Abstract
A mercury ion imprinted polymer material was prepared using a thiourea derivative functionalised graphene quantum dot (GQDTU) as the functional monomer using suspension polymerisation technique. This ion imprinted polymer (GQDTU-IIP) material was used to modify the surface of a glassy carbon electrode by drop casting method. The modified electrode was used for the detection of mercury using cyclic voltammetry (CV) and differential pulse voltammetric (DPV) techniques. The sensing performance was compared with a non-imprinted polymer (NIP) modified electrode. The GQDTU-IIP modified electrode showed a linear response towards concentration of Hg(II) ions in the range 5 × 10–8 M to 2.3 × 10–5 M using DPV with a limit of detection 23.5 nM. CV also showed linear response ranges of 6 × 10–8 M to 8.5 × 10–7 M and 1.4 × 10–6 M to 7 × 10–6 M with a limit of detection 30.2 nM. The electrode was used for the analysis of Hg(II) ions in real water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rise of various industries has been accompanied by a sharp increase in environmental contamination by toxic substances like heavy metals. Heavy metals are those metals having relatively large density like mercury, lead, arsenic, cadmium etc. discharged into the earth primarily through industrial effluents, volcanic emissions, fuel combustion etc. Interaction of these metal ions with the thiol group of biomolecules results in their cyanogenic impact on animals [1]. This builds the demand for the development of various refinement techniques and sensors for such pollutants. To monitor the presence of these metals, instrumental methods like Atomic Absorption Spectroscopy (AAS), Ion chromatography, Industrially Coupled Plasma Mass Spectrometry (ICP-MS) etc. are generally used.
Electrochemical sensors are good alternative for the spectroscopic and chromatographic techniques as the latter methods require complex methodology and significant expenses [2]. Various kinds of heavy metal sensors are available recently, created based on the chemical interactions between the electrode and metal ions. In order to improve selectivity in sensing application, chemically modified electrodes are used. Nanoparticles, organic molecules, polymer materials etc. and their composites can be used as electrode modifiers [3, 45, 6]. Graphene based nanoparticles like graphene oxide and graphene quantum dots show awesome electrical, optical and mechanical properties [7,8,9,10,11]. Several graphene based electrode modification were reported till date towards metal ion detection. The oxygen functionalities present in graphene oxide can act as metal binding site as well as it can be modified covalently or non-covalently, with a suitable molecule which can interact with the specific analyte [12,13,14,15,16,17,18,19].
Ion imprinting is a technique by which specific cavities can be introduced in the material. This ensures specificity and selectivity for a particular target analyte [20,21,22,23,24]. Combination of ion imprinting with nanomaterials is a good choice for developing sensors with high selectivity and sensitivity since nanomaterials improve electrical conductivity and imprinting provides better selectivity to the material. T. Alizadeh et al. have reported an imprinted polymer-MWCNT composite towards the detection of mercury(II) ions using itaconic acid as the monomer and fabricated a carbon paste electrode with the ITA-IIP/MWCNT composite [25]. An IIP composite with rGO was used by Motlagh et al. [26] for mercury(II) detection. The molecule 2,2′-(9E,10E)-1,4-dihydroxyanthracene-9,10-diylidene)bis(hydrazine-1-carbothioamide) (DDBHCT) was synthesised by them for chelating with mercury(II) ion. Methyl methacrylic acid was taken as the functional monomer, EGDMA as cross linker and ammonium persulfate as the initiator for imprinted polymer formation. They achieved a detection limit of 0.02 μg/L using SWASV. In these reported cases either the ligand synthesis involved many steps or the cost of CNT would be a concern.
Compared to carbon nanotubes, graphene is a cheaper and easily synthesizable material. Herein we report a novel nanosensor based on GQD composite with an ion-imprinted polymer, synthesized by a simple method which is used for modification of glassy carbon electrode for the electrochemical sensing of mercury(II) ions.
Experimental
Materials and reagents
Graphite fine powder was obtained from Loba Chemie. 4-vinyl aniline, Ethylene glycol dimethyl acrylate (EGDMA), Polyvinyl alcohol (PVA), Azobisisobutyronitrile (AIBN), and metal salts were purchased from Aldrich and Nafion was purchased from Merck. All other reagents utilized were of analytical grade. Acetate buffer solution was prepared by mixing sodium acetate and glacial acetic acid in appropriate quantities. It was used for all electrochemical measurements.
Apparatus
The materials synthesised were characterised by Fourier Transform Infrared spectroscopy (JASCO FT-IR- 5300 spectrometer) using KBr pellets. The surface morphology of the material was determined using SEM (Jeol JSM-6390LV/JED–2300) and TEM (HR-TEM Jeol/JEM 2100) imaging. To analyse the elemental composition of the material, CHNS analyser was used (Elementar Vario ELIII CHNS analyser). Electronic spectra of the sample is recorded using Thermo scientific Evolution 220 UV–Vis spectrophotometer. Electrochemical measurements were carried out in CHI 660E electrochemical workstation (CH instruments) with a three-electrode system in which GQDTU-IIP modified glassy carbon was used as the working electrode, Ag/AgCl as reference electrode and a Pt wire as counter electrode in acetate buffer solution (pH 5). ICP-MS analysis was done using Thermo Scientific, iCAP RQ ICP-MS instrument (Helium KED mode). For the GQD synthesis, probe sonicator (SONICS vibra cell model-VCX 750) was used. Thermogravimetric analyses were done using a Metler 851 Model SDTA/TGA instrument at a heating rate 20 °C min−1. X-ray photoelectron spectroscopy was recorded using PHI 5000 Versaprobe Scanning Esca Microprobe.
Synthesis of Graphene Quantum Dot (GQD)
GQD was synthesised from Graphene Oxide (GO) using probe sonication method according to a reported procedure. For this GO was synthesised initially using Tour method [27]. 1 g of NaOH was dissolved in 30 mL of ethyl acetoacetate by stirring for 30 min. It was filtered and to the filtrate about 0.2 g of GO was added and kept for ultra-sonication for 2 h under 10 kHz frequency (50%). The dispersion obtained was centrifuged to remove unexfoliated GO. The centrifugate was then dialysed and the solution outside was collected as GQD solution which exhibited strong fluorescence. It was lyophilised to obtain GQD in powder form [28].
Functionalization of GQD to form the ligand monomer (GQDTU)
About 0.1 g of GQD powder was treated with 4 mL thionyl chloride to convert the acid functionalities (-COOH) of GQD into acid chlorides [29]. The acid chloride modified GQD (GQD-COCl) was dissolved in dried acetone. About 0.08 g of dry ammonium thiocyanate was dissolved in dried acetone and cooled to 0–5 ˚C. To this, a cold solution of GQD-COCl in dried acetone was added drop wise by keeping the temperature below 5 ˚C. After complete addition it was stirred for 1 h to permit complete precipitation of ammonium chloride. The ammonium chloride formed was sifted through rapidly. The filtrate was taken in a round bottom flask, cooled and kept for stirring. To this a cold solution of 4-vinyl aniline (0.24 g in dried acetone) was added drop wise. Mixing was continued for 60 min. The resultant mixture was poured over a large quantity of ice water. A yellow brown precipitate was formed which was separated and dried in air [30].
Polymerisation of functionalised GQD monomer (GQDTU)
The polymer was prepared via suspension polymerisation method [31]. 0.1 g of polyvinyl alcohol (PVA) was dissolved in about 30 mL hot water and cooled to room temperature. The polymerization mixture was prepared by taking Template (Hg2+): Monomer (GQDTU): Cross linker (EGDMA) in the proportion 1:2:4. About 0.5 mL of free radical initiator Azobisisobutyronitrile (AIBN) was added to it. This mixture taken in a round bottom flask was subjected to N2 purging. To this, PVA solution was added drop wise with stirring. It was kept in an oil bath with vigorous stirring at 80 ˚C in N2 environment for 2 h. The stirring was continued overnight. The beads obtained were collected, washed and dried to get the polymer GQDTUP [32, 33].
Removal of the imprint ion from the polymer
Hg(II) ions were leached out from the polymer beads by stirring with 100 mL of 50% HCl for 18 h [31]. The beads were dried in an oven to get the ion imprinted polymer material (GQDTU-IIP). To confirm the removal of the template Hg(II) ions, ICP–MS analysis was carried out by digesting the material in nitric acid followed by filtration and dilution.
Electrode fabrication
Glassy carbon electrode (GCE) was cleaned using alumina powder (0.05 micron).Then adsorbed alumina was removed by sonication using methanol, 1:1 HNO3, acetone and distilled water successively and the surface was dried in air. For electrode modification about 0.1 g of GQDTU-IIP was dispersed in 100 μL nafion solution. 3 µL of this solution was used for drop casting in the cleaned GCE surface and kept for drying in air for 2 h. The modified electrode was kept at 4 ˚C until use [34, 35].
Electrochemical detection of Hg(II) ions
The detection of Hg(II) ions was carried out using the three electrode system in which the modified GCE was used as the working electrode, Ag/AgCl as the reference electrode and Pt wire as the counter electrode. The electrode characterisation was done using Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS) in a solution of [Fe(CN)6]4−/3− (5 mM) and 0.1 M KCl. Differential Pulse Voltammetry (DPV) measurements were carried out in acetate buffer electrolyte of pH 5 from -0.4 V to 0.6 V. Pulse amplitude, width and period used were 0.05 V, 0.05 s and 0.5 s respectively [36].
Real sample analysis
For the real sample analysis, water was collected from Periyar river near industrial area, Eloor, Kochi, Kerala, India and tap water sample was collected from residential area. The samples were filtered using Whatman No.1 filter paper to remove bigger particles. The pH of the samples were then adjusted to 5. Different concentrations of Hg(II) ions were spiked into the samples and analysed using the developed sensor [37, 38].
Results and discussion
Synthesis and characterisation of GQDTU-IIP
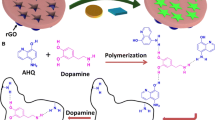
The synthesis of functionalised GQD and subsequent polymerisation to form the electrode modifier GQDTU-IIP is shown in Scheme 1. The polymer before (GQD–TUP) and after the removal (GQDTU–IIP) of the ions were digested and ICP–MS analysis was carried out to confirm the removal of the imprinted Hg(II) ions. The result obtained is given in Table 1, which shows the removal of Hg(II) ion from the polymer material. In order to understand the absorption capacity of GQDTU–IIP, 0.08 g of the polymer material was dispersed in 20 mL of 25 ppm mercury solution and stirred for about 6 h. Then the material was filtered out, washed with distilled water multiple times and dried in an oven to get GQDTU–IIP2. The amount of Hg(II) ions up taken was determined by ICP-MS analysis after the digestion of the polymer. The result obtained as given in Table 1 which shows that the material has good absorption capacity, confirming the coordination of Hg(II) ions into the imprint sites.
The modified GQD and the polymer material were characterised using FT-IR analysis. Figure 1 shows the FT-IR spectra of GQD (a), GQDTU monomer (b) and GQD-TUP polymer(c). The peaks in the range of 1723 cm−1, 1604 cm−1, 1345 cm−1, 1116 cm−1 etc. in the case of GQDs corresponds to C \(=\) O stretching, C \(=\) C stretching, O \(-\) H bending of carboxyl group and C \(-\) O stretching vibrations respectively [37, 38]. The amide C \(=\) O bond formation is confirmed by the peak observed at 1670 cm−1 in the functionalised GQD (b). In this spectrum, the peaks near 3500–3420 cm−1 is due to antisymmetric stretching vibrations of N \(-\) H bond and that between 3420–3340 cm−1 is due to the symmetric N \(-\) H stretching. C \(-\) N stretching is found near 1350 cm−1 [39, 40, 30, 41, 42]. It provides information regarding the functionalisation of GQDs. The IR spectra of polymer material confirms the EGDMA cross-linked polymer formation. The peaks at 2959 cm−1 and 2870–2840 cm−1 in the spectrum (c) corresponds to antisymmetric and symmetric stretching vibrations of CH2 bonds respectively. The peak at 1725 cm−1 is due to C \(=\) O bond [43].
SEM and TEM analysis were used to carry out surface characterisation. Figure 2a and b show the TEM images of GQDs and GQDTU monomer [44]. The mean particle size of GQD is found to be 9.5 nm and that of functionalised GQD is about 18 nm in diameter. The SEM image of the polymer material is also shown in Fig. 2c. The suspension polymerisation resulted in bead like polymer material having almost uniform size. This is used to modify the electrode surface. The FE–SEM image of modified electrode using about 3 µL of the polymer dispersion in nafion is shown in Fig. 2d. The image shows the uniform distribution of polymer beads on the electrode surface [43, 45].
The composition of the functionalised GQD was verified using CHNS analysis. The result obtained is given in Table 2 indicating the presence of N and S atoms which could be attributed to the successful functionalisation of GQDs [46].
The chemical composition of GQD and GQDTU-IIP were studied using X-ray photoelectron spectroscopy. The wide spectrum of GQD shows two prominent peaks at 284.9 eV and 531.3 eV binding energy corresponding to C1s and O1s respectively. In the polymer material (GQDTU–IIP) in addition to C1s peak (at 284.6 eV) and O1s peak (532.6 eV) two more peaks are present at 398.2 eV and 166.2 eV corresponding to N1s and S2p signals respectively (Fig. 3a and b). De-convoluted C1s spectrum of GQD and GQDTU–IIP is shown in Fig. 3c and d. For GQD, the peaks corresponding to C \(-\) C/C \(=\) C appears at 284.8 eV, that of C \(-\) O appears at 285.7 eV and C \(=\) O signals appears at 288.2 eV. In the polymer material, C \(-\) C/C \(=\) C appears at 284.6 eV, C \(-\) O at 286.2 eV and C \(=\) O at 288.5 eV binding energy. O1s signal formed at 532 eV and 533.5 eV for GQD (Fig. 3e and f). While for GQDTU-IIP it is shifted to 531 eV and 535 eV. The peak corresponding to N1s and S2p are very weak (Fig. 3g and h). This may be due to the formation of the cross linked polymer material which interrupt the signal from sulphur and nitrogen. Compared to the weight percentage of other elements in the matrix, the weight percentage of sulphur and nitrogen is very low. This also can be a reason for the weak signal in the spectrum [45,46,47,48].
Thermogravimetric curve of the material GQDTU–IIP, GQD and functionalised GQD monomer (GQDTU) are shown in Fig. 4. The initial weight loss of GQD below 100 °C is due to the elimination of moisture or solvent. In the case of GQDTU the initial weight loss is much lower than that of GQD. The weight loss above 150 °C is due to the decomposition of oxygen functional groups. In the case of GQDTU, the weight loss above 150 °C is only about 10% which can be due to the modification of oxygen functional groups. Compared to GQD, GQDTU shows much higher thermal stability. The polymer composite, GQDTU–IIP also showed thermal stability more than that of GQD. Degradation of the polymer material occurs in the temperature range 290–380 °C with maximum weight loss at ~325 °C. Thus the polymer GQDTU–IIP is found to be stable up to ~300 °C [49,50,51,52].
Electrochemical characterisation of GQDTU-IIP modified glassy carbon electrode
The electrochemical characterisation of the proposed sensor was carried out using CV and EIS measurements. In the impedance spectra the semi-circular part corresponds to the electron transfer process and the linear part corresponds to the diffusion process. The radius of semi-circular part of Nyquist plot gives the charge transfer resistance. For comparison another imprinted polymers BAIIP and GOIIP was also prepared using benzoic acid and graphene oxide respectively instead of GQD by following the same procedure. Non imprinted polymer (NIP) was prepared corresponding to GQDTU-IIP in the absence of the template ion during the synthesis. The modified electrodes using these polymers were also characterised using EIS. Figure 5 shows the Nyquist plot for GQDTU–IIP–GCE, BAIIP–GCE, GOIIP–GCE and NIP–GCE electrodes taken in a 1:1 solution of [Fe(CN)6]3−/4− (5 mM) and KCl (0.1 M) [51]. The charge transfer resistance of GQD–TUIIP was found to be lower when compared to that of GOIIP and BAIIP. For the non-imprinted polymer (NIP) also the charge transfer resistance is higher which can be due to the inhibition of electron transfer between [Fe(CN)6]3−/4− and GCE surface.
Figure 6 shows the CV plot of bare GCE, GQDTU–IIP–GCE and NIP-GCE towards Hg(II) ions [52,53,54,55]. The peak shifted towards lower potential (0.38 V to 0.32 V) with enhanced current response of about six times (3 μA to 17 μA) obtained with the GQDTU–IIP–GCE which confirms effective modification, whereas no considerable response was seen in the case of NIP–GCE. This can be due to the presence of imprinted cavities at the GQDTU–IIP modified GCE.
Sensing performance of the modified electrode
The detection of Hg(II) ions using the GQDTU-IIP modified electrode was studied using CV as well as DPV techniques. CV and DPV were taken in acetate buffer solution of pH 5. The cyclic voltammograms of the modified electrode with increasing concentration of Hg (II) ions is shown in Fig. 7a. The cyclic voltammogram shows an oxidation peak near 0.32 V in the presence of Hg(II) ions. The results show linear increase in current with increase in concentration of Hg(II) ions. In the absence of Hg2+ ions, no redox peaks were observed in the voltammogram. The corresponding calibration plot is shown in Fig. 7b. The linear response was obtained from 6 × 10–8 M to 8.5 × 10–7 M and 1.4 × 10–6 M to 8 × 10–6 M with regression equations,
and
From this the LOD was calculated using the equation,
where σ is the standard deviation of blank and S is slope of the calibration plot. Thus the detection limit obtained was 30.2 nM. There is a very small reduction peak found at the potential 0.15 V which shows that the interaction of the Hg(II) ions with the imprint sites on the electrode is quasi reversible [56,57,58].
The DPV graph of the sensor performance towards Hg(II) ions is shown in Fig. 8a. The DPV also shows a linear increase in current with increase in concentration of Hg(II) ions in the range 5 × 10–8 M to 2.3 × 10–5 M (R2 = 0.984, y = 0.0001x + 1.084). The corresponding calibration plot is shown in Fig. 8b. The LOD obtained from DPV technique is 23.5 nM. The mechanism of the sensing was studied using scan rate and the result is shown in Fig. 9a and b. The plot of peak current versus square root of scan rate shows a linear relation graph. This shows the electron transfer is diffusion controlled process. Table 3 shows comparison of recent reports on Hg(II) detection by ion imprinted polymer composite based electrode.
It is found that the modified electrode shows no much change in response after 10 days (RSD value 7.1%). The successive measurement upto three times also shows comparable results with RSD value 4.78%. This indicates that the sensor shows good stability and repeatability. Reproducibility was checked by taking the response of three different electrodes and the RSD value 2.93% shows the results are reproducible.
Optimisation studies
Buffer was chosen as the supporting electrolyte for the electrochemical analysis. Different buffers like acetate buffer, phosphate buffer, citrate buffer and HCl buffer of pH 5 were made and corresponding electrochemical response towards 4 µM concentration of Hg(II) ions were measured using DPV technique. The result shows that acetate buffer shows better current response compared to other buffers and hence it was chosen for all other studies (Fig. 10a). Then the pH of the buffer was optimised by measuring corresponding electrochemical response at different pH and was found that buffer of pH 5 exhibited highest current response and hence it was chosen for further studies (Fig. 10b). The incubation time was studied from 0 to 10 min and found that about 8 min gave highest current response and above that time the current remained same and then decreased after some more time (Fig. 10c). The effect of graphene quantum dots in the analytical performance was studied by comparing the resulting current response with imprinted polymer made using the same procedure using benzoic acid (BAIIP) and graphene oxide (GOIIP). The result shows that a well defined peak was obtained by using GQDTU-IIP compared to the other polymer material modified electrode and bare electrode. The study was done by using DPV technique (Fig. 10d).
(a) The DPV graphs of GQDTU-IIP modified electrodes towards Hg(II) ions in different buffers of pH 5 as supporting electrolytes (b) Effect of pH on peak current (c) Effect of incubation time on peak current (d) DPV response of electrodes modified using GOIIP, BAIIP, GQDTU–IIP and Bare GCE towards Hg(II) ions
Selectivity studies
The selectivity of the developed sensor was analysed in the presence of interfering ions like Pb2+, Cd2+, Cu2+, Ni2+, Mn2+, Co2+, Zn2+, As3+, Al3+ and Fe2+ using DPV. Initially the modified electrode was taken in the buffer solution and the response was measured using DPV. Then 5 µM concentration of Hg(II) was added into it and the corresponding voltammogram was recorded. Then the current response at 0.28 V towards each metal ions of concentration equivalent to that of Hg(II) was measured separately. The result is summarised in the Fig. 11. The study shows the peak current obtained by the common interfering ions relative to Hg(II) is much lower than that of Hg(II) ion in the potential range.
Real sample analysis
The modified electrode was used to study the presence of mercury ions in real water samples containing 0.2 µM Hg(II) ions using spike recovery test. The result obtained is summarised in Table 4. The study shows a satisfactory result to use the sensor towards real sample analysis.
Conclusion
Nanobeads of Graphene Quantum Dot based ion imprinted polymer (GQDTU–IIP) has been prepared through a simple synthesis procedure. GQDTU–IIP based electrochemical sensor has been successfully developed. The ion imprinted material was used for the sensing of mercury ions with a linear range of response from 5 × 10–8 M to 2.3 × 10–5 M using DPV. The sensor provided a limit of detection of 23.5 nM using DPV technique with reproducible results. The imprinting process has enhanced the selectivity of the material towards Hg(II) ions with negligible interference from other heavy metal ions. The electrochemical sensor was used for the analysis of water samples collected from river and tap. The results obtained show that the sensor can be reliably used for the sensing of Hg(II) ions in real samples.
References
Gumpu MB, Sethuraman S, Krishnan UM, Rayappan JBB (2015) A review on detection of heavy metal ions in water - An electrochemical approach. Sens Actuator B Chem 213:515–533
Bansod B, Kumar T, Thakur R, Rana S, Singh I (2017) A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron 94:443–455
Li M, Chen T, Gooding JJ, Liu J (2019) Review of carbon and graphene quantum dots for sensing. ACS Sens 4:1732–1748
Lu Y, Liang X, Niyungeko C, Zhou J, Xu J (2018) A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 178:324–338
Mohamed MG, Kuo SW (2018) Functional silica and carbon nanocomposites based on polybenzoxazines. Macromol Chem Phys 1800306. https://doi.org/10.1002/macp.201800306
Samy MM, Mohamed MG, Kuo SW (2020) Pyrene functionalized tetraphenylethylene polybenzoxazine for dispersing single-walled carbon nanotubes and energy storage. Compos Sci Technol 199: 108360
Geim AK (2009) GRAPHENE: STATUS AND PROSPECTS. Science 324(5934):1530–1534. https://doi.org/10.1126/science.1158877
Chang J, Zhou G, Christensen ER, Heideman R, Chen J (2014) Graphene-based sensors for detection of heavy metals in water: a review. Anal Bioanal Chem 406:3957–3975. https://doi.org/10.1007/s00216-014-7804-x
Li M, Chen T, Gooding JJ, Liu J (2019) Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens 4:1732–1748
Tian P, Tang L, Teng KS, Lau SP (2018) Graphene quantum dots from chemistry to applications. Mater Today Chem 10:221–258
Ambrosi A, Chua CK, Bonanni A, Pumera M (2014) Electrochemistry of graphene and gelated materials. Chem Rev 114:7150–7188. https://doi.org/10.1021/cr500023c
Jianguang F, Hongzhou D, Liyan Y, Lifeng D (2017) Optical and electronic properties of graphene quantum dots with oxygen-containing groups: a density functional theory study. J Mater Chem C 5:5984–5993. https://doi.org/10.1039/C7TC00631D
Quintana M, Spyrou K, Grzelczak M, Browne WR, Rudolf P, Prato M (2010) Functionalization of graphene. ACS Nano 4:3527–3533
Wang N, Lin M, Dai H, Ma H (2016) Functionalized gold nanoparticles / reduced graphene oxide nanocomposites for ultrasensitive electrochemical sensing of mercury ions based on thymine – mercury – thymine structure. Biosens Bioelectron 79:320–32
Chem JM, Liu J, Gooding JJ (2012) Strategies for chemical modification of graphene and applications of chemically modified graphene. J Mater Chem 22:12435–12452. https://doi.org/10.1039/c2jm31218b
Wang Y, Li S , Yang H and Luo J (2020) Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv 10:15328-15345
Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp K, Hobza P, Zboril R, Kim KS (2012) Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev 112:6156–6214
Liu Y, Liua Y, Zhoua J, Zhang X, Liub Z, Wana X, Tianb J, Wanga T, Chena Y (2009) Synthesis, characterization and optical limiting property of covalently oligothiophene-functionalized graphene material. Carbon N Y 47:3113–3121
Sebastian D, Soman S, Kala R (2021) A nanohybrid system based on covalently functionalized Graphene Quantum dots with Dithienopyrrole derivative for the sensitive and selective fluorometric detection of Pb (II) ions. Lumin Accepted Author Manuscript. https://doi.org/10.1002/bio.4116
Horacio S, Ana M, Díez-P EL, Soledad V, Marián A, Gomez-Fatou MA (2014) Chemical sensors based on polymer composites with carbon nanotubes and graphene. the role of the polymer. J Mater Chem A 2:14289–14328. https://doi.org/10.1039/C4TA02159B
Gui R (2019) Preparation and applications of electrochemical chemosensors based on carbon-nanomaterial- modified molecularly imprinted polymers. Nanoscale Adv 1:3325–3363. https://doi.org/10.1039/c9na00455f
Prasad BB, Kumar A, Singh R (2017) Synthesis of novel monomeric graphene quantum dots and corresponding nanocomposite with molecularly imprinted polymer for electrochemical detection of an anticancerous ifosfamide drug. Biosens Bioelectron 94:1–9
Shakerian F, Kim KH, Kwon E, Szulejko JE, Kumar P, Dadfarnia S, Shabani AMH (2016) Trends in analytical chemistry advanced polymeric materials: synthesis and analytical application of ion imprinted polymers as selective sorbents for solid phase extraction of metal ions. Trends Anal Chem 83:55–69
Rao TP, Kala R, Daniel S (2006) Metal ion-imprinted polymers – novel materials for selective recognition of inorganics. Anal Chim Acta 578:105–116
Alizadeh T, Hamidi N, Ganjali MR, Rafiei F (2018) Determination of subnanomolar levels of mercury (II) by using a graphite paste electrode modified with MWCNTs and Hg(II) -imprinted polymer nanoparticles. Microchim Acta 185:16. https://doi.org/10.1007/s00604-017-2534-3
Motlagh MG, Taher MA, Heydari A, Motlagh RG, Vinod KG (2016) A novel voltammetric sensor for sensitive detection of mercury (II) ions using glassy carbon electrode modified with graphene-based ion imprinted polymer. Mater Sci Eng C 63:367–375
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Suprabhat S, Dayakar G, Yeduru V, Prakriti RB, Sutapa G, Yang Y, Sunil M (2016) Graphene quantum dots from graphite by liquid exfoliation showing excitation-independent emission, fluorescence upconversion and delayed fluorescence. Phys Chem Chem Phys 18:21278–21287. https://doi.org/10.1039/C6CP01528J
Qian Z, Ma J, Shan X, Shao L, Zhou J (2013) Surface functionalization of graphene quantum dots with small organic molecules from photoluminescence modulation to bioimaging applications: an experimental and theoretical investigation. RSC Adv 3:14571–14579
Reddy NSG, Rao KM, Rao KSVK, Ha CS (2016) Synthesis of 1-acryloyl-3-phenyl thiourea based pH sensitive hydrogels for removal of samarium and terbium. Macromol Res 24:494–501
Daniel S, Prabhakara RP, Prasada RT (2005) Investigation of different polymerization methods on the analytical performance of palladium (II) ion imprinted polymer materials. Anal Chim Acta 536:197–206
Metilda P, Prasad K, Kala R, Gladis JM, Prasada Rao T, Naidu GRK (2007) Ion imprinted polymer-based sensor for monitoring toxic uranium in environmental samples. Anal Chim Acta 582:147–153
Naguib HF, Al Sagheer FA, Ali AAM, Elsabee MZ (1996) Polymerization behaviour of a new vinyl thiourea derivative. Eur Polym J 32:985–991
Elgrishi N, Rountree KJ, McCarthy BD, Rountree ES, Eisenhart TT, Dempsey JL (2018) A practical beginner’s guide to cyclic voltammetry. J Chem Educ 95:197–206
Hashemzadeh N, Hasanzadeh M, Shadjou N (2016) Graphene quantum dot modified glassy carbon electrode for the determination of doxorubicin hydrochloride in human plasma. J Pharm Anal 6:235–241
Liu Y, She P, Gong J, Wu W, Xu S, Li J, Zhao K, Deng A (2015) A novel sensor based on electrodeposited Au-Pt bimetallic nano-clusters decorated on graphene oxide (GO)-electrochemically reduced GO for sensitive detection of dopamine and uric acid. Sens Actuator B Chem 221:1542–1553
Liu Y, Deng Y, Li T, Chen Z, Chen H, Li S, Liu H (2018) Aptamer-based electrochemical biosensor for mercury ions detection using AuNPs-modified glass carbon electrode. J Biomed Nanotechnol 14:2156–2161
Lu Z, Lin X, Zhang J, Dai W, Liu B, Mo G, Ye J, Ye J (2019) Ionic liquid/poly-L-cysteine composite deposited on flexible and hierarchical porous laser-engraved graphene electrode for high-performance electrochemical analysis of lead ion. Electrochim Acta 295:514–523
Du FP, Cao NN, Zhang YF (2018) PEDOT: PSS/graphene quantum dots films with enhanced thermoelectric properties via strong interfacial interaction and phase separation. Sci Rep 8:6441. https://doi.org/10.1038/s41598-018-24632-4
Jovanović SP, Syrgiannis Z, Budimir MD, Milivojević DD, Jovanovic DJ, Pavlović VB, Papan JM, Bartenwerfer M, Mojsin MM, Stevanović MJ, Marković BMT (2020) Graphene quantum dots as singlet oxygen producer or radical quencher - The matter of functionalization with urea/thiourea. Mater Sci Eng Mater Biol Appl 110539 https://doi.org/10.1016/j.msec.2019.110539
Hande P, Samui AB, Kulkarni PS (2017) Selective nanomolar detection of mercury using coumarin based fluorescent Hg (II)–Ion imprinted polymer. Sens Actuator B Chem 246:597–605. https://doi.org/10.1016/j.snb.2017.02.125
Arslan H, Külcü N, Flörke U (2003) Synthesis and characterization of copper (II), nickel (II) and cobalt (II) complexes with novel thiourea derivatives. Transit Met Chem 28:816–819. https://doi.org/10.1023/A:1026064232260
Ardalani M, Shamsipur M, Besharati-seidani A (2020) A new generation ofhighly sensitive potentiometric sensors based on ion imprinted polymeric nanoparticles/multiwall carbon nanotubes/polyaniline/graphite electrode for sub-nanomolar detection of lead (II) ions. J Electroanal Chem 879:114788. https://doi.org/10.1016/j.jelechem.2020.114788
Liqing S, Jingjing S, Jun L, Chao L (2015) Structure observation of graphene quantum dots by single-layered formation in layered confinement space. Chem Sci 6:4846. https://doi.org/10.1039/c5sc01416f
Li L, Liu D, Shi A, You T (2018) Simultaneous stripping determination of cadmium and lead ions based on the N-doped carbon quantum dots-graphene oxide hybrid. Sens Actuator B Chem 255:1762–1770
Estrada-Aldrete J, Hernández-López JM, García-León AM, Peralta-Hernández JM, Cerino-Córdova FJ (2020) Electroanalytical determination of heavy metals in aqueous solutions by using a carbon paste electrode modified with spent coffee grounds. J Electroanal Chem 57:113663. https://doi.org/10.1016/j.jelechem.2019.113663
Khosrokhavar R, Motaharian A, Reza M, Hosseini M (2020) Screen-printed carbon electrode (SPCE) modified by molecularly imprinted polymer (MIP) nanoparticles and graphene nanosheets for determination of sertraline antidepressant drug. Microchem J 159:105348
Yang Y, Xie Y, Pang L, Li M, Song X, Wen J, Zhao H (2013) Preparation of reduced graphene oxide / poly (acrylamide) nanocomposite and its adsorption of pb (II) and methylene blue. Langmuir 29:10727–10736. https://doi.org/10.1021/la401940z
Wen G, Zhao W, Chen X, Liu J, Wang Y, Zhang Y, Huang Z, Wu Y (2018) N-doped reduced graphene oxide /MnO2 nanocomposite for electrochemical detection of Hg2+ by square wave stripping voltammetry. Electrochim Acta 291:95–102. https://doi.org/10.1016/j.electacta.2018.08.121
Li L, Yaru Q, Yun F, Yusheng L, Keyan W, Liande Z (2020) Stripping voltammetric analysis of mercury ions at nitrogen-doped reduced graphene oxide modified electrode. J Electroanal Chem 865:114121. https://doi.org/10.1016/j.jelechem.2020.114121
Junling Z, Zengwei Z, Junwei D, Yumei L, Weifeng L, Yifeng T (2015) A sensitive sensor for trace Hg2+ determination based on ultrathin g-C3N4 modified glassy carbon electrode. Electrochim Acta 186:192–200. https://doi.org/10.1016/j.electacta.2015.10.173
Liu Y, Zhou J, Zhang X, Liu Z, Wan X, Tian J, Wang T, Chen Y (2009) Synthesis, characterization and optical limiting property of covalently oligothiophene-functionalized graphene material. Carbon 47:3113–3121. https://doi.org/10.1016/j.carbon.2009.07.027
Shoujun Z, Junhu Z, Chunyan Q, Shijia T, Yunfeng L, Wenjing Y, Bo L, Lu T, Fang L, Rui H, Hainan G, Haotong W, Hao Z, Hongchen S, Bai Y (2011) Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem Commun 47:6858–6860
Sapari S, Hidayah N, Razak A, Aishah S, Yook L (2020) A regenerable screen-printed voltammetric Hg (II) ion sensor based on tris-thiourea organic chelating ligand grafted graphene nanomaterial. J Electroanal Chem 878:114670
Wang Z, Zhang D, Xiao X, Su C, Li Z, Xue J, Hu N, Peng P, Liao L, Wang H (2020) A highly sensitive and selective sensor for trace uranyl (VI) ion based on a graphene-coated carbon paste electrode modified with ion imprinted polymer. Microchem J 155:104767
Anand SK, Rachel M, Radecki J, Radecka H, Kumar KG (2020) Individual and simultaneous voltammetric sensing of norepinephrine and tyramine based on poly (L-arginine)/ reduced graphene oxide composite fi lm modified glassy carbon electrode. J Electroanal Chem 878:114531
Mohammad RG, Ali RR, Reza S, Abbas F, Mohammad K (2019) A highly sensitive and selective electrochemical mercury (II) sensor based on nanoparticles of Hg (II)-imprinted polymer and graphitic carbon nitride (g-C3N4). Int J Electrochem Sci 14:6420–6430. https://doi.org/10.20964/2019.07.80
Azam B, Abbas BS, Abdolkarim A, Mojtaba S (2015) A highly selective voltammetric sensor for nanomolar detection of mercury ions using a carbon ionic liquid paste electrode impregnated with novel ion imprinted polymeric nanobeads. Mater Sci Eng C Mater Biol Appl 48:205–212. https://doi.org/10.1016/j.msec.2014.12.005
Acknowledgements
This work was supported by Cochin University of Science and Technology (CUSAT). We thank DST-SERB for financial support (EMR/2016/005601) STIC, IUCND, PS&RT, CUSAT and NIIST, Thiruvananthapuram for analytical facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soman, S., P.V, A. & R, K. Covalently modified graphene quantum dot using a thiourea based imprinted polymer for the selective electrochemical sensing of Hg(II) ions. J Polym Res 28, 359 (2021). https://doi.org/10.1007/s10965-021-02716-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02716-6