Abstract
In this study, a new methacrylate monomer, methyl-4-{[(methacryloyloxy)acetyl]oxy}benzoate (MMOAB) was synthesized and copolymerized with glycidyl methacrylate (GMA) by the free radical polymerization at 65 °C in the 1,4-dioxane solvent. The compounds are characterized by FTIR, 1H- and 13C-NMR techniques. The copolymer composition was determined from the 1H-NMR data. The microstructures of the copolymers were found from the mean sequence length and run number. Thermal properties of polymers were determined by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) methods. The thermal decomposition activation energy (Ea) values were determined by Kissinger, Kissinger–Akahira–Sunose (KAS), and Flynn–Wall–Ozawa (FWO) methods. Some copolymers showed good biological activity against various microorganisms. Dielectric constant, dielectric loss factor, and AC conductivity values of the copolymers were estimated with the 100 Hz–20 kHz frequencies range. The dielectric study reveals that MMOAB units improve the dielectric properties of copolymers. The variation of electrical conductivity with frequency showed that the σ value increased linearly with increasing MMOAB content. The frequency dependence of the conductivity indicates that the transmission occurs through the barrier-hopping process. In addition, impedance measurements were performed over a wide frequency range. The obtained results showed that poly(MMOAB) and its copolymers could be used to miniaturize electronic devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Functional polymers can be obtained by modifying a monomer or polymer using chemical synthesis methods. Functional polymers are used in every field in our daily life, from construction to painting industry, from sports fields to recreation areas, from the automobile industry to textile industry, from food to agriculture. The ability to adapt the structural properties as desired results from the monomers themselves and the numerous bonding patterns and binding amounts with other monomers. Also, polymers with desired functions can be easily prepared by using monomers with appropriate functional groups [1,2,3].
Copolymers are not a mixture of two homopolymers, and different monomer units form chemical bonds in a copolymer molecule. A copolymer is obtained if chain polymerizations are made with a mixture of two monomers. Copolymerization is of great technological importance. We can design and prepare a product with the desired properties with greater freedom of movement by using copolymerization reactions. While a homopolymer can be easily broken and impact resistant, the products obtained by copolymerization of the monomer used in this polymer expand the usage areas and this product becomes more useful [4, 5].
Parabens, which are esters of para-hydroxybenzoic acid, are known as compounds with antimicrobial activity [6]. Parabens are used in skin cosmetics, medicine, food, and engineering fields due to their superior antibacterial activity and chemical stability [7,8,9,10]. The antimicrobial activity of parabens increases with the length of their aliphatic chains, but in this case, their solubility decreases [11]. Although it has been stated in various genotoxic studies that parabens are not mutagenic, it has been shown that ethyl and methyl parabens increase chromosomal abnormalities in hamster ovary cells but do not cause fetal anomalies even at toxic doses [12]. Methylparaben, the subject of our study, is also widely used as a preservative in the cosmetics and food industries. Acute toxicity studies in animals have shown that methylparaben does not cause significant subchronic and chronic toxicity [13, 14]. Polymethyl methacrylate (PMMA) is a high transparency thermoplastic polymer obtained by polymerization of methylmethacrylate monomer. PMMA is an alternative to glass because of its transparency, aesthetics, and scratch resistance. Methacrylate polymers are widely used commercially in the paint, adhesive, and coating industries due to their many good characteristics such as optical transmittance, UV stability, high electrical resistance, and high hydrolytic stability [15, 16].
Detailed information can obtain about the thermal behavior of polymers with thermogravimetric test methods. Degradation mechanisms of various polymers can be explained with the information obtained from the thermograms. In the determination of the thermal properties of polymers, melting and glass transition and thermal decomposition temperatures are priorities [17].
In this study, a new methacrylate monomer (MMOAB) was synthesized, which carries methylparaben in the side branch and is not available in the literature. Copolymers of MMOAB monomer and GMA commercial monomer have not been synthesized before, and their copolymerization kinetics have not been determined.
The copolymers of MMOAB with GMA were prepared by the free radical polymerization method. In addition, the monomer reactivity ratios of the poly(MMOAB-co-GMA) system were determined, the thermal and dielectric properties and biological activities of the copolymers were investigated, and the results were discussed.
Experimental
Materials
Sodium methacrylate, glycidyl methacrylate, chloroacetyl chloride, potassium carbonate, and methylparaben were supplied from Aldrich. 1,4-dioxane and dichloromethane were purified by distillation over magnesium sulfate. Azobisisobutyronitrile (AIBN) was recrystallized from a chloroform–methanol mixture (v/v:3/1).
Characterization techniques
FTIR spectra were recorded with Perkin-Elmer Spectrum BX FT-IR(USA) as KBr disk. 1H and 13C-NMR spectra were recorded using Bruker GmbH DPX-400 400 MHz spectrometer (USA). The glass transition temperatures of the polymers (Tg) were determined by DSC 60H (Shimadzu, Japan) instrument using approximately about 10 mg of sample in aluminum jacks at a nitrogen flow of 5 L/hr and a heating rate of 10 °C/min. The thermal stability of the polymers was determined at a heating rate 20 °C/min and about with 8–10 mg sample up to 600 °C by a TGA TG60H (Shimadzu, Japan) device. The molecular weights (Mw and Mn) of the polymers were determined by gel permeation chromatography (GPC). The photostability of the copolymers was determined by UV–Visible Spectrometer (UV-1700 Shimadzu, Japan). The dielectric and AC conductivity measurements were carried out with a Hioki LCR Meter impedance analyzer. The dielectric constant, ε′, the dielectric loss factor, ε′′ and ac conductivity of polymers were measured for the frequency range of 100 Hz to 20 kHz, at 300 K temperature. For these measurements, copolymer samples (0.15 g) were formed into discs of 13 mm diameter under appropriate pressure (5 tons).
Synthesis of MMOAB monomer
MMOAB monomer was synthesized according to the procedure in the literature to obtain in 90% yield [18]. The reaction flow is shown in Scheme 1.
Preparation of poly(MMOAB-co-GMA)
Six different poly(MMOAB-co-GMA) copolymers were obtained by taking different MMOAB and GMA monomers. The polymerization was carried out at 65 °C in an oil bath using 1,4 dioxane as solvent and AIBN (1% of total monomer amount) as initiator. The copolymerization process was completed in about 10 h. After the polymerization was completed, all copolymers were precipitated in ethanol. For purification, dried copolymers were redissolved in CH2Cl2 and precipitated in ethanol. After the precipitated polymers were filtered, they were dried in a vacuum oven for 24 h. The synthesis of the copolymer is shown in Scheme 2.
Biological activity of the compounds
Disk diffusion tests are the most commonly used method to determine the antibiotic susceptibility of molecules [19]. Biological activity of molecules have investigated two different concentrations (50 and 100 µg) against Staphylococcus aureus ATCC 29,213, Escherichia coli ATCC 25,922, Pseudomonas aeruginasa ATCC 27,853, Salmonella enteridis and Klebsiella pneumoniae as bacteria and candida albicans CCM 31 as fungi. The results were standardized with Penicillin G as standard.
Results and discussion
Characterization of MMOAB
In the FTIR spectrum of MMOAB, signals of ester carbonyls at 1725–1730 cm−1 and stretching vibrational bands of double bonds (C = C) at 1635 cm−1 were observed. In 13C-NMR, the signals of three carbonyl carbons were observed at 169–171 ppm. The 1H and 13C NMR spectrums with peak identification of the MMOAB is shown in Fig. 1. Detailed FTIR spectrum evaluation of MMOAB and poly(MMOAB) is given in Table 1.
Characterization poly(MMOAB)
As seen in Table 1, in the FTIR spectrum of Poly(MMOAB), ester carbonyl peaks (OC = O) at 1730–1742 cm−1, aliphatic C-H vibrations at 2992 cm−1, and ArC-H vibrational bands at 3108 cm−1 were observed. With the polymerization, the peak disappeared at 1635 cm−1 of the -C = C- stretching vibration bands. Another indicator of polymerization in the FTIR spectrum is the shift of the stretching vibration band of the ester carbonyl groups of methacrylic to 1742 cm−1. In the 1H-NMR spectrum of poly(MMOAB), Ar–H protons at 7.2 ppm and methyleneoxy (OCH2) protons at 4.7 ppm were observed. In the 13C-NMR spectrum of poly(MMOAB), signals at 169–172 ppm are attributed to ester carbonyl carbons, signals at 104–158 ppm are attributed to aromatic ring carbons, and signals at 65 ppm are attributed to methyleneoxy (OCH2) carbons.
Characterization of the poly(MMOAB-co-GMA)
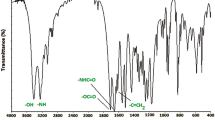
Spectroscopic characterizations of the copolymers were performed by FTIR, 1H- and 13C-NMR techniques. The FTIR spectrum of a copolymer sample is shown in Fig. 2. The peaks seen in the 1732–1744 cm−1 region are due to ester carbonyl stretching vibrations. Aromatic and aliphatic C-H stretching vibration bands are observed at 3093 cm−1 and 2905 cm−1, respectively. Also, the symmetrical stretching of the epoxy group is observed at 1242 cm−1. The C–O stretching is observed at 1161 cm−1. Another band seen at 908 cm−1 is due to the asymmetric stretching of the epoxy group of GMA. The 1H- and 13C-NMR spectrum and peak evaluation of the poly(MMOAB-co-GMA)(0.47:0.53) copolymer are shown in Fig. 3.
Determination of monomer reactivity ratios
The copolymer compositions were determined with the help of 1H-NMR spectroscopy data [20]. Thus, the mole fraction of MMOAB in the copolymer was determined from the ratio of the integrated values of the intensities of the peak due to the aromatic protons (4H, 7.8,8,2 ppm) of MMOAB units and the methylenoxy protons (2H, 3.4,4.2 ppm) of GMA units.
Let m1 be the mole fraction of MMOAB and m2 = (1-m1) that of the GMA unit.
With the help of this relationship, the following relation was formed;
On simplification;
Table 2 shows the molar fractions of the MMOAB calculated by Eq. (2) in the composition.
The reactivity ratio is defined as the ratio of the rate constant for the addition of one monomer of the type at the chain end to the rate constant for the addition of the other monomer. This value can be determined in different methods. The most widely used methods are Fineman Ross(FR), and Kelen Tudos(KT) [21, 22].
In order to calculate r1 and r2 values with both methods, the following relations are used by using mixture and composition ratios.
M1 and M2 are monomer molar compositions in the feed and m1 and m2 are copolymer molar compositions,
By using the F and f values, the G, H, η and ξ values required for the F-R and K-T equations can be calculated. The obtained values are shown in Table 3.
Linear η—ξ graph was obtained with the help of KT Eq. (4) given below.
The η—ξ graph was given in Fig. 4(a). The rMMOAB and rGMA values were obtained 0.38 and 0.67, respectively.
The FR equation is expressed as G = Hr1-r2. The r1 and r2 values were calculated from the linear graphic equation obtained with the calculated G and H values. G-H graph is given in Fig. 4(b). From this, rMMOAB and rGMA values were calculated 0.31 and 0.52, respectively..
The r1.r2 values of the Poly(MMOAB-co-GMA) system determined by both methods are 0.25 and 0.16, respectively. The r1.r2 value being close to zero shows that these copolymer types have an alternative tendency. One such copolymer is a copolymer containing two monomeric units distributed in alternating order. Alternating copolymers can be thought of as homopolymers with a structural unit of two different monomers. An alternative copolymer consists of two different repeating units in the polymer chain, with the active end of one monomer favoring the other monomer. This replication of structural units is possible if monomers have a strong preference for reacting with each other. The r-value of MMOAB was average (r1avr. = 0.35) and r-value of GMA is average (r2avr. = 0.60). From this, it can be concluded that GMA will be more involved in the copolymer structure. The most important reason for this situation is the steric effect of methylparaben in the side branch of MMOAB. Therefore, MMOAB-terminal radicals tend to join more GMA-terminated radicals. In such copolymeric systems, alternating copolymers containing predominantly methacrylate monomers were formed [23]. Information on the calculated reactivity rates is given in Table 4.
Mean sequence length, run number, and Dyad fraction of the copolymers
The mean sequence lengths(μ) values of the MMOAB and GMA monomers in the copolymers are calculated using the following equation: [24]
The calculated mean sequence length distributions, μMMOAB/μGMA ratio are shown in Table 5. [M1] = 30% and [M2] = 70%, and [M1] = 50% and [M2] = 50%, for copolymer samples each copolymer segment with M2 units is approximately twice as long as its adjacent segment with M1 units whereas for [M1] = 90% and [M2] = 10%, segments containing M1 units were 4 times longer than those containing M2 units. For the [M1] = 90% and [M2] = 10% copolymer example, the rank can be expressed as AAAAB, where B represents GMA, and A represents MMOAB. The number of GMA units in the copolymer increases due to the higher r-value. In addition, when the results obtained are compared with the 1/r1 and 1/r2 values, the low 1/r2 value shows the tendency of the GMA monomer to be more homopolymerized.
From the reactivity ratios of the monomers, the order number, RN, was determined as the average number of monomer changes per 100 monomeric units in a copolymer chain according to the Harwood and Reichy Eq. (7): [25]
RN is another parameter that gives information about the monomer distributions in the copolymer chains. If the sequence in a copolymer is known, more accurate interpretations can be made about the physical properties of the copolymer. The RN change determined using the calculated r values for the MMOAB-GMA system is shown in Fig. 5a. The maximum RN value for this copolymer system was found to be 67.8 at X = 1.0.
To determine the microstructure of the MMOAB-GMA copolymer system, the statistical distribution of MMOAB-MMOAB, GMA-GMA and MMOAB-GMA dyad monomer sequences was calculated using the method proposed by Igarashi [26].
The variation of the dyad fractions with the MMOAB mole fraction in the copolymers is depicted in Fig. 5b. The results obtained showed that the probability of GMA-GMA is higher than MMOAB-MMOAB. It was also observed that MMOAB tended to be more copolymerized than homopolymerized.
Thermal stability of the polymers
The TGA curve of polymers is shown in Fig. 6. Poly(MMOAB) gave a one steps degradation curve. From the TGA graph, the initial thermal decomposition temperature is 318 °C, and the final temperature is 440 °C. The 50% mass loss temperature of Poly(MMOAB) was determined as 380 °C from the thermogram. Also, the residual amount left by the homopolymer at 600 °C was around 16%. Since the aromatic benzene core of the methylparaben group in the side branch of Poly(MMOAB) could not thermally transform into volatile products, the amount of residue may have increased. The thermal degradation of poly(GMA) started at 184 °C and finished at 600 °C and left 2% residue. The high thermal stability of Poly(MMOAB) is the functional group diversity due to the side branch methylparaben. In particular, dipole–dipole interactions of ester carbonyl groups create secondary forces between the polymer chains, increasing thermal stability. This also increases the thermal stability with the rise of MMOAB units in the copolymer. It has been observed that the thermal stability of the copolymer is between the thermal stability of the homopolymer [27]. PMMA is also a thermoplastic. In thermoplastic polymers, intra- and inter-chain interactions hold the polymer chains together, such as dipole–dipole polar interactions, hydrogen bonds, London forces, and even stacking of aromatic groups. In addition, there are inter-and intra-chain bending and entanglement that hold the thermoplastic polymer chains together. These chain loops and twists greatly influence the physical properties of thermoplastic polymers, especially on the mechanical response. The thermal properties obtained from the TGA graphs are shown in Table 6.
Tg values of the polymers
Tg values of poly(MMOAB) and poly(GMA) determined by DSC were estimated as 103 °C and 74 °C, respectively. From the DSC curves seen in Fig. 7, it was determined that the glass transition temperatures of the copolymers increased with the increase of MMAOB units in the composition. The dipole–dipole interaction caused by polarized ester groups in the side branch of MMOAB causes the free volume to decrease and the Tg value to increase. The dipole–dipole effect arises with the electrostatic attraction between the opposite poles of the molecules. This situation, which occurs at the end of a chemical process, affects the physical properties of molecules. Increasing the polarity of the molecules also strengthens the dipole–dipole interaction. Therefore, the Tg value increases with the increase of MMAOB units in the copolymer.
The Gibbs-diMarzio theory is used to determine the experimental glass transition temperature in copolymers, taking into account the weight ratio of the composition.
The Gibbs-Di Marzio equation is as in 11; [28]
where, \(\Phi\) is the mole fraction and Tg is the glass transition temperature of the related homopolymer.
Alternatively, the theoretical Tg values of the copolymers were determined by the Fox Eq. (12) [29].
where W is the weight fractions in the copolymer. As seen in Fig. 8, the theoretical Tg values obtained by both methods were found to be parallel. It appears that the real Tg values obtained from DSC are between the theoretical Tg values up to 0.55 mol fraction and then deviate and increase. Many parameters affect the Tg values of copolymers. Especially the type of copolymer is the most important factor. Using instrumental methods is the most accurate approach to determine the real Tg values of copolymers. However, it is possible to estimate the Tg values of statistical copolymers with some theoretical methods and compare them with the real values. As shown in Table 7, it is clear that the values estimated from the Gibbs-diMarzio and Fox models are in line with the experimental results.
While Fox theory mainly focuses on order–disorder transitions of polymers under the influence of heat, Gibbs and diMarzio concentrate on the concept of glass transition of polymers. Recognizing that there is a van der Waals interaction between non-chemically bonded monomers, Fox suggested that the empty lattice regions are equivalent to broken van der Waals bonds. For this reason, he defined the melt consisting of the polymers themselves and the holes between them as compressible. On the other hand, Gibbs-DiMarzio, is to model polymers as irreversible random walks and reduce the equation of state to a van der Waals-like form. The unique point of the Gibbs-diMarzio theory is that entropy takes negative values at small temperature values. At low temperatures, the entropy value is increased to lower temperatures than the experimentally observed values, and the entropy that does not occur experimentally becomes negative. Therefore, it can be shown that the glass transition temperature shifts to a lower temperature in the Gibbs diMarzio theory.
Thermal degradation kinetics
The activation energies of the thermal degradation (Ea) were determined by TGA using non-isothermal models. Thermograms of polymers were obtained at heating rates of 5.0, 10.0, 15.0, and 20.0 °C/min to determine the Ea values. The Kissinger, Kissinger–Akahira–Sunose (KAS), and Ozawa, which are non-isothermal methods, were used to determine the Ea values of the polymers.
The kissinger method
With the Kissinger method [30], the activation energy (Ea) can be determined using the following Eq. (13);
where β is the heating rate, Tp is the temperature that corresponds to the position of the rate peak maximum, and R is the ideal gas constant. A graph of 1000/Tp versus ln(β/Tp2) was drawn from the TGA data, and Ea values were calculated. The DTG curves of the poly(MMOAB-co-GMA)(0.47:0.53) are shown in Fig. 9. The Ea value of Poly(MMOAB) was determined as 129.03 kJ/mol. The Kissinger plot of the copolymer containing 47% MMOAB in its composition is shown in Fig. 10. The obtained Ea values by the Kissinger method of the copolymers are between 121.22 kJ/mol and 104.31 kJ/mol. The obtained Ea values of the copolymers are given in Table 8.
The Kissinger–Akahira–Sunose (KAS) method
With the KAS equation [30], the activation energy can be calculated without knowing the solid-state decomposition reaction mechanism. The KAS method is based on taking the differential of Eq. (14).
According to this,
where β is the heating rate, T is the temperature, and R is the ideal gas constant. ln(β/T2) against (1/T) gives activation energy from the slope. The Ea value of Poly(MMOAB) was determined as 126.44 kJ/mol. The KAS plot of the poly(MMOAB-co-GMA)(0.47:0.53) is shown in Fig. 11. The Ea values of the copolymers are given in Table 9. The obtained Ea values by the KAS method of the copolymers are between 117.58 kJ/mol and 99.28 kJ/mol.
Poly(MMOAB) is polarized due to the functional groups it contains. Therefore, the potent dipole–dipole interactions between poly(MMOAB) units cause more molecules to hold together. With the increase in MMOAB units in the copolymer, there was an increase in Ea values. The possible reason for this is the intermolecular dipole–dipole interactions arising from the polarized ester groups present in the MMOAB units.
Flynn–Wall–Ozawa (FWO)
Alternatively, Ea values were calculated using the Flynn–Wall–Ozawa (FWO) method [31]. For this purpose, thermograms at different heating rates were used.
The FWO method is defined as:
R is the gas constant; β, the heating rate ( oC/min) and Ea is the activation energy. Using the TG data at different heating rates (5,10,15, and 20 °C), log β and counter 1/T graphs were created. When the results of the FWO analysis were plotted, parallel lines were obtained. (Fig. 12). The Ea values of the polymers calculated at different conversions are listed in Table 10. The mean Ea value of Poly(MMOAB) was 125.39 kJ/mol by the FWO method. As shown in Fig. 13, the average Ea values increased as the number of MMOAB units in the copolymers increased. The main reason for this result is the intermolecular secondary interaction forces between the polar groups in the MMOAB units. While the Ea value of the copolymer sample containing 78% MMOAB in its composition was 123.15 kJ/mol, 102.48 kJ/mol was found for the sample containing 17% MMOAB.
Our results are consistent with the thermal stability and calculated Ea values of methacrylate polymers containing different functional groups in the side branch in the literature [32]. It is seen that the interactions between the polar groups in the side branch affect the thermal stability of the polymers [33].
Molecular weights of polymers
GPC were used to determine the molecular weights of polymers by using polystyrene as standard and tetrahydrofuran as solvent. Table 11 lists the Mw and Mn values and polydispersity index(PI) (Mw/Mn) of the polymers. The PI values of the copolymers showed a random distribution independent of the composition. The smallest PI value of 1.52 belonged to the copolymer containing 55% MMOAB in the composition, and the largest PI value, 1.73 of belonged to the copolymer containing 78% MMOAB. The PI value of Poly(MMOAB) was also found to be 1.52. The theoretical value of Mw/Mn is 1.5–2.0 means that the termination is disproportionate [34]. When the poly (MMOAB) polydispersity index was examined, the disproportionate chain termination was higher than the union.
An important parameter used in the characterization of polymers is their average molecular weight and molecular weight distribution. The average molecular weight and distribution of a polymer affects all its properties and directly determines its uses. With increased molecular weight in polymers, interpenetration and intermolecular attraction forces in the polymer structure increase. This situation causes significant changes in the mechanical, thermal, electrical, optical, and chemical properties of the polymer.
Antimicrobial screening of the polymers
By the disc diffusion method were determined the biological activities [35]. In this method, the inoculated microorganism is first spread on the solid nutrient medium in Petri dishes. Then, the substance to be tested is impregnated with commercially available paper discs and placed in petri dishes and zone formation is observed in the presence of activity. The results of the biological activity tests are shown in Tables 12 and 13. All polymers were most effective against Staphylococcus aureus bacteria. This bacterium is gram-positive and is a hospital (hospital infection) agent. It is also found commensal in the human skin flora. The effectiveness of poly(MMOAB) against the microorganisms used in the study is higher than the copolymers formed with GMA. According to the results, it was observed that the biological activity increased with the increase of polymer concentration and MMOAB unit in copolymers. The possible reason for this is the methylparaben group carried by the polymer. Parabens have long been used in injections and ophthalmic preparations because of their antimicrobial activity. It has a wide range of antimicrobial activity against parabens, especially gram-positive bacteria and fungi, and is chemically stable and effective over a wide pH range. It is an actual result in polymer science that our new methacrylate derivative polymers, which carry a pharmacophore group such as methylparaben, are effective on some bacteria.
The biological activity of a molecule is closely related to the functional groups it contains. The presence, position, and stereochemistry of atoms and groups called pharmacophores play a leading role in biological activity. The lipophilic character is essential for that drug to pass through living cell membranes and interact with the receptor. The electronic effect is one of many physicochemical parameters on which the bioactivity of a compound depends. The ionization and polarity of a drug are affected by the electronic effects of different substituents. A drug may have a static effect at low concentrations and a cidal effect at more extreme dilutions. Although growth in static density is not visible to the naked eye, growth can be observed or some colonies may occur when sowing in liquid or solid media that does not contain antibiotics. However, not much reproduction may be found. There is no reproduction in sowing made from dilution showing cidal effect. To determine the cidal or static effect, it is more appropriate to try their cultivation in a liquid medium without antibiotics. Because the antibiotic contained in the inoculum taken for sowing is diluted in the liquid medium and its effectiveness is lost. A drug may be cidal for one microorganism and static for another. This situation depends on the resistance mechanism of the host microorganism. Because microorganisms show resistance to drugs in various ways (extrachromosomal, chromosomal, and other factors). According to the results obtained, poly(MMOAB-co-GMA) polymers can be used as biomaterials.
PMMA is preferred as a biomaterial in different areas of the health sector. It has been used in dentistry for many years, especially as a denture base material. Since the introduction of PMMA, its physical and chemical properties have been greatly improved. In addition, PMMA-based cross-linked resins, fiber-specific resins, radiopaque resins, and high-impact resins are also available. PMMA is widely used as a biomedical material in intraocular and hard contact lenses due to its good light transmittance, hardness, and stability.
Antimicrobials caused by microorganisms are drugs used to treat infectious diseases. Their common disadvantages are that they have side effects and cause the development of resistance in microorganisms. Antibiotics act on bacteria through different mechanisms of action. These antimicrobials, which have bactericidal effects, stop cell wall synthesis by binding to transpeptidase and carboxypeptidase enzymes, which are located in the cell wall of bacteria and serve in the last step of peptidoglycan synthesis. These molecules also bind to ribosomes and inhibit protein synthesis. Some antibiotics, on the other hand, inhibit DNA replication by inhibiting the nNA gyrase (topoisomerase II) enzyme.
Photo-stability of the copolymers
The exposure times to sunlight and some artificial lights of the samples, whose behavior against external environmental effects were examined, have a negative effect on their lifetime. UV radiation causes the breakdown of chemical bonds in polymeric structures. Many polymers have bond energies in the wavelength range of 280–400 nm at the molecular level and are therefore severely affected by this portion of solar radiation. Photodegradation begins when the photon energy is absorbed by the polymer and the molecular chain is activated.
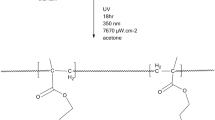
Most synthetic polymers undergo photodegradation by absorbing UV light through functional groups. In the UV–Vis spectra of the poly(MMOAB-co-GMA) copolymers, the maximum wavelength of the peaks belonging to the π-π* transition of the aryl and carbonyl chromophore groups C = C and C = O was observed around 280 nm. Significant changes were observed in the absorption values of the carbonyl region of the copolymers exposed to UV light for 12,18,24,36,48,60, and 72 h. Methacrylate polymers showed photodegradation [36]. All copolymers were soluble in polar solvents such as CH2Cl2, Acetone, DMF, DMSO, and 1,4-dioxane before exposure to UV light, while none were dissolved in a solvent after 72 h due to crosslinking. This result shows that the polymer undergoes photodegradation by absorbing UV light. After photodegradation in the UV spectrum, peaks appeared, indicating conjugate unsaturation at 245 nm and carbonyl groups at 290 nm. The results obtained from UV stability studies of poly(MMOAB-co-GMA) copolymers showed that these polymers have similar photodegradation behavior with other methacrylates in the literature [37].
Polymers frequently encounter factors and substances such as high heat and UV-rays, humidity, ozone, and chemicals during production, processing, or use. The mentioned factors and substances can change the physical properties or chemical structures of polymers at a certain level or in a way that completely prevents the use of polymeric materials. Photodegradation causes a decrease in the molecular weight of the polymer by breaking the bonds, which leads to changes in the mechanical properties of the polymer over time. The change in these properties causes the material to degrade and the bonds to break, increasing the chemical reactivity of the system.
The energy of the beam, which has a wavelength of 280 nm, is high enough to break the carbon–carbon bonds. Therefore, UV-rays with wavelengths lower than 300 nm are more effective in the degradation of polymers. UV-Stabilizers should be used against this degradation. These are generally peroxide scavengers and free radical scavengers, UV absorbers, and excited-state scavengers, which are believed to be the most effective. These materials effectively reduce the rate of radical formation by absorbing UV radiation before the photochemical reaction starts. The main mechanisms of action are based on absorbing harmful UV radiation and converting it into much less harmful heat energy. It can be said that poly(MMOAB) and its copolymers doped with such a system will gain photostability. It is planned to carry out studies in this direction in the future.
Dielectric properties of the polymers
Dielectric materials do not allow the free movement of charged particles. In this case, a dielectric material can generally be thought of as an insulator. Dielectrics are electrically insulating materials, store charge under an external electric field, and have high polarization capability. It is essential to measure the dielectric properties of materials under different temperature and frequency conditions to prevent leakage currents in a circuit and produce high-performance materials and devices. The resulting electric dipoles are electrically conductive on the surface of the dielectric material, allowing charge accumulation. For this, they are used in the construction of capacitors. They are used as insulators to prevent charge transfer in the electrical circuit. Dielectric materials do not allow the free movement of charged particles. If a dielectric material is placed in an electric field, it is called polarization when it changes infinitely small depending on the frequency but independent of the electric field strength.
These polarizations occur in different ways depending on the structure of the material. They are divided into electronic, ionic (atomic), dipolar (orientation), and interface (space-charge) polarization. Depending on the type of material, its polarization mechanisms may be different, may or may not include all mechanisms. The dielectric constant of a substance with respect to vacuum is expressed as the ratio of the capacitance of a capacitor filled with that substance to its capacity in a vacuum under a certain external field. Some materials contain permanent dipoles in their structure and are oriented in the direction of the applied area when an electric field is applied. When the applied electric field is removed, the dipoles remain oriented, causing polarization. Permanent dipoles are also found in polymeric structures containing asymmetric units.
Dielectric loss is the loss of electric field energy as heat that cannot be recovered in the dielectric.
The dielectric constant of a polymer is calculated by the following Eq. (16) [38].
Here “C” is parallel capacitance, “Ɛ0” is permittivity in free space (Ɛ0 = 8.854.10−12 F/m), “d” is the thickness of the polymer sample and A is the surface area of the sample.
The dielectric loss factor can be calculated by Eq. (17) [38].
Here Df is the loss factor and Ɛ'' is the dielectric loss.
Figure 14a, b shows the variation of dielectric constant (ε′) and dielectric loss factor (ɛ′′) of poly(MMOAB) and poly(MMOAB-co-GMA) copolymers with frequency. It was observed that ε′ and ε′' values were high at low frequencies. It is known that ε′ and ɛ′′ values of polymers are more stable at 1 kHz. [39]. It can be said that this situation is due to polarization effects. It is known that orientation and interface polarization are effective in the high ε′ and ɛ′′ values at low frequencies [40]. The ε′ and ɛ′′ values of Poly(MMOAB) at 1 kHz were found to be 5.89 and 0.091, respectively. It was observed that the ε′ values of the copolymers decreased with the decrease of MMOAB units in the copolymer composition and varied between 5.67 and 3.98. The same trend was observed for the ε′' values of the copolymers. The main reason for this result is the aromatic ring and polar ester groups of MMOAB units. The dipole–dipole interactions formed by these groups increase in the polarity of the copolymers, thus increasing the ε′ and ɛ′′ values [41].
The reason for this increase can be because of polar groups [42] such as C–O and OC = O groups in polymer structure. The rotational motion of the polar molecules in the structure of a dielectric sample at low frequencies is fast thanks to the applied field. The event that causes this situation can be described by dielectric relaxation. When the frequency rises, the impact of the dipoles ups, resulting in polarization and ε′ and ε′' value decreases. Similar results have been recorded in the litarature [43].
Polarization is one of the electrical insulating materials characteristics and is due to their structure. Each polymer, under the influence of an electric field, is polarized. The degree of polarization in a material, ε (dielectric constant), shows. The degree of polarization depends on the density and size of the dipoles formed in the material and is oriented by the effect of the external electric field.
The conductivity measurements of the copolymer system were studied at 100 Hz and 20 kHz, depending on the alternating current (AC) conductivity. The visual figure of the device, on which dielectric measurements are made, is shown in Fig. 15. It has been observed that conductivity values increase with frequency [44]. The σac value for Poly(MMOAB 17%-co-GMA) is 2.27 × 10−9, while the same value is 3.93 × 10−9 at 25 °C and 1 kHz for Poly(MMOAB 78%-co-GMA). The electrical conductivity of a polymer depends on the free electrons and ions in its structure [45]. The obtained σac values show that the copolymer is close to the semiconductor band. The ɛ′, ɛ′′ and σac values obtained for the copolymer are presented in Table 14. Polymers with dielectric properties are used as electrical insulators, dielectric capacitors, or parts of microwave devices. The polarization degree of the material is the most important parameter affecting the dielectric properties. The properties of dielectric materials are commonly expressed in terms such as dielectric constant, dielectric loss factor, and resonance frequency temperature coefficient. These properties of dielectric materials, which have a wide range of application areas, must have the desired values in terms of the design and performance of the devices.
Conclusions
A new methacrylate containing methylparaben group in the side branch and its copolymers with GMA were synthesized by radical solution polymerization. All molecules were characterized by FTIR and NMR techniques. The copolymer composition was determined by 1H-NMR spectrum data. FR and KT methods were used to determine the monomer reactivity ratios. The mean rMMOAB value determined by both methods was 0.35, and the rGMA value was 0.60. It was concluded that the rGMA value was greater than rMMOAB and thus, the copolymer was rich in GMA. The rMMOAB.rGMA value of 0.20 indicated that this copolymer trend is alternative. Poly(MMOAB) was found to be more thermally stable than poly(GMA) due to the dipole–dipole interactions formed between the carbonyl functional groups carried by the MMOAB units. Thermal degradation activation energy (Ea) values were calculated for polymers using the methods of Kissinger–Akahira–Sunose, Flynn–Wall–Ozawa and Kissinger. The Ea values determined by the three methods for poly(MMOAB) are close to each other. It was observed that the thermal stability increased as the amount of MMOAB in the copolymer increased. By DSC obtained Tg values of the copolymers were between of the its homopolymers. It was observed that the biological activities of the copolymers depended on the MMOAB units and were effective against some microorganisms. It was determined that the copolymers exposed to UV light for a certain time (72 h) underwent photodegradation. The dielectric properties of poly(MMOAB-co-GMA) were measured using an impedance analyzer as a function of temperature and frequency. It is observed that the ɛ′ and ɛ′′ value increased with the MMOAB content in the copolymers. Also, the conductivity (σ) indicates a strong dependence on the frequency of the applied field. The σ value increased linearly with increasing MMOAB content. In addition, the impedance value decreases with increasing frequency due to the increase in conductivity. Poly(MMOAB) and its copolymers can be used in applications such as electronic devices and capacitors, high voltage electrical insulators, air capacitors, transformers due to their dielectric properties.
References
Cheng C, Li J, Yang F, Li Y, Hu Z, Wang J (2018) Renewable eugenol-based functional polymers with self-healing and high temperature resistance properties. J Polym Res 25(2):57
Soykan C, Erol I (2003) Synthesis, Spectral and Thermal Properties of Homo- and Copolymers of 2-[(5-methylisoxazol-3-yl)amino]-2-oxo-ethyl methacrylate with Styrene and Methyl Methacrylate and Determination of Monomer Reactivity Ratios. Eur Polym J 39:2261–2270
Solmaz A, İlter Z, Kaya I (2021) Synthesis, characterization and thermal properties of chalcone methacrylamide polymers containing methoxy group in side chain. J Polym Res 28:233
Abdollahi H, Najafi V, Amiri F (2021) Determination of monomer reactivity ratios and thermal properties of poly(GMA-co-MMA) copolymers. Polym Bull 78:493–511
Aydogdu Y, Erol I, Yakuphanoglu F et al (2003) Electrical conductivity and optical properties of copolymers based on 4-vinylpyridine and tetralincyclobutylhydroxyethylmethacrylate. Synth Met 139:327–334
Lee JW, Lee HK, Moon HB (2019) Contamination and spatial distribution of parabens, their metabolites and antimicrobials in sediment from Korean coastal waters. Ecotoxicol Environ Saf 180:185–191
Bolujoko NB, Unuabonah EI, Alfred MO et al (2021) Toxicity and removal of parabens from water: A critical review. Sci Total Environ 792:148092
Smarr MM, Sundaram R, Honda M, Kannan K, Louis GMB (2017) Urinary Concentrations of Parabens and Other Antimicrobial Chemicals and Their Association with Couples’ Fecundity. Environ Health Perspect 125(4):730–736
Petric Z, Ružić J, Žuntar I (2021) The controversies of parabens – an overview nowadays. Acta Pharm 71(1):17–32
Nowak K, Jabłońska E, Ratajczak-Wrona W (2021) Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ Res 198:110488
Geer LA, Pycke BFG, Waxenbaum J, Sherer DM, Abulafia O, Halden RU (2017) Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater 323:177–183
Zhu Q, Jia J, Wang Y, Zhang K et al (2019) Spatial distribution of parabens, triclocarban, triclosan, bisphenols, and tetrabromobisphenol A and its alternatives in municipal sewage sludges in China. Sci Total Environ 679:61–69
Chen J, Ahn KC, Gee NA et al (2007) Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmacol 221(3):278–284
Chen J, Hartmann EM, Kline J et al (2018) Assessment of human exposure to triclocarban, triclosan and five parabens in U.S. indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry. J Hazard Mater 360:623–630
Erol I, Soykan C, Ahmedzade M (2002) Monomer reactivity ratios of the 2-(3-mesityl-3-methylcyclobutyl)-2-hydroxyethyl methacrylate and styrene system from 1H-NMR. J Polym Sci Part A-Polym Chem 40:1756–1763
Hong S, Kim J, Kim MS, Kim BW (2012) Radical polymerization of acrylate copolymer-based GMA for use as a UV-curable layer via thin coating. Adv Polym Technol 31:271–279
Erol I, Soykan C, Ilter Z et al (2003) Thermal degradation of poly 2-[3-(6-tetralino)-3-methylcyclobutyl]-2-ketoethyl methacrylate. Polym Degrad Stab 81:287–295
Coskun M, Erol I, Coskun MF, Demirelli K (2002) Thermal degradation behaviour of two methacrylate polymers with side chain amide groups. Polym Degrad Stab 78:49–55
Connerton IF (1994) In Analysis of Membrane Proteins, Ed by Gould G.W.: Portland, London, 177
Erol I et al (2009) Synthesis and Characterization of Novel Fluorine-Containing Methacrylate Copolymers: Reactivity Ratios, Thermal Properties, and Antimicrobial Activity. J App Polym Sci 3351–3359
Fineman M, Ross SD (1950) Linear method for determining monomer reactivity ratios in copolymerization. J Polym Sci 5:259–262
Kelen T, Tudos F (1974) Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphical method. J Mac Sci Part A-Chem 9:1–27
Ham G (1964) Copolymerization, High Polymers. Interscience, New York
Arshady R, Kenner GW (1974) Laboratories RR. Phenolic Resins for Solid-Phase Peptide Synthesis: Copolymerization of Styrene and p-Acetoxystyrene. J Polym Sci 12:2017–2025
Harwood HJ, Richey WM (1964) The characterızatıon of sequence distribution in copolymers. J Polym Res Part-B Polym Lett 2:601–607
Igarashi S (1963) Representation of composition and blockiness of the copolymer by a triangular coordinate system. J Polym Sci Polym Lett Ed 1:359–363
Coskun M, Demirelli K, Erol I, Ahmedzade M (1998) Thermal degradation of poly[2-(3-aryl-3-methylcyclobutyl)-2-hydroxyethyl methacrylate]. Polym Degrad Stab 61:493–497
Di Marzio EA, Gibbs JH (1963) Molecular interpretation of glass temperature depression by plasticizers. J Polym Sci A: Gen Papers 1:1417–1428
Fox Jr TG, Flory PJ (1950) Second order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys 21:581
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Ozawa T (1965) A New Method of Analyzing Thermogravimetric Data. Bull Chem Soc Jpn 38:1881–1886
Erol İ, Özer M (2021) Copolymers of a novel amphiphilic methacrylate monomer based on the hydroxyl group: copolymerization kinetics, thermal properties, biological activity, and swelling behavior. J Polym Res 28:372
Zhang K, Jiang Z, Qiu Z (2021) Effect of different lengths of side groups on the thermal, crystallization and mechanical properties of novel biodegradable poly(ethylene succinate) copolymers. Polym Deg Stab 187:109542
Melville HW, Noble B, Watson WF (1949) Copolymerization. II. Molecular weight distribution and mean molecular weights in copolymerization systems. J Polym Sci 4:629–637
Ringertz S, Rylander M, Kronvall G (1991) Disk Diffusion Method for Susceptibility Testing of Neisseria Gonorrhoea. J Clin Microbiol 29:1604–1609
Kaczmarek H, Kaminska A, Van herk A (2000) Photooxidative Degradation of Poly(alkyl methacrylate)s. Eur Polym J 36:767–777
Shirai M, Yamamoto T, Tsunooka M (1999) Ablative Photodegradation of Poly(Methyl Methacrylate) and İts Homologues by 185- nm Light. Polym Degrad Stab 63:481–487
Yakuphanoglu F et al (2005) The dielectric spectroscopy and surface morphology studies in a new conjugated polymer poly(benzobisoxazole-2,6-diylvinylene). Physica B 365:13–9
Yakuphanoglu F, Erol I (2004) A novel organic semiconducting material: 2-(3-mesityl-3-methylcyclobutyl)-2-keto-ethyl methacrylate (MCKEMA). Physica B-condends Matter 352:378–382
Belakere NN, Misra SCK, Ram MK et al (1992) Interfacial polarization in semiconducting polypyrrole thin-films. J Phys Condens Matter 4:5747–5756
Ramesh S, Yahana AH, Aroof AK (2002) Dielectric behaviour of PVC based polymer electrolytes. Solid State Ion 291:152–153
Wang C, Zhang Z, Pejić S, Li R, Fukuto M, Zhu L, Sauvé G (2018) High Dielectric Constant Semiconducting Poly(3-alkylthiophene) s from Side Chain Modifcation with Polar Sulfnyl and Sulfonyl Groups. Macromolecules 51:9368–9381
Biryan F, Pıhtılı G (2021) Study on the thermal and electrical properties of novel poly[propyl acrylate-co-phenyl methacrylate] containing acetamido group. J Polym Res 28:382
Ku CC, Liepins R (1989) Electrical properties of polymers. Chem Principles 30(11):2141
Zuo F et al (1989) AC conductivity of emeraldine polymer. Phys Rev B 39:3570–3578
Acknowledgements
The author is grateful to Dr. Zeki GÜRLER for the study of biological behavior.
Funding
This study was supported by Afyon Kocatepe University Scientific Research Projects Coordination Unit. The Project Number is 15-FENBİL-38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erol, I., Akbıyık, H. Kinetic parameters, thermal stability, biological activity, and dielectric properties of new methacrylate-based copolymers functionalized with methylparaben. J Polym Res 29, 93 (2022). https://doi.org/10.1007/s10965-022-02950-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-02950-6