducing the fire risk of EP.
Based on the above description of the PNB added to the resin, which could promote the formation of a protective layer and dilution of combustible gases, thus achieving the effect of retarding spread of fire, a possible mechanism of PNB/EP was illustrated in Fig. 10. During the combustion process, PNB played a major part in the following ways: (1) The non-flammable gases N2, NH3 and NO2 were mainly generated by the pyrolysis of PNB, which would help to reduce the concentration of oxygen and combustible gases around the sample; (2) PNB generated P· and PO· radicals into the gas phase during pyrolysis, and interrupted the reaction by reacting with the active radicals (such as H· and OH·) after entering the gas phase; (3) The formed phosphoric acid effectively catalyzed the dehydration and carbonization of the substrate, thus forming a dense char residue, which further hindered the exchange of flame retardant substances. In summary, PNB had excellent flame retardant effect, opening up applications for epoxy resins in many fields with high fire safety performance requirements.
Mechanical properties
The versatility of polymer materials was the key to make them marketable. Therefore, in addition to the excellent fire safety, the mechanical properties of EP should meet the requirement of various applications. The mechanical properties had also been systematically studied and shown in Table 3. By adding PNB, the mechanical properties of PNB/EP were improved to a certain extent. Specifically, the tensile and bending strength of PNB/EP-15 increased by 38.3% and 55.1%, respectively. In addition, the best impact strength was achieved when PNB was 10 phr, which increased impact strength by 22.5% compared with pure EP.
For the purpose of further investigated the reasons for the improvement of mechanical properties by adding PNB, the fracture morphology of PNB/EP were inspected by SEM (Fig. 11). There were a few cracks on the fracture surface of pure epoxy resin, as shown in Fig. 11a, which were typical of brittle fracture. Many cracks were clearly detected in PNB/EP, which improved the mechanical properties of PNB/EP. In addition, the impact strength of PNB/EP-15 was lower than that of PNB/EP-10, which was due to the agglomeration of PNB/EP-15 on the fracture surface, resulting in lower energy consumption (the red circle in Fig. 11d). The possible factors for the significant improvement in mechanical properties with the addition of PNB could be interpreted as follows. Firstly, the interaction between PNB and the epoxy matrix could be enhanced due to the multi-aromatic ring structure in PNB, which could form π-π stacking. Secondly, the -NH structure in PNB structure facilitated the diffusion of PNB in EP [33, 34].
Conclusion
In summary, PNB flame retardants were synthesized from PPDC and NBZ at room temperature and were applied to epoxy resins. PNB promoted the reactivity of the curing agent, the peak temperature (Tp) and apparent activation energy (E) of PNB/EP-10 were lower than EP-0. PNB/EP-10 had LOI of 37.9% and easily passes UL-94 testing to achieve a V-0 rating, showing excellent of fire safety. In addition, PNB/EP-10 had outstanding smoke suppression and flame retardant properties, with TSP and av-EHC values of PNB/EP-10 reduced by 17.4% and 34.6%, respectively. The introduction of PNB into the EP matrix resulted in much higher impact strength (22.5%), tensile strength (47.1%) and bending strength (34.5%) owing to the presence of rigid aromatic and reactive amino groups in PNB. Due to these advantages, PNB/EP broaden the value of epoxy resins for applications in aerospace and other fields, where low fire risk, excellent mechanical properties and good curing activity were very necessary.
References
Zhang J, Kong Q, Wang D (2018) Simultaneously improving the fire safety and mechanical properties of epoxy resin with Fe-CNTs via large-scale preparation. J Mater Chem A 6:6376–6386. https://doi.org/10.1039/c7ta10961j
Duan H, Chen Y, Ji S, Hu R, Ma H (2019) A novel phosphorus/nitrogen-containing polycarboxylic acid endowing epoxy resin with excellent flame retardance and mechanical properties. Chem Eng J 375:121916. https://doi.org/10.1016/j.cej.2019.121916
Dasari A, Yu Z, Cai G, Mai Y (2013) Recent developments in the fire retardancy of polymeric materials. Prog Polym Sci 38:1357–1387. https://doi.org/10.1016/j.progpolymsci.2013.06.006
Li Z, Chen M, Li S, Fan X, Liu C (2019) Simultaneously improving the thermal, flame-retardant and mechanical properties of epoxy resins modified by a novel multi-element synergistic flame retardant. Macromol Mater Eng 304:1800619. https://doi.org/10.1002/mame.201800619
Gaan S, Sun G, Hutches K, Engelhard M (2008) Effect of nitrogen additives on flame retardant action of tributyl phosphate: phosphorus-nitrogen synergism. Polym Degrad Stab 93:99–108. https://doi.org/10.1016/j.polymdegradstab.2007.10.013
Yang G, Wu W, Wang Y, Jiao Y, Lu L, Qu H, Qin X (2019) Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of epoxy resin. J Hazard Mater 366:78–87. https://doi.org/10.1016/j.jhazmat.2018.11.093
Yang S, Hu Y, Zhang Q (2019) Synthesis of a phosphorus-nitrogen-containing flame retardant and its application in epoxy resin. High Perform Polym 31:186–196. https://doi.org/10.1177/0954008318756496
Ma W, Xu B, Shao L, Liu Y, Chen Y, Qian L (2019) Synthesis of (1,4-methylenephenylphosphinic acid) piperazine and its application as a flame retardant in epoxy thermosets. Macromol Mater Eng 304:1900419. https://doi.org/10.1002/mame.201900419
Huo S, Yang S, Wang J, Cheng J, Zhang Q, Hu Y, Ding G, Zhang Q, Song P, Wang H (2020) A liquid phosphaphenanthrene-derived imidazole for improved flame retardancy and smoke suppression of epoxy resin. ACS Appl Energy Mater 2:3566–3575. https://doi.org/10.1021/acsapm.0c00577
Jian R, Wang P, Duan W, Xia L, Zheng X (2017) A facile method to flame-retard epoxy resin with maintained mechanical properties through a novel P/N/S-containing flame retardant of tautomerization. Mater Lett 204:77–80. https://doi.org/10.1016/j.matlet.2017.05.135
Bai Z, Wang X, Tang G, Song L, Hu Y, Yuen R (2013) Structure-property relationships of synthetic organophosphorus flame retardant oligomers by thermal analysis. Thermochim Acta 565:17–26. https://doi.org/10.1016/j.tca.2013.04.027
Chi Z, Guo Z, Xu Z, Zhang M, Li M, Shang L, Ao Y (2020) A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: synthesis, flame-retardant behavior and mechanism. Polym Degrad Stabil 176:109151. https://doi.org/10.1016/j.polymdegradstab.2020.109151
Ferdosian F, Zhang Y, Yuan Z, Anderson M, Xu C (2016) Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur Polym J 82:153–165. https://doi.org/10.1016/j.eurpolymj.2016.07.014
Li L, Liao X, Sheng X, Liu P, Hao Z, He L, Qin G (2020) Influence of surface modified graphene oxide on the mechanical performance and curing kinetics of epoxy resin. Polym Adv Technol 31:1865–1874. https://doi.org/10.1002/pat.4913
Jian R, Wang P, Xia L, Zheng X (2017) Effect of a novel P/N/S-containing reactive flame retardant on curing behavior, thermal and flame-retardant properties of epoxy resin. J Anal Appl Pyrol 127:360–368. https://doi.org/10.1016/j.jaap.2017.07.014
Fang F, Huo S, Shen H, Ran S, Wang H, Song P, Fang Z (2020) A bio-based ionic complex with different oxidation states of phosphorus for reducing flammability and smoke release of epoxy resins. Compos Commun 17:104–108. https://doi.org/10.1016/j.coco.2019.11.011
Huo S, Song P, Yu B, Ran S, Chevali V, Liu L, Fang Z, Wang H (2021) Phosphorus-containing flame retardant epoxy thermosets: recent advances and future perspectives. Prog Polym Sci 114:101366. https://doi.org/10.1016/j.cej.2019.123830
Zhao W, Liu J, Peng H, Liao J, Wang X (2015) Synthesis of a novel PEPA-substituted polyphosphoramide with high char residues and its performance as an intumescent flame retardant for epoxy resins. Polym Degrad Stabil 134:120–129. https://doi.org/10.1016/j.polymdegradstab.2015.04.023
He X, Zhang W, Yang R (2017) The characterization of DOPO/MMT nanocompound and its effect on flame retardancy of epoxy resin. Compos Part A Appl Sci Manuf 98:124–135. https://doi.org/10.1016/j.compositesa.2017.03.020
Shao Z, Deng C, Tan Y, Yu L, Chen M, Chen L, Wang Y (2014) Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J Mater Chem A 2:13955–13965. https://doi.org/10.1039/c4ta02778g
Zhu X, Zhang T, Sun Z, Chen H, Guan J, Chen X, Ji H, Du P, Yang S (2017) Black phosphorus revisited: a missing metal-free elemental photocatalyst for visiblelight hydrogen evolution. Adv Mater 178:1605776. https://doi.org/10.1002/adma.201605776
Shen L, Li Y, Zhao W, Wang K, Ci X, Wu Y, Liu G, Liu C, Fang Z (2020) Tuning f-doped degree of rGO: restraining corrosion-promotion activity of EP/rGO nanocomposite coating. J Mater Sci Technol 44:121–132. https://doi.org/10.1016/j.jmst.2019.09.043
Liu J, Dai J, Wang S, Peng Y, Cao L, Liu X (2020) Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos Part B-Eng 190:107926. https://doi.org/10.1016/j.compositesb.2020.107926
Hu X, Yang H, Jiang Y, He H, Liu H, Huang H, Wan C (2019) Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J Hazard Mater 379:120793. https://doi.org/10.1016/j.jhazmat.2019.120793
Luo H, Rao W, Zhao P, Wang L, Liu Y, Yu C (2020) An efficient organic/inorganic phosphorus-nitrogen-silicon flame retardant towards low-flammability epoxy resin. Polym Degrad Stabil 178:109195. https://doi.org/10.1016/j.polymdegradstab.2020.109195
Luo H, Rao W, Zhao P, Wang L, Liu Y, Yu C (2020) An efficient organic/inorganic phosphorus-nitrogen-silicon flame retardant towards low-flammability epoxy resin. Polym Degrad Stab 178:109195. https://doi.org/10.1016/j.polymdegradstab.2020.109195
Zhao W, Liu J, Peng H, Liao J, Wang X (2015) Synthesis of a novel PEPA-substituted polyphosphoramide with high char residues and its performance as an intumescent flame retardant for epoxy resins. Polym Degrad Stabil 118:120–129. https://doi.org/10.1016/j.polymdegradstab.2015.04.023
Wen Y, Cheng Z, Li W, Li Z, Liao D, Hu X, Pan N, Wang D, Hull T (2018) A novel oligomer containing DOPO and ferrocene groups: synthesis, characterization, and its application in fire retardant epoxy resin. Polym Degrad Stabil 156:111–124. https://doi.org/10.1016/j.polymdegradstab.2018.08.010
Qi Y, Weng Z, Zhang K, Wang J, Zhang S, Liu C, Jian X (2020) Magnolol-based bio-epoxy resin with acceptable glass transition temperature, processability and flame retardancy. Chem Eng J 387:124115. https://doi.org/10.1016/j.cej.2020.124115
Chen M, Lin X, Liu C, Zhang H (2021) An effective strategy to enhance the flame retardancy and mechanical properties of epoxy resin by using hyperbranched flame retardant. J Mater Sci 56:5956–5974. https://doi.org/10.1007/s10853-020-05691-3
Zhang J, Mi X, Chen S, Xu Z, Zhang D, Miao M, Wang J (2020) A bio-based hyperbranched flame retardant for epoxy resins. Chem Eng J 381:122719. https://doi.org/10.1016/j.cej.2019.122719
Li S, Chen M, Su L, Lin X, Liu C (2020) Highly efficient multielement flame retardant for multifunctional epoxy resin with satisfactory thermal, flame-retardant, and mechanical properties. Polym Advan Technol 31:146–159. https://doi.org/10.1002/pat.4758
Tan Y, Shao Z, Yu L, Long J, Qi M, Chen L, Wang Y (2016) Piperazine-modified ammonium polyphosphate as monocomponent flame-retardant hardener for epoxy resin: flame retardance, curing behavior and mechanical property. Polym Chem 7:3003–3012. https://doi.org/10.1039/c6py00434b
Qiao H, Su L, Liu C, Zhang H, Chen M (2022) From laboratory to industrialization: eco-friendly flame retardant endowing epoxy resin with excellent flame retardancy, transparency, and mechanical properties. Polym Advan Technol 1–11. https://doi.org/10.1002/pat.5632
Acknowledgements
We appreciate the National Natural Science Foundation of China (Grant No. 21805036) and the Natural Science Foundation of Fujian Province of China (Grant No. 2019J01668) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, W., Li, D., Chen, M. et al. A novel polyaromatic ring phosphor-nitrogen imidazole derivative endowing epoxy resin with remarkable flame retardancy and mechanical properties. J Polym Res 29, 306 (2022). https://doi.org/10.1007/s10965-022-03161-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03161-9