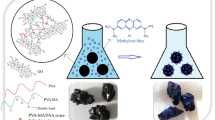
Abstract
In this work, pH-sensitive hydrogel nanocomposites based on the acrylic acid (AA), graphene oxide (GO) and monoacryloyl tetraammonium thiacalix[4]arene (MATCA) was conveniently prepared via heterogenious copolymerization using methylene bisacrylamide (MBA) and azobisisobutyronitrile (AIBN) as crosslinker and initiator, respectively. The prepared hydrogel nanocomposites showed excellent capability for water absorption up to 67.5 g/g. The pH-sensitive nanocomposite (MATCA-co-AA@GO) with tunable properties was utilized for removing dye pollutant such as malachite green (MG) and methylene blue (MB) dyes from wastewater. The dye removal efficiency up to 95% was achieved at optimum pH of 6.5. FTIR, 1H and 13C NMR spectroscopies, CHNSO elemental analysis, mass spectroscopy, TGA, XRD, CHNSO, DMTA and FESEM were used to characterize and study the synthesized materials. In isotherm studies of adsorption, the Freundlich isotherm provided a better fit to the data of MG adsorption. However, in the case of MB, the results are compatible to langmiur isotherm. Results obtained through the kinetic investigations showed that the adsorption of MG and MB on to MATCA-co-AA@GO nanocomposite followed a pseudo-second-order kinetic model. The maximum adsorption capacity (Qm) was 67.5 and 70.3 mg g−1 for MG and MB respectively. Besides, from the thermodynamic data, the adsorption process was suggested to be chemisorption, spontaneous, and endothermic. Reusability of the prepared sorbent was examined by consecutive adsorption/desorption experiments. The prepared adsorbent is recyclable and represents a good candidate for extremely effective sequential removal of MG and MB dyes from wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, most of industries use dyes and their sewages enter to the soil and under ground water which cause environmental hazards [1]. Two kinds of the risky dyes are malachite green (MG) and methylene blue (MB) cationic dyes, which are widely utilized in aquaculture, food, paint, papers and textile industries. But, toxicity effect of these two dyes is a serious problem. It has been reported that MG can effect on the various organs such as liver, kidney, intestine, eyes, lungs and bones. Also, it can causes several problems, including carcinogenic, mutagenic, teratogenic and respiratory toxicity after entering the food chain [2, 3]. Methylene blue has also been reported to have adverse effects such as eye damage, difficulty breathing, nausea, excessive sweating, mental confusion, and methemoglobinemia [4]. It is, therefore, timely to explore an efficient way to remove such noxious dyes from the environment. There are several separation methods to remove dyes from effluent such as filtration, precipitation, oxidation and coagulation [5]. However, these methods are neither economical nor sufficiently effective [6]. Adsorption is the best way to remove dye owing to its low cost, fastness, high performance, easy operation and recovery [7]. So far, various type of adsorbents have been introduced in the literatures, such as activated carbon derived from Borassus aethiopum flower biomass [5], neem sawdust [8], Supermagnetic/Thiacalix[4] arene tetrasulfonate self-doped/polyaniline nanocomposite [9], magnetic nanocomposite based on functionalized cross-linked poly(methylacrylate) [10], cellulose grafted with 2-acrylamido-2-methylpropane sulfonic acid and binary comonomer acrylonitrile [11], chemically modified rice husk [12], and carbon prepared from Arundo donax root [13].
In addition, the three-dimensional polymeric hydrogels having reactive functional groups and porosity depicted high water absorption capability [14]. Among them, stimuli responsive materials such as thermo and/or pH sensitive hydrogels as intelligent soft materials are hopeful, efficient and effective in many uses which have been widely investigated [15]. Hydrogels with suitable functional groups and host-gust capability have a good potential to adsorb cationic dyes [16]. So, various type of hydrogels have been reported as supper adsorbent for separation of MG from aqueous solutions [17]. In adsorption, macrocyclic compounds with host–guest chemistry play an important role as the receptor of dye molecules [18]. Thiacalixarenes are a type of stable macrocyclic compounds composed of phenolic rings bonded together with sulfur bridges, in which the cavity size, π-π staking, hydrogen bonds and hydrophilic-lipophilic interactions are substantial parameters in the removal of dye molecules [19]. A few attempts have been made to insert calixarenes and thiacalixarenes into polymer structures for convenient separation of pollutants from aqueous medium [17, 20].
In recent years, chemically modified GO, as a two-dimensional nanomaterial has been applied in polymer composites due to highly accessible surface area and enhanced active nanosize [21,22,23]. These polymeric composites are of interest owing to their unique properties. Recently, the non-covalent in situ polymerization technique has been used to produce some new GO-based polymeric composites that have been employed as adsorbent [24, 25].
In this paper, in continuation of our ongoing works on polymeric nanocomposites, the pH sensitive GO based hydrogel nanocomposite of hydrophilic tetraammonium thiacalixarene monomer has been designed to provide a suitable platform for adsorption of dye from aqueous medium. In order to achieve this purpose, MATCA as a new hydrophilic monomer with hust-gust property was copolymerized by AA in the presence of GO nanoparticles. The heterogeneous copolymerization was initiated by AIBN and cross-linked with MBA to produce a pH sensitive hydrogel nanocomposite (MATCA-co-AA@GO). The obtained hydrogel nanocomposite was investigated for the structure, morphology, thermal properties, swelling and gel content. The dye removal efficiency of synthetized adsorbent together with the adsorption kinetics, isotherms, thermodynamics, and the parameters possibly affecting the adsorption procedure were studied in details for MG and MB as a model of most common pollutants in textile wastewater. Also, desorption and recoverability of the prepared sorbent were explored.
Experimental
Materials
p-Tert-butyl phenol, AIBN, MBA, Phenol, Aluminium trichloride (AlCl3), Acryloyl chloride (AC) and Tin (Sn) were obtained from Merck Chem. Co. (Darmstasdt, Germany). MG and MB utilized in this research was prepared from the commercial manufacturing company Dy Star Co. (Germany) at 90% purity. AA (98%, Merck) was distilled under reduced pressure prior use. Dichloromethane (DCM) and toluene were dried with P2O5 and sodium respectively. All other solvents and reagents were prepared from Aldrich and Merck companies and utilized with no further purification, unless otherwise stated.
The applied p-tert-butyl thiacalix[4]arene (TTCA) was prepared based on the previously reported method [26]. ES-MS: m/z calcd M = 720, [M]+ = 720.4.
GO has been synthetized from Hummer’s method, the most common method used for preparing GO, by the treatment of graphite with strong mineral acids and oxidizing agents [27].
Synthesis of thiacalix[4]arene (TCA)
The following modified process was applied to synthesize of TCA. Firstly, TTCA (3.0 g, 4.17 mmol) was dissolved in toluene (90 ml) under heating. The reaction mixture was cooled down to room temperature and then phenol (4.0 g, 42.25 mmol) was poured into it. In the next step, AlCl3 (20.0 g, 150.0 mmol) was added during 3 min and the mixture was refluxed for 5 h. After cooling down the mixture, it was poured into 2 N HCl (250 ml) and stirred for 24 h. The sediment was separated via filteration, rinsed using chloroform (3 × 50 ml) and acetone (2 × 50 ml). The filtered product was dried under vacuum (0.01 mmHg) at 220 °C until sublimation of the volatile aluminum salts was complete. The gained product (1.80 g, 87%) was obtained as a beige crystalline [28]. Mp 300–305 °C. ES-MS: m/z calcd M = 496, [M]+ = 496.1.
Synthesis of the tetranitrothiacalix[4]arene (NTCA)
TCA (1.25 g, 0.0025 mol) was dissolved in CHCl3 (200 mL). Glacial acetic acid (7 mL) and aqueous solution of HNO3 (65% ~ 68%, 5 mL) were poured into the mixture while it was stirred at ambient temperature. In the following 30 h, the sediment was separated from the reaction mixture through filtration and rinsed several times using water to afford purified product (1.31 g, 76%) as an orange solid [29]. Mp > 350 °C. ES-MS (positive mode): m/z calcd M = 676.58, [M + H]+ = 677.9.
Synthesis of tetraamimonium thiacalix[4]arene (ATCA)
NTCA (0.5 g, 0.7 mmol) was poured into concentrated HCl (20 mL). Then, tin (2 g, 17 mmol) was introduced into this suspension. The reaction mixture was heated for 1 h and the white suspension was cooled down by placing it in an ice bath. The precipitate was filtered and rinsed with HCl, acetone and diethyl ether to produce pure product in 0.47 g, (95% yield) [30]. ES-MS (positive mode): m/z calcd M = 702, [M + H]+ = 702.8.
Synthesis of MATCA
The reaction was performed in a 250 mL three necked flask equipped with a magnetic stirrer and thermometer to which 50 mL dry DCM and ATCA (1 mmol) were added. Degassing the mixture was performed using three freeze–pump–thaw cycles. Then, it was heated to temperature of 60 °C for 30 min while being stirred. The reaction mixture was cooled down to ambient temperature and followed to 0–5 °C. Thereafter, the AC (1 mmol) was added dropwise over 60 min to the cooled mixture while the temperature was kept about 0–5 °C over the addition. Moreover, the mixture was stirred for 24 h and MATCA was centrifuged and washed using acetone for twice. The yield of reaction was 87%. ES-MS (positive mode): m/z calcd M = 756, [M-H]− = 727.8, this fragmentation can be attributed to degradation of vinyl group from MATCA.
Synthesis of hydrogel nanocomposite
The reaction was applied in a 250 mL four-necked flask equipped with a mechanical stirrer, a reflux condenser, a thermometer, and a nitrogen line. In a typical procedure, GO (0.005 g) was dispersed in 10 mL distilled water in an ultrasound bath. The AA (1 mL) and MBA (0.05 g) were added to a suspension of GO and MATCA (0.005 g) in 10 mL of acetone. The mixture was stirred and purged with N2 for 1 h to eliminate the dissolved oxygen. The mixture was heated to 70 °C, and AIBN (0.014 g) was consequently poured into the solution to start the polymerization and crosslinking reactions. After gelation was completed, the achieved product was washed with acetone and grounded into small pieces, then placed in a vacuum oven to be dried (80 °C, 9 h).
In the case of other hydrogel composites, all the synthetic steps mentioned above were repeated and the GO and MATCA contents are summarized in Table 1.
Characterization
For evaluation of thermal behavior, a TGA (thermogravimetric analysis) Polymer Laboratories England instrument at a heating rate of 10 °C min−1 under a nitrogen atmosphere was performed. Infrared (IR) spectra were carried out by using a Bruker Tensor 27 spectrometer (Bruker, Karlsrohe, Germany). X-ray powder diffraction (XRD) data were recorded on an XD-3A diffractometer through Cu Kα radiation (XRD D8-Advance, Bruker). The surface morphology of prepared hydrogel and GO were analyzed using a Field Emission Scanning Electron Microscopy (FESEM) with a Vega-II from TESCAN Co. 1H and 13C NMR spectra were obtained with a 400 MHz Bruker Avance DRX spectrometer (Bruker, Karlsruhe, Germany). For evaluation of the structure of TTCA, TCA, NTCA, MATCA and ATCA, elemental analysis was conducted using VarioEL III Elementar CHNSO analyzer (Germany). Mass spectra were collected on a 70 eV instrument (Agilent USA 5975C). Dynamic mechanical thermal analysis (DMTA) was performed using a Netzsch Dynamic Mechanical Analyzer model 242C in which the dried samples were analyzed in the temperature range of 25–160 ℃, with a heating rate of 5 ℃ min−1 and at frequency of 1 Hz. The amounts of the MG and MB in solution were estimated by using a UV–visible spectrophotometer (T90 + PG instrument) at wavelength of 618 and 668 nm respectively.
For evaluation of the water absorbency of the synthetized hydrogels, 0.10 g of the gel sample was put into a tea bag, submerged in 30 mL of water, and permitted to swell for 5 h for reaching equilibrium swelling. The weight of swollen gels was then measured, and the swelling capacity (g/g) was computed as the ratio of swollen weight to dried gel weight. Finally, the swelling ratio of the samples was calculated according to Eq. 1.
where W refers to the weight of samples after soaking of water and W0 refers to the primary weight of dried sample.
The gel content of the specimens was measured via gravimetric method by immersing 0.20 g of each sample into 250 mL distilled water for 72 h. Then, hydrated gel was filtrated, dried and weighted. Finally, the gel content was obtained according to Eq. 2.
where W refers to the samples weight following extraction and W0 refers to primary weight of dried sample.
Determination of dye removal capacity
The molecular structure corresponding to the MG and MB were demonstrated in the Scheme 1. In order to prepare the MG and MB stock solution (1000 mg L−1), exact amount of the dye was dissolved in distilled water. Different concentrations of working solutions were prepared by dilution of the stock solution with distilled water All batch adsorption tests were conducted by shaking samples on a shaker at a speed of 130 rpm. The influence of hydrogel sorbent quantity on removal of MG and MB dyes were assessed in 50 mL of dye solution (20 mg L−1, pH 6.5) using various amounts of adsorbent at temperature of 25 °C for 90 and 120 min respectively. For studying effect of primary pH on removal of MG and MB, the pH of the dye solutions ranged from 3.5 to 11.5 with 0.05 g of sorbent at temperature of 25 °C for 90 and 120 min was evaluated respectively.
The impact of temperature on sorption of MG and MB at 298, 308, 318 and 328 K was investigated in 50 mL of dye solution (40 mg L−1, pH 6.5) with 0.05 g of sorbent for 90 and 120 min respectively. It should be noted that, the pHs were set via adding 0.1 N HCl and 0.1 N NaOH solutions and measured by means of a pH-meter. To investigation of kinetic study, the impact of contact time on removal of MG and MB was performed. For this purpose, 50 mL of dye solution (20 mg L−1, pH 6.5) containing 0.05 g of sorbent was shaken continuously at temperatute of 25 °C for predetermined time intervals. In this work, to study removal capacity of dye using the hydrogel sorbent, 0.05 g of MATCA-co-AA@GO hydrogel composite (IV) was immersed in to 50 ml of the MG and MB dye solution (20- 80 mgL−1) samples at pH 6.5 for 90 and 120 min respectively.
The dye and sorbent samples were filtered and amounts of residual dye were determined by means of the UV–visible spectrophotometer (T90 + , PG Instruments) at maximum corresponding wavelength (618 and 668 nm for MG and MB respectively) using Beer-Lambert law (Eq. 3):
where A refers to the absorption of dye at a definite wavelength, ε is molar absorptivity, b is the length that the light travels through the solution (1 cm) and C represents the amount of dye in the solution. All tests were carried out in triplicates, the mean values were reported and the error bars were presented in the charts. At equilibrium point, the equilibrium adsorption capacity (Qe) was computed using Eq. 4.
where Qe refers to the concentration of dye adsorbed by the hydrogel (mg g−1), Ci is the primary amount of dye put in contact with the hydrogel (mg L−1), Ce is the amount of dye (mg L−1) following the adsorption process, W represents the hydrogel mass (g) and V refers to the volume corresponding the dye solution (L).
The concentration of the dye removal was measured using the difference between the primary and residual amount of dye solution Eq. 5.
Desorption experiments
Consecutive adsorption/desorption tests were conducted to evaluate the reusability of sorbent. Firstly, 50 mg of the prepared adsorbent was saturated with dye for 2 h at ambient temperature. Next, the sorbent was isolated from the solution, rinsed using distilled water, and consequently immersed in desorption media (5% acetic acid + methanol). The adsorption–desorption runs were performed five cycles. The amount of dye in the filtrate was measured by UV–vis spectrophotometer in each adsorption cycle.
Results and discussion
Preparation of the adsorbent
The practical procedure for the synthesis of monoacryloyltetraammonium thiacalix[4]arene-co-acrylic acid@Go hydrogel composite adsorbent (MATCA-co-AA@GO) includes following steps, which are schematically shown in Scheme 2 (a, b). (i) The TTCA was synthesized based on the kumagai et al. procedure. In this method, a combination of p-tert-butylphenol, elemental sulfur S8, and NaOH in tetraethylene glycol dimethyl ether was stirred under nitrogen and heated gradually to 230 °C. (ii) It is necessary to remove tert-butyl groups from para position to obtain upper rim functionalized TCA. De-tert-butylation corresponding to the parent TTCA has done with AlCl3, possessing hydrogen atoms in the para-positions of phenols. (iii) then, tetra nitro derivative of TCA was synthesized. For nitration of TCA, the reaction was conducted with mixture of glacial acetic acid and HNO3 (65% ~ 68%) to prevent oxidation of the bridging sulfur atoms. Finally, an orange solid separated out after 30 h in 85% yield. (iv) To obtain tetraammonium derivative of TCA, one of the most efficient methods is the reduction of NTCA with metallic Sn in acidic pH. Reaction of tin with HCl produces SnCl2 which act as reducing agent. The tetraammonium hydrochloric salt (ATCA) was achaived as a white powder. The yield of reaction was attractively good (95%). v) For synthesis of MATCA, AC was poured into the mixture at 0–5 °C, and the esterification completed after several hours. vi) The (MATCA-co-AA@GO) hydrogel nanocomposite was prepared from MATCA as a dye encapsulating molecule, AA as a co-monomer, MBA as a crosslinker, and AIBN radical initiator in the presence of GO as a hydrophilic carbon nanoadsorbent. GO was synthesized through Hummer’s method, using mixture of KMnO4, NaNO3, and H2SO4. The analyses confirmed that the GO sheets was successfully prepared. Hydrogel of MATCA with AA demonstrated noticeable weakness and brittleness. Therefore, GO was poured into the reaction mixture via non-covalent in situ free-radical polymerization in aqueous medium for modification of mechanical and thermal features. This technique involve mixing of GO aqueous dispersion, MATCA and acrylic acid as monomers followed by copolymerization in the existence of AIBN (initiator) and MBA (cross linker). As was expected, when GO added into the reaction, the resulting hydrogel composite became very tough and showed fairly good strength [31] Due to many oxygen-comprising hydrophilic functional groups on the GO surface, such as carboxyl, hydroxyl and epoxy groups, it cause uniform dispersion in water by forming exfoliated monolayer sheets to form a homogeneous hydrogel composite.
Spectroscopic studies
Characterization of the prepared materials was performed using FT-IR and 1H NMR spectra. FT-IR spectra of TTCA, TCA, NTCA, ATCA, MATCA, MATCA-co-AA@GO hydrogel nanocomposite and GO are presented in Fig. 1(a-g), respectively. The FT-IR spectrum corresponding to the synthesized TTCA (Fig. 1a) displays absorption peaks at 3322 and 2961 cm−1, which are assigned to O–H and C-H stretching vibrations of phenolic and tert-butyl groups respectively. In the spectrum corresponding to TCA in the Fig. 1b, the absorption peak of aliphatic C-H vibration at 2961 cm−1 has disappeared, representing that the p-tert-buyl was completely detached. Peaks appeared at 1521 and 1344 cm−1 are ascribed to NO2 vibrations in Fig. 1c. A typical band assigned to C-N stretching is seen at around 1238 cm−1. The infrared spectra, Fig. 1d, demonstrated obviously the replacement of the NO2 vibration peaks (697 cm-1) by NH3 + Cl- ones (3057, 2829, and 2562 cm-1) [29]. In the spectrum of MATCA, Fig. 1e, a band at 1707 cm−1 is assigned to the carbonyl group of ester which indicates that esterification had been successfully occurred. The peaks at 2915, 1566, 1228 and 3361 cm−1 are ascribed to C-H, C = C, C-O and Ar–O-H vibrations, respectively. The bands at 3130, 2829, 2551 cm−1 are due to the stretching vibrational modes of NH3+Cl−. For the MATCA-co-AA@GO hydrogel composite, as seen in Fig. 1f, the peaks at 3440, 1711, 1230 and 2918 cm−1 are related to O–H vibration, C = O vibration, and C-H vibration respectively. The C = C typical peak at 1566 cm−1 has disappeared, showing that the polymerization was completely accomplished. In the spectrum corresponding to GO (Fig. 1g), several characteristic vibrational peaks including: O–H (3428 cm−1), C = O (1710 cm−1), C = C (1629 cm−1), C–OH (1229 cm−1) and C–O–C (1021 cm−1) are observed.
The 1H and 13C NMR technique was employed for further characterization of the chemical structure of TTCA (Figs. S1.1, S2.1), TCA (Figs. S1.2, S2.2), NTCA (Figs. S1.3, S2.3), ATCA (Figs. S1.4, S2.4) and MATCA (Figs. S1.5, S2.5). The 1H NMR of TTCA and TCA was performed in CDCl3 as solvent. The 1H NMR spectrum of TTCA shows three resonance signals at 1.24 (singlet), 7.6 (singlet), and 9.62 (singlet) ppm. These peaks are ascribed to the methyl.
groups of p-tert-butyl, 8Ha of aromatic ring, and 4 H of OH phenolic. In the 13C NMR of TTCA, in addition to the aromatic ring signals (120.54, 136.400, 144.69, and 155.62 ppm), two signals appear at 31.24 and 34.210 ppm which assigned to aliphatic carbons of methyl groups [26]. In the recorded 1H spectrum of TCA, the signals at 6.7 (triplet), 7.6 (doublet), and 9.47.
(singlet) ppm are related to 4 H of aromatic ring, 8 H of aromatic ring, and 4 H of OH phenolic. Also, the signal at 1.24 ppm correspond to p-tert-butyl groups is disappeared. Moreover, the signals at 120.91, 121.69, 128.33, 139.25, and 157.926 ppm has been seen in the relevant 13C NMR spectrum, which correspond to aromatic carbon of phenolic ring [28]. This evidence support the synthesis of TCA and p-tert-butyl groups was completely detached. The 1H NMR spectrum of NTCA (DMSO-d6 as solvent) represented the signal at 8.43 (singlet) ppm, which are ascribed to 8 Ha of H-aromatic ring [29]. Also, the 13C NMR spectrum of NTCA showed four signals at 167.99 (ArC-OH), 137.80 (ArC-NO2), 132.60 (Ar–CH), and 122.18 (ArC-S-Ar) [30]. The 1H NMR peaks of ATCA (DMSO-d6 as solvent) appear at 4.08 (singlet) and 7.47 (singlet) ppm which are related to 12 H of NH3+ and 8 Ha of H-aromatic ring. Furthermore, the 13C NMR spectrum of ATCA showed several signals at 161.851 (ArC-OH), 130.99 (Ar–CH), 125.05 (Ar–CH), 122.41 (ArC-NH3+), 121.95 (ArC-S-Ar), and 120.99 (Ar–CH) [30]. For MATCA, 1H NMR signals were observed at 5.7 (doublet), 5.9 (doublet), 6.1 (triplet), 7.54 (singlet), 7.56 (singlet) and 7.6 (singlet) ppm that are related to 1 H of CH2 = C, 1 H of CH2 = C 1 H of CH2 = CH, 2 H of ArH, 4 H of ArH and 2 H of ArH. The 13C NMR spectrum signals of MATCA are 157.43 (= C-H, ArC-OH), 131.33 (H2C = C, Ar–CH), 122.24 (ArC-NH3+), and 122.02 (ArC-S-Ar) [17].
Also, the structures of TTCA, TCA, NTCA, ATCA, and MATCA were evaluated by CHNSO elemental analysis which the results are given in the Table S1. The obtained data were in good agreement with theoretical data and confirmed the structures of the synthesized materials.
Material study
TGA
The thermograms of MATCA-co-AA@GO (sample IV), MATCA-co-AA (sample II), and poly acrylic acid (PAA, sample I) are presented in Fig. 2a. In this experiment, the samples are heated up to 600 °C under nitrogen atmosphere at a heating rate of 10 °C/min and changes in mass loss was recorded. The details of the thermal decomposition characteristic of the three synthetized hydrogels and composites are shown in the Table 2. According to the TGA thermogram, the hydrogel composites IV (MATCA-AA@GO) showed high thermal stability with addition of small amount of GO. Also, thermal stability of hydrogel composite is significantly increased in comparison with poly acrylic acid (PAA) due to electrostatic interaction of ammonium groups with negative charges on GO. As reported previously, the higher cross-linking raise the thermal stability of hydrogel [31]. Indeed, MBA, MATCA and GO have important synergic effects on the crosslinking density, leading to the thermal stability trend of \(\mathrm{MATCA}-\mathrm{co}-\mathrm{AA}@\mathrm{GO}>\mathrm{MATCA}-\mathrm{co}-\mathrm{AA}>PAA\).
XRD
XRD has been utilized to analyze the crystalline structure of the prepared GO and MATCA-co-AA@GO hydrogel composite (Fig. 2b). The distance between two layers is a parameter in evaluating the structural information of the graphene. The XRD spectra of GO measured in a range of 2θ from 9.8° to 80° show (002) diffraction peak at 2θ = 10°, representing the distance between graphene layers, and (10) XRD diffractogram at 2θ = 43.03° representing a short range order in stacked graphene layers. A remarkably wide diffractogram appeared at 2θ ~ 25° (002) which specifies the destruction of the regular crystalline pattern of graphite during the oxidation. The XRD patterns indicate that the crystalline structure of GO during the synthesis of the hydrogel composite is well maintained and reveals the multilayer structure of GO [35].
FE-SEM morphology
Morphological feature of the resulting GO and the MATCA-co-AA@GO hydrogel nanocomposite (sample IV) has been verified by FESEM as shown in Fig. 3. As previously reported [36], the results of surface morphology corresponding to GO shows that its structure is sheet-like crumpled and rippled with a large thickness which can be assigned to deformation of upon the exfoliation and restacking process. The FESEM image of the MATCA-co-AA@GO exhibits that the morphology of the nanocomposite is quite uniform in large scale and the exfoliated GO sheets are well dispersed in the matrix of hydrogel nanocomposite. The FESEM observation indicates that the synthetized hydrogel contained many small spheres with approximate size of 20 nm. Aggregation of nanospheres can be attributed to rising of surface to volume ratio (aspect ratio) in nanoparticles. Roughen surface and nanoparticles flock causes a plenty of porosity on the surface of the synthetized hydrogel, leading to increasing of dye adsorption.
DMTA
Figure 4 shows the results of the temperature dependence of the storage modulus (É) and the damping factor (tan δ) of dried MATCA–co-AA(II) and MATCA-co-AA@GO (IV) hydrogels from DMTA measurements. Tan δ refers to the ratio of energy dissipated as heat (E″, the viscouse behaviour) to the maximum energy stored into the material (É, the elastic behaviour) during a loading cycle. From Fig. 4b, two relaxation peaks appeared for MATCA–co-AA(II) which labeled as α and β. The broad and relatively low intensity of β-relaxation was due to the motions of hydroxyl groups of trapped water molecules in the polymer networks. This event might be owing to water molecules absorbed from moisture by the MATCA–co-AA(II). This phenomenon is agreement with those proposed previuosly study [37]. Tg (glass transition temperature) of polymers and their composites is determined using the maximum of of tan δ peak (α relaxation) [38]. The Tg of MATCA–co-AA(II) and MATCA-co-AA@GO (IV) hydrogels appeard at 90 ℃ and 71 ℃ respectively. The relatively lower Tg of nanocomposite with the addition of GO sheets were due to the accumulation of GO sheets together which results in weakening of the intraction between the GO and the polymer matrix. Also, the covalent bonds formation of polymer networks during the in situ polymerization is limited with the addition of GO and the Tg decreases [37]. From Fig. 4a, the storage modulus of MATCA-co-AA@GO (IV) is lower than MATCA–co-AA(II). The higher loss modulus of nanocomposite with the addition of GO sheets shows the viscous behavior of nanocomposite which indicates the intermolecular bonding destruction is occurred. The lower damping factor of MATCA-co-AA@GO (IV) nanocomposite illustrates the improvement of interfacial bonding within the polymer matrix [39].
Adsorption studies
To investigation of dye adsorption and swelling, six samples were tested which have been effectively synthetized through in situ polymerization with different GO and MATCA contents.
as summarized in Table 3.
According to the results which are shown in the Table 3, the amount and ratio of GO along with MATCA have significant effect on swelling, gel content and dye removal. In comparison with samples IV, V and VI, the swelling ratio of samples I, II and III are lower significantly. As seen, in parallel to swelling, dye removal percentage is changed. For sample I, there is very low dye adsorption which confirmed that poly acrylic acid alone absorb very small amount of dye. In the case of samples II and III, dye removal percentages are relatively small which indicated that the presence of both GO and MATCA is necessary for efficient dye absorption. The observed behavior of swelling is a result of the number of ionizable groups in nonocomposite matrix. The adsorption experiments indicated that the synthetized hydrogel containing 0.005 g GO and 0.005 g MATCA (sample IV) has a great adsorption capacity for dye comparing to other synthetized hydrogels. It can be assigned to the flexible cavity of MATCA macrocycles, the π–π interactions between the aromatic rings of the MATCA host parts and hydrophobic residuals of dyes, the electrostatic interactions between the -O- and –NH- groups in GO, MATCA and the iminium groups in MG and MB dyes. The electrostatic repulsion between ionizable group such as NH3+, COOH and OH is responsible for the network swelling which enhance repulsive forces with adjacent chains by means of decrease in the hydrostatic pressure within the network. AA, MATCA and GO networks linked each other via hydrogen bonding and other secondary valence forces between neighboring polymer chains. A balance between GO and MATCA and maximum dye removal is occurred. But this balance in the sample of V and VI is disrupted and dye removal is decreased severely. Due to the existence of ammonium groups, MATCA has positive charge. Therefore, MATCA can strongly adsorb negatively charged GO sheets via electrostatic interactions. In addition, formation of multiple hydrogen bonds between GO sheets and MATCA chains increase the bonding force amongst GO sheets. Consequently, MATCA acts as an effective crosslinking agent which improves the creation of the GO hydrogel [25].
As can be observed from Table 3, the equilibrium swelling ratio of the MATCA-co-AA@ GO nanocomposite decreases with enhancing GO and MATCA concentrations (sample V and VI). Increasing of crosslinking agent content causes to gradually decreasing of the swelling ratio, as adding GO, MATCA and MBA will enhance the number of effective cross linked points. It is clear that the swelling ratio of hydrogels substantially is influenced by the effecient crosslink density of the hydrogels. It was concluded that the GO sheets and MATCA could play role as a multifunctional cross-linker to create more connections in the MATCA-co-AA@ GO and enhance the crosslink density, causing the decrease of swelling capacity and dye removal [31]. Summing up, the sample IV is selected for adsorption of MG and MB dye, and further examination of other parameters.
The adsorption method can be performed by the host–guest property of the MATCA host, which allows the adsorption of dyes by its flexible cavity and through π—π interactions between aromatic rings of GO, the aromatic cavity and hydrophobic residues of dyes and hydrogen bonding between OH at the lower rim of the calix and the edges of GO with the MG and MB cationic dyes. Also, there is electrostatic intraction between –COO− negative group of the AA and the positively charged nitrogen atom in the MG and MB dyes [17, 40]. Scheme 4 demonstrates the proposed adsorption mechanism between the MATCA-co-AA@ GO (IV) host segment and cationic dyes guest segment.
Effect of adsorbent dosage
The amount of adsorbent is a substantial factor in adsorption process. Therefore, to evaluate the effect of adsorbent dosage on adsorption of dye, different amounts (0.01- 0.1 g) of MATCA-co-AA @ GO (IV) adsorbent were used under the following conditions: 50 ml of dye solution containing 20 mg L−1 dye at pH 6.5 for 90 and 120 min (for MG and MB respectively) at room temperature. As can be seen in Fig. 5a, the adsorption capacity enhances with increasing amount of adsorbent from 0.01 to 0.05 mg. After increasing the adsorbent content to 0.05 mg, due to saturation of the active sites, no significant change in the amount of adsorption was observed. The early sharp rise can be assigned to fast adsorption of dye on active site of the adsorbent which consist of increasing the area of absorbent surface and the accessibility of adsorption sites. Finally, 0.05 mg of adsorbent was used as the optimal content for subsequent experiments.
Effect of pH
The optimum pH value of dye solution is an essential parameter for efficient dye removal. Thus, to investigate more about the adsorption event and to examine the influence of pH, we considered the adsorption capacity of the dye with 0.05 g adsorbent (MATCA-co-AA@GO (IV)) in 50 ml dye solution (20 ppm) during 90 and 120 min (for MG and MB respectively) over pH range of 3.5 to 11.5 which are shown in Fig. 5b. The pH value was set by HCl 0.1 mol L−1 and NaOH 0.1 mol L−1 at a certain amount and measured using a pH meter. The results indicated that the dye adsorption occurred efficiently at pH 6.5. At this pH value, MG and MB molecules are completely reachable by the reactive functional groups in the hydrogel network. At higher pH-values (7.5–9.5), a deswelling is observed. In fact, further ionization of carboxyl groups on GO sheets and poly acrylic acid is occurred and hydrogel deswelling quickly reduces the adsorption efficiency. This is because electrostatic repulsion forces between GO sheets and poly acrylic acid enhanced due to increasing of negative charge density in their surfaces which prevent the hydrogen bonding. At pH value higher than 9.5, when ionization is completed, the.
added sodium hydroxide increases the osmotic pressure of the external solution and cause the hydrogel to collapse significantly. Thus, at pH higher than 6.5, the diffusion of the dyes into the network slowdown, then the rate of the adsorption of dyes by the hydrogel decrease considerably. The result indicate that at pH 5.5 and also lower values, most of the carboxyl group of acrylic acid and GO become fully protonated which causes the hydrogel to shrink [15, 41]. Also more NH3+Cl− groups may repel the cationic MG and MB dyes. At pH 6.5 ammonium groups in upper rim of the thiacalix[4]arene, and carboxylate group of polymer chains are more ionized and interact more effectively with the cationic dye. Thus, the synthesized pH-sensitive hydrogel nanocomposite enable us to modulate the adsorption activity through a thermodynamic transition that take place within the network. Consequently, the best pH for adsorption of MG and MB is about 6.5 (Qe = 19 mg g−1), therefore, further studies were conducted with dye solutions at this pH.
Influence of initial concentration of dye (Isotherm studies)
The removal of MG and MB dyes by the MATCA-co-AA@GO hydrogel nanocomposite (0.05 gr) at the initial concentraions ranging from 20 to 80 mg/L, was studied at room temperature. Having said that, all other parameters keep constant (at pH 6.5 and t = 90 and 120 min). As can be observed in Fig. 5c, the removal percentage of MG dye reduced with increasing the initial concentrations. The dye removal efficiency is steadily reduced. It can be because of the total available adsorption sites in the adsorbent are confined at higher initial concentrations of dye [42]. The Qm was 67.5 and 70.3 mg g−1 (for MG and MB respectively) which calculated using Eq. 6. This indicates that the synthetized hydrogel nanocomposite has great potential for elimination of cationic dye from polluted water.
An adsorption isotherm demonstrate the correlation between the extent of adsorbate adsorbed per unit weight of sorbent (Qe, mg g−1) and the amount of adsorbate in the bulk solution (Ce, mg L−1) at a definite temperature under equilibrium conditions. According to these parameters, it is possible to obtain information about the development of the adsorption mechanism and how the dye molecule interact with the adsorbent [43]. For determination of the mechanistic factors related with MG and MB adsorption, the data from the adsorption tests were studied based on the Langmuir and Freundlich isotherm models [17]. The basic assumption of the langmuir model is that the molecular adsorption being a monolayer and assumes that the adsorption occurs on the certain identical and energetically equivalent homogeneous adsorption places. This model shows that each site can adsorbed only one molecule, and decrease of the intermolecular forces take place with increasing of distance. The Eq. 6 has been applied for Langmuir isotherm.
where Qe refers to the equilibrium adsorption capacity of adsorbent (mg g−1), Ce represents the equilibrium dye amount in solution (mg L−1). The Qm and b refer to maximum adsorption capacity (mg g−1) and langmuir adsorption constant related to adsorption energy (L mg−1), respectively. The values of Qm and b were computed by the intercept and slope of the linear plot of 1/Qe versus 1/Ce (Fig. 6a) and are shown in Table 4 together with the value of R2.
Whether adsorption process is favorable or not, the separation factor RL is determined. RL is given by Eq. 7:
RL values show the kind of adsorption as either unfavorable (\({R}_{L}>1\)), linear (\({R}_{L}\)=1), favorable (\(0<{R}_{L}<1\)), or irreversible (\({R}_{L}=0\)). In our study, the MG and MB adsorption is favorable type because the RL in all cases lies between 0 and 1 as depicted in Fig. 6c.
The Freundlich model is appropriate for applying with heterogeneous surfaces and involves the formation of multilayers. This model is used for high and intermediate amounts and result in poor data at low concentration values. The Freundlich isotherm is given by the Eq. 8:
where Qe and Ce are explained above, Kf refers to the Freundlich constant (mg g−1) and n is the heterogeneity factor which is associated with the capacity and intensity of adsorption respectively. The values of Kf and 1/n were achaived from the intercept and slope of a linear plot of ln Qe versus ln Ce (Fig. 6b).
The data achaived from the Langmuir and Freundlich isotherm Equations were completely fitted with the Freundlich model for MG (R2 = 0.9851) and Langmuir model for MB (R2 = 0.9946). The calculated maximum adsorption capacities for MG from Freundlich model (Kf, cal) was close to the observed values (Qm, exp). Figure 6 demonstrates the adsorption isotherms of the examined MG and MB dye at ambient temperature. As can be seen, the obtained data for MG is better fitted to the Freundlich model. This indicated that adsorbent surface is heterogeneous under the applied experimental conditions [44] and confirms the multilayers adsorption of MG by the adsorbent. Also, the data of MB is better fitted to the Langmuir model, which confirms the monolayer adsorption of MB and homogeneous adsorption places.
As can be observed in Fig. 6d, the color of MG and MB solution was disappeared completely after treatment with the adsorbent representing the high efficacy of the prepared adsorbent in the MG and MB dye removal. The GO nanosheets with large surface area, and reactive functional groups like hydroxyl and carboxylic acid and π-π interactions of the aromatic rings of GO with MG, enhance the adsorption capacity of MG and MB cationic dye onto the adsorbent.
The Qm of MG and MB dyes corresponding to the synthetized hydrogel derived from the Langmuir Equation was estimated as 67.5 and 70.3 mg g−1 respectively which are comparable with those of other sorbent (Table 5).
Effect of contact time (Kinetics studies)
In order to investigation of adsorption kinetic, the impact of contact time on the concentration of adsorbed MG and MB dyes upon prepared hydrogel was experimentally studied for various times. Also initial MG and MB solution was setted at 20 mg L−1 concentration and pH 6.5.
As revealed in Fig. 5d, it is clear that the removal percentage of MG and MB dyes enhanced quickly initially and gradually increased with prolonging the contact time until equilibrium. As about 95% of adsorption took place over the first 90 min for MG and 120 min for MB; therefore, The optimal contact time was choiced at 90 min for MG and 120 min for MB. This shows that the synthetized hydrogel have fast adsorption kinetic for MG and MB solution. The quick adsorption at the early contact time can be related to the high amount of the MG and MB dyes and the abundant availability of the active sites on the surface of the prepared MATCA-co-AA@GO (IV) adsorbent [50]. By using Eq. 4, the equilibrium adsorption capacity (Qe) was determined to be 19 mg g−1 for MG (after 90 min) and MB (for 120 min) dyes. To study the mechanism controlling the adsorption procedure on the synthetized hydrogel, three kinds of kinetic models were used [51].
Moreover, the pseudo-first-order model, considered as the Lagergren kinetic Equation is on the basis of the assumption that the absorption rate associates to the number of the unoccupied adsorptive sites. This is given as follows (Eq. 9):
where Qe and Qt refer to the adsorption capacity (mg g−1) at equilibrium and at time t, respectively, k1 refers to the rate constant for the pseudo first-order adsorption (min−1). The values of K1 and Qe were obtained from the slope and intercept of linear plots of ln (Qe − Qt) versus t (Fig. 7a). The rate constant K1, Qe and the correlation coefficient corresponding to this kinetic model are given in Table 4.
The pseudo second-order model assumes that the chemical adsorption is a rate controlling step and presented by Eq. 10:
where K2 (g mg−1 min−1) refers to the pseudo-second-order rate constant of adsorption. In this model, K2 and Qe (Table 4) are obtained from the intercept and the slope of the linear plot of t/Qt versus t (Fig. 7b).
The basic assumption of intra-particle diffusion model is that linking of contamination to the active surface of the adsorbent depends on the mass transfer resistance and explained by Eq. 11.
where C refers to the intercept (mg g−1) and kp (mg g−1 min−1/2) represents the intra-particle diffusion rate constant, which can be calculated from the slope of the linear plot of Qt versus t1/2 (Fig. 7c).
The results summarized in Table 4 showed that adsorption of the MG and MB on the synthetized hydrogel nanocomposite follows the Pseudo-second-order kinetic model (R2 = 0.9982 and 0.9901) in comparison with Pseudo-first-order kinetic model (R2 = 0.944 and 0.928) and intra-particle diffusion model (R2 = 0.9139 and 0.9733). In fact, from these data, chemisorption is the rate determining step and the total rate of MG and MB adsorption procedure appears to be controlled via the chemical procedure [43]. Also, the computed values of Qe for the pseudo-first-order model for the MG and MB adsorption were 12.44 and 25.09 mg g−1 respectively. The Qe for the the pseudo- second -order model for the MG and MB adsorption were 20.75 and 23.20 mg g−1. Evidently, in the case of the pseudo-second-order model, the calculated values of Qe (20.75 and 23.20 mg g−1 for the MG and MB respectively) is greatly fitted with the obtained value (19 mg g−1).
Thermodynamics of adsorption
In order to study the impact of temperature, the MG and MB dye adsorption tests on the MATCA-co-AA@GO (IV) adsorbent was conducted at 298, 308, 318, and 328 K with 50 mL of 40 mg L−1 dye solution. As revealed (Fig. 8a), the uptake of MG and MB is improved with increase of temperature. This behavior relates to raising of the dye molecules mobility and diffusion rate of adsorbate molecules through the surface of cells in the hydrogel nanocomposite with enhancing temperature. Also, the rise of the dye adsorption capacity with enhance in temperature confirms that the dye adsorption is an endothermic procedure [12]. The adsorption capacity were 38.5 and 37.3 mg g−1 (for MG and MB respectively) and the removal percentage were 96.25% and 93.25% (for MG and MB respectively), at room temperature (25 ℃). The thermodynamic parameters including Gibbs free energy change (∆Gº), standard enthalpy change (∆Hº), and standard entropy change (∆Sº) were computed by the subsequent Eqs. (12, 13):
where R refers to the universal gas constant (8.314 J mol−1 K−1), T represents the absolute temperature (K), and KL refers to the ratio of dye concentration adsorbed upon the sorbent to the residual amounts in solution at equilibrium (Ce). Also, the slope and intercept corresponding to the plot of ln KL versus 1/T result in ∆Hº and ∆Sº respectively (Fig. 8b). ∆Gº has negative values for adsorption of the examined MG and MB dyes on MATCA-co-AA@GO (IV) adsorbent. This result demonstrate that the adsorption procedure is thermodynamically possible and spontaneous at ambient temperature. Moreover, the reduction of ΔGº values with enhancing temperature indicates that the degree of spontaneity and possibility of adsorption on adsorbent beads rises at higher temperature. The value of the change in enthalpy (∆Hº) was calculated 23.900 and 34.290 kJ mol−1 with positive value for MG and MB respectively which are in the range of ∆Hº for chemisorption (20.9- 418.4 kJ mol−1) (Table 6). The positive value of ∆Hº confirmed that the adsorption of MG and MB dye on the adsorbent was endothermic. Furthermore, positive value of ΔSº shows an irregular rise of the randomness at the MATCA-co-AA@GO (IV) adsorbent–solution interface over adsorption [42].
Desorption studies
It is important in terms of ecological environmental and economic aspects to recycle the adsorbent, which contaminated with pollutants. For this purpose, consecutive adsorption–desorption cycles were performed five times under the similar conditions by using 5% acetic acid + methanol as desorption medium. The removal percentage of MG and MB dyes was computed for each cycle by using Eq. 5. As revealed in Fig. 9, the dye removal percentage was not changed remarkably after five adsorption/desorption cycle. The results show the synthesized [MATCA-co-AA@GO (IV)], could be an affordable and efficient adsorbent for removal of MG and MB dyes because of the excellent regeneration and reusability performance.
Conclusion
This study represents the synthesis of a novel pH sensitive hydrogel composite based on ATCA by in situ polymerization technique. After functionalization with AC, the GO/thiacalixarene based hydrogel nanocompsosite was synthetized through heterogeneous polymerization of AA and MATCA with GOusing AIBN and MBA as initiator and cross linking agent, respectively. The features of the hydrogel can be progressed by setting the GO and MATCA contents. The formation of the synthesized monomer was confirmed by using FTIR, CHNSO, mass spectroscopy, 1H and 13C NMR. Synthetized hydrogel was characterized and analyzed by means of FTIR, TGA, XRD, DMTA and FESEM spectroscopy. The prepared hydrogel was used as new sorbent for removing MG and MB from aqueous environment by batch adsorption method. The effects of diverse operation factors like the dosage of the adsorption, content of GO, content of MATCA, the primary pH, the temperature and the contact time were examined. The result indicated that pH value of 6.5 is the best pH for abstraction of MG dye. At the higher and lower pH of 6.5, the adsorption of MG and MB is decreased significantly. Langmuir and Freundlich isotherms were utilized to investigate the equilibrium adsorption data. It is concluded that the data of MG and MB were greatly fitted by the Freundlich isotherm (R2 = 0.9851) and the Langmuir isotherm (0.9946). The adsorption of MG and MB onto hydrogel followed pseudo-second-order kinetic model. The adsorption procedure was considered to be chemisorption, spontaneous and endothermic. The Qm was 67.5 and 70.3 mg g-1 for MG and MB respectively. These properties showed that the synthesized hydrogel nanocomposite can be an effective sorbent owing to its good recoverability, cost effective and excellent efficiency in removing MG and MB cationic dyes from water.
References
Hieu C, Juang R (2019) Efficient removal of cationic dyes from water by a combined adsorption-photocatalysis process using platinum-doped titanate nanomaterials. J Taiwan Inst Chem Eng 99:166–179. https://doi.org/10.1016/j.jtice.2019.03.017
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66:319–329. https://doi.org/10.1016/j.aquatox.2003.09.008
Mohd Noor NF, Yusoff SFM (2020) Ultrasonic-enhanced synthesis of rubber-based hydrogel for waste water treatment: Kinetic, isotherm and reusability studies. Polym Test 81:106200. https://doi.org/10.1016/j.polymertesting.2019.106200
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: A review. J Hazard Mater 177:70–80. https://doi.org/10.1016/j.jhazmat.2009.12.047
Nethaji S, Sivasamy A, Thennarasu G, Saravanan S (2010) Adsorption of Malachite Green dye onto activated carbon derived from Borassus aethiopum flower biomass. J Hazard Mater 181:271–280. https://doi.org/10.1016/j.jhazmat.2010.05.008
Preethi S, Sivasamy A, Sivanesan S et al (2006) Removal of Safranin Basic Dye from Aqueous Solutions by Adsorption onto Corncob Activated Carbon. Ind Eng Chem Res 45:7627–7632. https://doi.org/10.1021/ie0604122
Zhang PB, Tang AQ, Wang ZH, et al (2018) Tough poly(L-DOPA)-containing Double Network Hydrogel Beads with High Capacity of Dye Adsorption. Chinese J Polym Sci (English Ed 36:1251–1261. https://doi.org/10.1007/s10118-018-2163-2
Khattri SD, Singh MK (2009) Removal of malachite green from dye wastewater using neem sawdust by adsorption. J Hazard Mater 167:1089–1094. https://doi.org/10.1016/j.jhazmat.2009.01.101
Lakouraj MM, Norouzian RS, Balo S (2015) Preparation and Cationic Dye Adsorption of Novel Fe3O4 Supermagnetic/Thiacalix[4]arene Tetrasulfonate Self-Doped/Polyaniline Nanocomposite: Kinetics, Isotherms, and Thermodynamic Study. J Chem Eng Data 60:2262–2272. https://doi.org/10.1021/acs.jced.5b00080
Pourjavadi A, Abedin-Moghanaki A (2016) Ultrafast and efficient removal of cationic dyes using a magnetic nanocomposite based on functionalized cross-linked poly(methylacrylate). React Funct Polym 105:95–102. https://doi.org/10.1016/j.reactfunctpolym.2016.05.016
Kumar R, Sharma RK, Singh AP (2019) Synthesis and characterization of cellulose based graft copolymers with binary vinyl monomers for efficient removal of cationic dyes and Pb(II) ions. J Polym Res 26. https://doi.org/10.1007/s10965-019-1790-9
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168. https://doi.org/10.1016/j.desal.2010.07.047
Zhang J, Li Y, Zhang C, Jing Y (2008) Adsorption of malachite green from aqueous solution onto carbon prepared from Arundo donax root. J Hazard Mater 150:774–782. https://doi.org/10.1016/j.jhazmat.2007.05.036
Narimani F, Lakouraj MM (2015) Swelling behavior and characterization of alcohol-specific superabsorbing gels based on acrylic acid and allyl tetrasodium thiacalix[4]arene tetrasulfonate. J Polym Res 22:1–6. https://doi.org/10.1007/s10965-015-0670-1
Bai H, Li C, Wang X, Shi G (2010) A pH-sensitive graphene oxide composite hydrogel. Chem Commun 46:2376–2378. https://doi.org/10.1039/c000051e
Mohamed MG, Ahmed AFM, EL-Mahdy, Meng TS et al (2020) Multifunctional Hypercrosslinked Porous Organic Polymers Based on Tetraphenylethene and Triphenylamine Derivatives for High-Performance. Polymers (Basel) 12:2426. https://doi.org/10.3390/polym12102426
Narimani F, Mansour Lakouraj M (2015) Synthesis and characterization of super dye adsorbent hydrogels based on acrylic acid and acryloyl tetrasodium thiacalix[4]arene tetrasulfonate. Polym Int 64:1482–1490. https://doi.org/10.1002/pi.4946
Kamboh MA, Solangi IB, Sherazi STH, Memon S (2011) A highly efficient calix[4]arene based resin for the removal of azo dyes. Desalination 268:83–89. https://doi.org/10.1016/j.desal.2010.10.001
Mohammadi A, Lakouraj MM, Barikani M (2014) Preparation and characterization of p-tert-butyl thiacalix[4]arene imbedded flexible polyurethane foam: An efficient novel cationic dye adsorbent. React Funct Polym 83:14–23. https://doi.org/10.1016/j.reactfunctpolym.2014.07.003
Lakouraj MM, Tashakkorian H (2013) Synthesis and thermophysical properties of nanocrystalline polycalix[4]amides and polycalix[4]arene containing mesogenic triazole units and their heavy metal sorption. Supramol Chem 25:221–232. https://doi.org/10.1080/10610278.2012.758366
Duan J, Xie Y, ning, Yang J hui, et al (2016) Graphene oxide induced hydrolytic degradation behavior changes of poly(L-lactide) in different mediums. Polym Test 56:220–228. https://doi.org/10.1016/j.polymertesting.2016.10.015
Fan L, Luo C, Sun M et al (2012) Preparation of novel magnetic chitosan/graphene oxide composite as effective adsorbents toward methylene blue. Bioresour Technol 114:703–706. https://doi.org/10.1016/j.biortech.2012.02.067
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240. https://doi.org/10.1039/b917103g
Potts JR, Dreyer DR, Bielawski CW, Ruoff RS (2011) Graphene-based polymer nanocomposites. Polymer (Guildf) 52:5–25. https://doi.org/10.1016/j.polymer.2010.11.042
Chen Y, Chen L, Bai H, Li L (2013) Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J Mater Chem A 1:1992–2001. https://doi.org/10.1039/c2ta00406b
Kumagai H, Hasegawa M, Miyanari S et al (1997) Facile synthesis of p-tert-butylthiacalix[4]arene by the reaction of p-tert-butylphenol with elemental sulfur in the presence of a base. Tetrahedron Lett 38:3971–3972. https://doi.org/10.1016/S0040-4039(97)00792-2
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Kasyan O, Swierczynski D, Drapailo A et al (2003) Upper rim substituted thiacalix[4]arenes. Tetrahedron Lett 44:7167–7170. https://doi.org/10.1016/S0040-4039(03)01809-4
Hu X, Zhu Z, Shen T et al (2004) Synthesis of the tetranitro derivative of thiacalix[4]arene and its acid-base properties. Can J Chem 82:1266–1270. https://doi.org/10.1139/v04-090
Desroches C, Parola S, Vocanson F et al (2002) Nitration of thiacalix[4]arene using nitrosium nitrate complexes: Synthesis and characterization of tetranitro-, tetraamino-, and tetra(4-pyridylimino)tetrahydroxythiacalix[4]arene. New J Chem 26:651–655. https://doi.org/10.1039/b110609k
Shen J, Yan B, Li T et al (2012) Mechanical, thermal and swelling properties of poly(acrylic acid)-graphene oxide composite hydrogels. Soft Matter 8:1831–1836. https://doi.org/10.1039/c1sm06970e
Chen W, Yan L, Bangal PR (2010) Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon N Y 48:1146–1152. https://doi.org/10.1016/j.carbon.2009.11.037
Maurer JJ, Eustace DJ, Ratcliffe CT (1987) Thermal Characterization of Poly(acrylic acid). Macromolecules 20:196–202. https://doi.org/10.1021/ma00167a035
Park Y, Lee S, Ha SS et al (2019) Crosslinking effect on thermal conductivity of electrospun poly(acrylic acid) nanofibers. Polymers (Basel) 11:1–13. https://doi.org/10.3390/polym11050858
Akceylan E, Bahadir M, Yilmaz M (2009) Removal efficiency of a calix[4]arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J Hazard Mater 162:960–966. https://doi.org/10.1016/j.jhazmat.2008.05.127
Ai L, Zhang C, Chen Z (2011) Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J Hazard Mater 192:1515–1524. https://doi.org/10.1016/j.jhazmat.2011.06.068
Huang Y, Zeng M, Ren J et al (2012) Preparation and swelling properties of graphene oxide / poly (acrylic acid-co- acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surfaces A Physicochem Eng Asp 401:97–106. https://doi.org/10.1016/j.colsurfa.2012.03.031
Zeng M, Huang Y, Lu L et al (2011) Effects of filler-matrix morphology on mechanical properties of corn starch-zein thermo-moulded films. Carbohydr Polym 84:323–328. https://doi.org/10.1016/j.carbpol.2010.11.038
Boparai KS, Singh R (2018) Thermoplastic composites for fused deposition modeling filament: challenges and applications. Ref Modul Mater Sci Mater Eng 1–14. https://doi.org/10.1016/b978-0-12-803581-8.11409-2
Yan H, Tao X, Yang Z et al (2014) Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J Hazard Mater 268:191–198. https://doi.org/10.1016/j.jhazmat.2014.01.015
Zhao T, Zhang K, Chen J et al (2019) Changes in heavy metal mobility and availability in contaminated wet-land soil remediated using lignin-based poly(acrylic acid). J Hazard Mater 368:459–467. https://doi.org/10.1016/j.jhazmat.2019.01.061
Sekhavat Pour Z, Ghaemy M (2015) Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly(vinyl alcohol)/carboxymethyl starch-g-poly(vinyl imidazole). RSC Adv 5:64106–64118. https://doi.org/10.1039/c5ra08025h
Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res 4:550–562. https://doi.org/10.1007/s12274-011-0111-3
El-Sayed GO (2011) Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination 272:225–232. https://doi.org/10.1016/j.desal.2011.01.025
Ramírez E, Burillo SG, Barrera-Díaz C et al (2011) Use of pH-sensitive polymer hydrogels in lead removal from aqueous solution. J Hazard Mater 192:432–439. https://doi.org/10.1016/j.jhazmat.2011.04.109
Chen Z, Zhang J, Jianwei Fu MW et al (2014) Adsorption of methylene blue onto poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanotubes: kinetics, isotherm and thermodynamics analysis. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2014.03.053
Bagheri N, Lakouraj MM, Hasantabar V, Mohseni M (2021) Biodegradable macro-porous CMC-polyaniline hydrogel: synthesis, characterization and study of microbial elimination and sorption capacity of dyes from waste water. J Hazard Mater 403:123631. https://doi.org/10.1016/j.jhazmat.2020.123631
Naseeruteen F, Hamid NSA, Suah FBM et al (2018) Adsorption of malachite green from aqueous solution by using novel chitosan ionic liquid beads. Int J Biol Macromol 107:1270–1277. https://doi.org/10.1016/j.ijbiomac.2017.09.111
Rangabhashiyam S, Lata S, Balasubramanian P (2018) Biosorption characteristics of methylene blue and malachite green from simulated wastewater onto Carica papaya wood biosorbent. Surfaces and Interfaces 10:197–215. https://doi.org/10.1016/j.surfin.2017.09.011
Baldez EE, Robaina NF, Cassella RJ (2008) Employment of polyurethane foam for the adsorption of Methylene Blue in aqueous medium. J Hazard Mater 159:580–586. https://doi.org/10.1016/j.jhazmat.2008.02.055
Masoumi A, Ghaemy M (2014) Removal of metal ions from water using nanohydrogel tragacanth gum-g-polyamidoxime: Isotherm and kinetic study. Carbohydr Polym 108:206–215. https://doi.org/10.1016/j.carbpol.2014.02.083
Acknowledgements
We appreciate the council of University of Mazandaran for the partial financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no any competing financial interests or personal relationships.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niknezhad, M., Mansour Lakouraj, M. Development of pH-sensitive hydrogel nanocomposite based on acrylic acid/ graphene oxide/acryloyl tetra ammonium thiacalix[4]arene for separation of cationic dyes. J Polym Res 28, 209 (2021). https://doi.org/10.1007/s10965-021-02510-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02510-4