Abstract
The primary aim of this study was to identify the polymers recovered from keyboard waste, and formulate a ternary blend from the recovered polymers, with improved mechanical, thermal and morphological characteristics, and encouraging product-based recycling. The recycling and reuse of polymers recovered from E-waste is one of the prime topics in solid waste management. The major drawbacks of utilizing recycled polymers are its lower mechanical strength. This was majorly evidenced in styrene based polymers such as acrylonitrile butadiene styrene (ABS) and high impact polystyrene (HIPS). In our case, the polymers ABS, HIPS and polystyrene (PS) were recovered from waste keyboards, and their impact strength was 29 J/m, 42 J/m and 20 J/m respectively. After formulating a ternary blend with PS as a major phase, the impact strength observed was 66 J/m. The thermal stability of the blends also improved from 380℃ to 396℃, after the addition of PS to the blends, but the glass transition temperature had negligible effect. The morphology of ternary blend shows salami structures after the addition of ABS/HIPS blends. This shows that the r-PS morphology had a brittle to ductile transition which leads to the improvement of impact properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In this rapidly growing era, the accumulation of electronic waste (E-waste) or waste electrical and electronic equipment (WEEE) is progressively increasing due to the proliferation of latest technologies [1, 2]. Most of the E-waste consists of computer peripherals such as monitors, keyboards, printers etc. Based on their operating conditions, they are either sent to second hand market for resale, or to the recyclers [3]. These equipments contain a mixture of various materials such as metals, plastics and glass, which are dismantled during recycling. Among these, metals are consumed immediately due to their higher purity and economic values. The polymers are least considered for recycling due to the different types of polymers involved in EEE applications, their mechanical property after recycling, and their economic value. The computer keyboards are an example of equipments which are directly considered as E-waste, because 90% of the material used in keyboards is plastic [4]. Lin et al. has mentioned keyboards as one of the three products in top priorities, that have the need to recycle [5]. The styrene based plastics such as acrylonitrile butadiene styrene (ABS), high impact polystyrene (HIPS), polystyrene (PS) and polycarbonate/acrylonitrile butadiene styrene (PC / ABS) are used for over 70%, and hence it becomes really important to recycle these polymers [6, 7].
The recycling of WEEE plastics should be encouraged in order to enhance the efficiency of waste management system and to replace the use of virgin plastics in secondary applications [8]. Recycling of plastics can be done by either mechanical, chemical or thermal techniques, but mechanical recycling is preferred over other two recycling techniques, due to its cost consumption and effectiveness [9]. One of the major hurdles for recycling is the degradation of the polymeric materials due to several factors such as environmental effects, ageing or recycling [10, 11]. The recycled polymers are generally not considered for important applications as the mechanical property is found to be very low. In case of ABS and HIPS, impact strength is one of the key properties due to which they are employed in casings of electrical and electronic equipments (EEE). During degradation, the polybutadiene phase of ABS and HIPS are deteriorated and the impact strength of the polymers is reduced drastically [12]. Hence value addition of recycled polymers is an important in order to employ them in various applications.
The value additions of plastics recovered from WEEE are carried out in multiple routes such as incorporation of additives, virgin polymers or blending with another WEEE plastic. Among these, the latter is found to be effective as it utilizes maximum quantity of waste plastics and improves its properties [13,14,15]. There are a number of studies in which ABS and HIPS recovered from housings of WEEE’s are blended together, such as, in a study by Vazquez et al., it was observed that the mechanical properties of ABS improved with the addition of 50% of HIPS in the blends [16]. A similar study undertaken by Brennan et al. shows that ABS and HIPS recovered from WEEE housings formed a successful blend with minimum ratio of a polymer added to another polymer [17]. Another study by Desouza et al. investigated the influence of shot size and particle size on the mechanical properties of ABS/HIPS blends, and it was concluded that, the smaller particles gave better mechanical properties as compared to larger particles [18]. In most of the studies, the ABS and HIPS were recovered from different products such as computers and keyboards housings, but there are very limited studies, where the polymers recovered from similar products are blended with each other for improving the overall properties of the blend.
In the current study, the keyboards casings were sorted and segregated according to the resin identification code (RIC). The sorted polymeric casings were recycled and blended with each other in different proportions. The FTIR-ATR and mechanical performance of the blends were analyzed in order to discover an optimized blend ratio with higher impact strength. Other than the mechanical properties, the thermal and morphological properties of the optimized ternary blends were analyzed and compared with the individual polymers.
Materials and methods
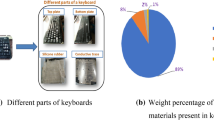
The waste keyboards of various brands such as HP, HCL, TVS, Dell, Logitech and etc. were procured from M/s Roshan industries, Bhubaneshwar, India. The keyboards are made up of 90 weight % (wt %) of engineering plastics such as ABS, HIPS and PS. The plastics used in the keyboards were identified using the resin identification code (RIC) embossed on them, and the RIC were embossed inside the keyboards. Hence, the screws in the keyboards have to be unscrewed initially, to identify the RIC embossed inside them. After the identification, the keyboards have to be isolated according to the polymers. Before grinding, the keyboards have to be cleaned with a fabric material to remove the dirt and other foreign particles. The keyboards were then grinded individually using scrap grinder (M/s Arun, India) and again cleaned in a water bath. The samples were then pre-dried in a hot air oven at 60℃ for 6 to 8 h before further processing. The grinded material was processed into specimens for being tested by tensile and impact analysis, using the micro-compounder DSM Xplore-15 (M/s Xplore, The Netherlands) at a temperature of 180˚C and 60 rpm with a residence time of 120 s. The flow chart for recycling of keyboard waste plastics is illustrated in Fig. 1.
Composition of the blends
The polymers, recycled acrylonitrile butadiene styrene (r-ABS) and recycled high impact polystyrene (r-HIPS) were initially blended together in different ratios using the batch mixer Haake Polylab os Rheodrive (M/s Thermo Scientific, United States). The optimised processing conditions were 180˚C with a mixing speed of 60 rpm for a period of 300 s. The prepared polymeric blends as shown in Table 1, were again grinded using M/s Arun Scrap grinder, and made into tensile and impact specimens using the micro-compounder DSM Xplore-15 (M/s Xplore, The Netherlands) at a temperature of 180˚C and 60 rpm with a residence time of 120 s. From the impact and tensile results of the binary blend, the optimized binary blend ratio was chosen, and it was further blended with different ratios of recycled polystyrene (r-PS) as shown in Table 1, making it a ternary blend. The ternary blends were also tested for tensile and impact properties, and an optimized blend was chosen from the results. The optimized binary and ternary blends were further characterized with differential scanning calorimetry (DSC), thermo gravimetry analysis (TGA), dynamic mechanical analysis (DMA) and scanning electron microscopy (SEM), along with r-ABS, r-HIPS and r-PS.
Fourier transform infrared analysis
The engineering plastics, r-ABS, r-HIPS, r-PS and different compositions of binary and ternary blends were analyzed using Fourier transform infrared- attenuated total reflection spectroscope (FTIR-ATR) Nicolet 6700 (M/s Thermo Scientific, United States) in the range between 400 to 4000 cm−1 at 64 scans.
Mechanical testing
Impact test
ASTM D256 -10 is the standard test method for determining the impact resistance of plastics using IZOD method. The dimensions of impact test specimens were 63.5 × 12.5 × 3.2 mm, and they were prepared according to ASTM D256 -10 standards. The samples were notched using a notch cutter (M/s Tinius Olsen, USA) at an angle of 45˚ and a depth of 2.54 mm. The reported impact value was an average of six tested specimens. The impact test was analyzed employing an impact testing machine IT 504 (M/s Tinius Olsen, USA).
Tensile test
Important tensile properties of plastics such as tensile strength, elongation at break, tensile modulus were evaluated from tensile tests. ASTM D638-14 is the standard test method for determining the tensile properties of plastics using universal testing machine. The dumbbell shaped specimen of dimension 150 × 12.7 × 3.2 mm was subjected to tensile test according to ASTM D 638 -14. The tensile test was conducted on seven samples from each individual plastic and ternary blends using the Universal Testing Machine UTM 3382 (M/s Instron, United States) fitted with a 10 KN load cell capacity and operated at crosshead speed of 10 mm/min.
Thermal analysis
Differential scanning calorimetry
Differential scanning calorimeter (DSC) was done using DSC Q 20, (M/s TA Instruments, USA) as instructed in ASTM D3418 using 5 mg of the sample. The glass transition temperature (Tg) of the blends and recycled polymers were analysed in DSC. The samples were tested at a temperature range of 30°C to 300°C with a ramp temperature of 10°C /minute under the nitrogen atmosphere.
Thermogravimetry analysis
Thermogravimetry analysis (TGA) was performed using TGA Q 50, (M/s TA Instruments, USA) according to ASTM D3850 with 10 mg of smaple. The rate of decomposition, final char content and other important thermal characteristics were identified from TGA. The samples were characterised in the range of 30°C to 800°C with a ramp temperature of 10°C /min in a nitrogen atmosphere.
Dynamic thermal mechanical analysis
Dynamic thermal mechanical analysis (DTMA) was performed in three point bending mode using dynamic mechanical analyzer (M/s TA Instruments, DMA Q 800 USA) according to ASTM D4650 -12. The samples were tested at a temperature range between 30°C to 150°C with a ramp temperature of 10°C/min and a frequency of 1 Hz. The dimensions of the samples were 63.5 × 12.5 × 3.2 mm. The storage modulus (G’), loss modulus (G”) and tan δ were evaluated from the results.
Morphological analysis using scanning electron microscopy (SEM)
The impact fractured surface of the samples were analyzed using SEM EVO MA 15 (M/s Carl Zeiss, UK), to analyze the miscibility of various polymers, surface morphology, and nature of crack and stress absorption regions at a voltage of 15 kV. The fractured samples were gold sputtered. The image resolution was taken at 20 µm with a magnification of 800 X for all the samples.
The sample surfaces were etched initially to remove the polybutadiene (PB) material from the surface. The etching of the sample surface helps to understand the miscibility of the polymers with each other and the dispersion of different phases in the polymer. The surfaces of the samples were etched using potassium chromate solution for 30 min, in order to etch out the PB material from the surface.
Results and discussion
FTIR-ATR analysis of binary blends
The FTIR spectra of r-ABS, r-HIPS and its blends are shown in Fig. 2 Table 3. The characteristic peaks of r-ABS, r-HIPS and its blends were analyzed using FTIR-ATR technique.
The FTIR graph shown in Fig. 2 shows the intense peak of the functionalities present in the polymers such as for acrylonitrile at 2240 -2260 cm−1, for C = C at 1600–1800 cm−1 and 690–750 cm−1 for phenyl groups [19]. A high intense peak could be observed at 696 cm−1 and 712 cm−1 and it shows the presence of aromatic groups, and these peaks are observed in ABS, HIPS and its blends. It is common that, polybutadiene (PB) and polystyrene (PS) phases are present in both ABS and HIPS, and hence the characteristic peaks of both PS and PB are observed in both the individual polymers and its blends. The peaks in the range 1250–1275 cm−1 represents the vibrations of C–C bond present in PS, and cis units of PB. The peaks at 960 cm−1 and 905 cm−1 were attributed to polybutadiene phase in HIPS, a similar report was also indicated by Vilaplana et al. [20]. The peaks at 1600 cm−1 indicated the C = C linkages present in the aromatic ring of polystyrene. A similar vibration in the range 1584 cm−1 to 1602 cm−1 was observed by Oksman et al. [21]. The peaks at 1450 cm−1 represents the scissoring mode of ethane (C2H6). The aromatic and aliphatic stretches of C-H bond were represented at 2925 cm−1and 3025 cm−1, which was reported by Muntennu et al. in their work of spectral and thermal characterization of styrene butadiene co polymers with different architectures [22]. The characteristic peak of acrylonitrile was represented at 2230 cm−1and the intensity of the peak was observed to be very weak.
Impact and tensile performance of developed r-ABS/r-HIPS blends
Table 2 and Fig. 3 shows the impact and tensile properties of r-ABS, r-HIPS and its blends. The impact strength of r-ABS was found to be 29.3 J/m and for the blend K-2 (r-ABS/r-HIPS 70:30) was 28.8 J/m, which was almost equal to the impact strength of r-ABS. On increasing the weight percentage of r-HIPS in r-ABS, the blend shows an improvement in impact strength of 23% from the impact strength of r-ABS, which was 36.2 J/m. Further, increase in weight percentage of r-HIPS to 70%, increases the impact strength of the blend to 42.8 J/m, which was 44% higher than the impact strength of r-ABS. The increase in impact strength shows that the blends follow the rule of mixtures. The improvement of impact strength with the addition of r-HIPS in r-ABS was also observed by Yamila et al., in particular for the blend ratio r-HIPS/r-ABS (80:20) [16]. Similarly, Denise et al. observed an increase in impact resistance for the blends containing 25% of ABS in HIPS, which results in improved impact strength in the blends having HIPS as major phase [23]. In constrast, Brennan et al. and Tarantili et al. have also studied the different compositions of recycled ABS/HIPS, and they observed that the tensile strength is maintained across the composition range, but the impact strength and ductility of the specimen’s decreases with the increase of HIPS weight percentage [17, 24]. It was also claimed by De Souza et al. that, even the lowest concentration of HIPS in ABS showed detrimental effect on its impact strength [18]. The impact resistance of a polymer is mainly bestowed from the polybutadiene phase present in r-ABS and r-HIPS, these PB phases are liable to thermo oxidative degradation during the exposure to open environment after discarding, and it leads to mechanical failure of the polymeric material [25]. The processing method was also considered to be an important criterion for the deterioration of mechanical properties in the blends and the impact strength was mostly affected during the extrusion process. The mechanical recycling of polymers in large quantity involves extrusion initially, and the extruded material is further subjected to injection moulding for product development or its analysis. Thus, the polymers are exposed to thermo- mechanical operations twice, which may also be a strong reason for the decrease of impact strength. The observation also indicates that the impact strength of r-HIPS was 42.5 J/m and of r-ABS/r-HIPS (30:70) was 42.6 J/m. It shows that, the blend performs well with a minimum quantity of r-ABS in r-HIPS and, there is a possibility of synergistic action between these polymers.
As a thumb rule, the increase in impact strength reduces the tensile property. The tensile strength of r-ABS and all the blends are almost 30 ± 2 MPa, but the tensile strength of r-HIPS is 22.6 ± 2 MPa. This is due to the rubbery PB phase present in r-HIPS, which imparts ductility to the sample thereby decreasing the tensile strength of the sample [16]. The elongation at break for K-4 blends was 12% and for r-HIPS was 24%. In our case, the prime reason for the increase in elongation at break was due to the fact that the prepared blends had to undergo thermo-mechanical operations twice as discussed in the earlier section, whereas the recycled individual polymers were exposed to thermo-mechanical operations only once. This may be the possible reason for the progressive increase of the elongation at break of r-HIPS. However, Brennan et al. had observed that the elongation at break property decreases for the polymers after recycling due to increase in stiffness of the polymers [17]. The increase in stiffness may be owing to the effects of crosslinking, which is initiated on the onset of degradation, during processing of polymer, and it leads to reduction in ductile properties. Brennan et al. also observed that the increase in stiffness of the material also increases the tensile modulus of the material and it stated that the tensile strength and modulus value did not had any major effect due to recycling of polymeric material [17].
The tensile modulus values of the blends were unpredictable as the modulus values were increasing upto the blend ratio K-3, but after that the modulus value decreases from 2206 to 2144 MPa and it again increases to 2171 MPa for r-HIPS. The effective compatibility of the blends is dependent upon the adhesion of different phases of the polymeric blend system. Tensile modulus is a low strain property which greatly depends upon the internal structure in the case of individual polymers, and ratio of different polymers in the case of blends. Whereas, properties like tensile strength and elongation at break have high strain behavior, that the improvement in properties indicates better adhesion between different phases with effective compatibilization [16]. From the impact and tensile results it is clear that the blend K-4 (r-ABS/r-HIPS 30:70) is the optimized blend composition because the impact strength is highest for that particular blend ratio and also the tensile strength is maintained with the addition of r-ABS. The blend K-4 is further mixed with r-PS and its FTIR and mechanical properties are discussed below.
The major reasons for choosing K-4 as optimized blend was its impact strength. The materials ABS and HIPS are known for their impact strength, due to which they were used in casings of monitors, keyboards and other electronic equipments. Their impact strength decreases during recycling due to thermo mechanical degradation leading to chain scission of polybutadiene phase with polystyrene phase. Hence, impact strength serves as the prime property for the blends formulated from ABS and HIPS. In our case, the blend K-4 had higher impact property as compared to other blend compositions. Hence K-4 was chosen as the optimized blend ratio.
FTIR-ATR analysis of ternary blends
Figure 4 shows the FTIR-ATR graph of the K-4 blend (r-ABS/r-HIPS, 30:70) with different ratios of recycled polystyrene (r-PS) added to it. The characteristic peaks observed in the ternary blends were related to the binary blends due to similar chemical constituents, and these peaks have been already explained in the previous section. This section explains the reason for variable intensity observed in the FTIR-ATR graph of the blend, after the addition of r-PS. A broad peak at 1016 cm−1represents the stretching vibrations of polystyrene, and the intensity of the peaks were increasing in the consecutive blends. It was noted that, the 100 wt % r-PS does not show a larger intensity at the 1450 cm−1 region and hence, the peak corresponds to the vibrations of ethane molecule. In case of K-4 blends, the intensity of the peaks at 1450 cm−1 was higher as compared to K-4/r-PS (0:100) and this suggests that the peak at 1450 cm−1 could be the result ethane group present in the polybutadiene (PB) phase of r-ABS and r-HIPS. It was observed that there were no major chemical shifts observed in the FTIR spectra, but the intensity of some peaks were increased in the blends due to the addition of r-PS to the system.
The degradation present in the polymer could be identified with the help of two important peaks present in 1700 cm−1 and 3250 cm−1 known as carbonyl and hydroxyl groups’ respectively [26]. The peak at 1700 cm−1 represents the carbonyl bond which is formed due to the oxidation of the polymer either during its service time or during thermal exposure to the atmosphere after end of life. The broad peak at 3250 cm−1 represents the hydroxyl group usually formed by the absorption of moisture from the atmosphere to a negligible extent. The acrylonitrile group has high moisture absorptivity due to the presence of polar groups present in it resulting higher moisture content in ABS material. In our case, the intensity of both carbonyl and hydroxyl peaks are minimum in all the individual polymers and blends, hence it represents lower level of degradation in the sample.
Optimization of ternary blends and analysis of its mechanical properties
The tensile and impact analysis of K-4 blend and K-4 blend with different proportions of r-PS are represented in Fig. 5 and Table 3. The impact properties of the blend K-4 increases, as the wt percentage of r-PS were increased in the blend. The impact strength of the blend K-4 was 46.68 J/m and it was increased for more than 20% and reached till 66.12 J/m for the blend ratio K-4/r-PS (30:70). The test result shows that the addition of r-PS in K-4 blends improved the impact property of r-PS rather than K-4 blends. On the whole, the synergism between the r-PS and K-4 blends improved the mechanical property of the blends. This phenomenon was also observed by Chen and Juilian, wherein the increase in impact strength of PS was observed after blending with ABS [27]. The physical characteristics of PS and ABS were described by Kolawole et al. as the hard and soft segments of the polymeric blend [26]. The improvement in the impact property of blend as well as PS could be justified by PB phase being intercalated in PS matrix. This could be later verified by analyzing the morphology of impact fractured specimen through SEM. This characteristic was also observed by Arnold et al. and Denise et al. and their results indicated that the presence of ABS dominated the domain structure of ABS/HIPS blend system [23, 28]. It was commented that, the SAN forms the domain within ABS, when ABS was the major phase, and PS forms the domains within HIPS, when HIPS was the major phase. In the present study, we have considered a ternary blend of ABS/HIPS/PS, but as all polymers are co polymers of PS, they can be compared to the ABS/HIPS system. Experiment work done by Didier et al. with blending of PS with ABS and HIPS suggests that, the compatibilizing effect of PS with HIPS was positive, whereas the same was not observed with ABS, resulting in deteriorating the mechanical properties of the ABS/HIPS blends [29]. The present investigation suggests improvement in impact property, which may be due to the compatibility effect among the polymers with a right combination.
However, the tensile strength seems to follow a decreasing trend as the weight percentage of r-PS increases in the blend and it does not follow the rule of mixing, in case of tensile properties. The tensile strength of K-4 blend was 29.5 MPa and it decreased to 28.7 MPa for the blend ratio K-4/r-PS (30:70). As there is only a marginal change in the tensile strength, it could be concluded that the incorporation of r-PS in the blend has not much influence in its tensile properties. But, the elongation at break increases progressively for the blend ratio K-4/r-PS (70:30) from 12% to 18.9%, as compared to K-4 blend system. This increase in elongation property may be due to the synergistic action between PB and PS phases in the blend. But the elongation property starts decreasing as the weight percentage of PS was increased in the blend, and it contributed to the negative transition of elongation at break in the blends. The tensile modulus was unsteady, but the modulus could be correlated with the elongation at break and tensile strength of the blends. It could be observed that, as the percentage of elongation increases the strain rate also increases, thereby decreasing the tensile modulus.
From the above results and previous discussions it is clear that K-4/r-PS (30:70) is the optimized blend composition due to higher impact strength. The binary blend K-4 was the optimized composition, and it was convincing since the impact strength of r-HIPS was almost similar to the blend K-4. But, in this case, the impact strength of r-PS was lower as compared to K-4 blend system. Hence, it was understood that there was a compatible effect between the polymers r-ABS, r-HIPS and r-PS recovered from keyboard waste, which was later verified using thermal and morphological analysis. The compatibility effect was not exposed in FTIR results as there were no major chemical shifts, which also suggests no major bond formation. The physical intercalation of PS and PB phases were the major reasons for improved mechanical property. This was also evidenced by Kobita et al. that major phase of PS with minor ABS phase results in good mechanical performance [30].
Thermal analysis
The thermal analyses of the blends have been done by three different methods namely DSC, TGA and DMA. Initially the characteristics about the optimized binary blend have been discussed, and later the characteristic about the binary blend after the addition of r-PS has been discussed.
DSC analysis of binary and ternary blend
The glass transition temperature is particularly related to the mobility of the polymeric chains and determines the transition between glassy and rubbery polymeric state. Hedenqvest et al. has mentioned that the glass transition temperature is dependent upon several aspects such as structure of the polymers, degree of crosslinking, molar mass and molecular degradation of the polymeric chains [31]. The blend system K-4 consists of r-ABS and r-HIPS, hence the glass transition temperature of the blend should lie in intermediate to the glass transition temperatures of r-ABS and r-HIPS. As observed from the Fig. 6, the Tg of the blend is found to be on the lower side as compared to individual polymers. Some of the researchers who have worked in recycled ABS and HIPS have reported that the Tg of both the polymers are in the range of 85 to 100◦ C [18, 32]. In general, The Tg of r-HIPS was slightly higher than that of r-ABS, due to the higher weight percentage of polystyrene group in HIPS as compared to ABS, and it is also well known that the aromatic groups in polystyrene leads to slightly higher glass transition temperature.
The DSC curve of the blend K-4 indicates a broad endothermic peak in the range of 100 to 120◦ C. In general the peaks are broadened due to, (1) higher heating rate, and (2) improper size and distribution of the crystallites. The heating rate given to the polymer may be too high, which tends to eliminate the internal stresses due to which the peaks look broad. This may also be explained due to the size of crystallites, which leads to the broadness of peaks in DSC. Another parameter that supports the claim is that the enthalpy of the polymeric blend was 1.9 J/g, which suggests that the blend had very few numbers of crystallites present in them. The lower the crystallites, the less energy is required for the material to heat up. Brennan et al. had observed that the Tg of the polymers ABS and HIPS were lowered after recycling and improper blending may reduce the chance of homogeneity which has detrimental effects on the Tg value and mechanical properties [17]. But in contrary, a study by Kim et al. claimed that recycling doesn’t affect the Tg of the polymer [33].
It was noticed that the Tg of the K-4 blends was slightly improved with the addition of r-PS. In general, the Tg of PS occurs in the range of 100 to 115°C and it was observed that it is higher than its co polymers because of its structural configuration. It was discussed by Muntennu et al. that the Tg of PS in styrene butadiene styrene (SBS) and styrene ethylene butadiene styrene (SEBS) was recorded and reported as 60°C and 80°C respectively [22]. It shows that the Tg of the polymer is governed by the co polymers attached to it, and hence, the Tg of the ternary blend doesn’t show a drastic improvement after the addition of r-PS due to the presence of PB phase with them. The degradation of the polymer also affects the Tg of the polymer, wherein our present study represent no such degradation behavior of individual polymer resulting better mechanical properties.
TGA of binary and ternary blend
The thermogravimetry analyses were employed to the samples for determining the thermal stability of the blends and its effects due to the addition of r-PS. The polymers and its blends were analysed at a temperature range from 50◦C to 700◦C. As the polymers were thermoplastics and styrenic based polymers, they tend to degrade completely at a temperature of 750◦C to 800℃. The TGA of individual polymers and the blends shows a single step degradation process and it occurs on a narrow temperature range with an onset degradation point at 350℃ to an endset of 470℃. The maximum degradation temperature (Tmax) of both binary and ternary blend was at 433 and 434℃ respectively. The onsets of thermal degradation for binary and ternary blends were at 389℃ and 398℃ respectively and the same for r-PS was 413℃, which shows the higher thermal stability of the polymer and the addition of r-PS to binary blend increased the thermal stability of the blends. Denise et al. had observed that the virgin ABS and HIPS had an onset degradation temperature of 360°C and the endset of 440°C, with lower percentage of final residue [19]. However, the recycled ABS and HIPS indicates higher degradation behavior starting from 380℃ to 450℃. The reason for slight improvement of thermal stability in recycled polymers as compared to the virgin polymers was due to the presence of different additives which improves the performance characteristics of recycled plastics. The addition of thermal stabilizer during the processing of a polymer for EEE application becomes mandatory and hence the thermal stability seems to be higher for recycled polymers. The thermal stability of r-HIPS was higher than that of r-ABS, and the addition of r-ABS in r-HIPS, improved the thermal stability of r-ABS as represented in Fig. 7. In ternary blends, the PB phase was diffused into PS, which lead PS to the formation of allylic type radicals that had lower reactivity, and hence PS was stabilized in the presence of PB phase and this PB phase was bestowed from ABS and HIPS. It was also discussed by Kolawale et al. that 75 wt% PS with 25 wt% ABS had higher thermal stability in natural weathering conditions [26].
In case of r-ABS the char residue found after the complete degradation of the polymer was around 4–5%, which wasn’t observed in r-HIPS, r-PS, K-4 or K-4/r-PS-3 blend, the higher char residue may be due to additives added to them during processing. The additive such as metal oxides may be added to the polymer during its polymerization process, wherein they act as a catalyst during polymerization process and also these metal oxides acts as an active center during the degradation of polymers.
Similarly, the K-4 and K-4/r-PS-3 blend contain combination of PB, PS and polyacrylonitrile (PAN) in its backbone, the thermal decomposition of these blends involves multiple chemical components. The TGA curve begins at 164℃ with the disintegration of constituents like C-H bond from styrene rings followed by C-H bond of butadiene segment. The release of styrene congeners was high at the initial stage of the thermal degradation, however, with the increase in temperature, liberation of butadiene parts was initiated. The evolution of hydrogen cyanide (HCN) and ammonia from polyacrylonitrile depends on the weight percentage of PAN present in the system. In our case, r-HIPS forms the major phase and hence it results in lower weight percentage of PAN in the system. HIPS, on the other hand are grafted co-polymers, with an arrangement of PS and PB. Similarly, in the decomposition of r-ABS, the components are fragmented to C-H bond along with some other oligomers like, benzene and toluene [34]. The degradation of PS was well studied by several authors. Studies from, Suzuki et al. represented the degradation of PS with the evolution of styrene monomers, oligomers, benzene and toluene [35]. It can be observed from Fig. 7, that the TGA and DTG graphs of the K-4 blend did not completely follow the same pattern that of r-HIPS, but the graph was intermediate to r-ABS and r-HIPS, unlike in DSC, where the thermogram of the blend was similar to that of r-HIPS, being r-HIPS as the major phase. Further the changes in the TGA and DTG graph of K-4 blend due to the addition of r-PS has been explored.
Further, single step degradation pattern represents the better compatibility among r-ABS, r-HIPS and r-PS polymeric materials. The level of degradation after end of life for the blended polymers was very low which is well supported by FTIR results. The maximum degradation temperatures for all the polymers and its blends were found to be around 435 ± 5℃. In accordance to the above claims, the optimized blends ratio of 70 wt% of r-PS and 30 wt % of K-4 blends(r-ABS/r-HIPS 30:70) has taken for a consideration. The thermal stability of the K-4/r-PS-3 blend was marginally improved with the addition of r-PS to the blend K-4 and the improvement could be noticed by the difference in the onset temperature of the K-4/r-PS-3 blends. The homogeneity of the blends is further verified by imaging the morphology of impact fractured specimens in our forthcoming section.
DMA analysis of binary and ternary blend
Figure 8 shows the storage modulus and tan delta graph of r-ABS, r-HIPS, r-PS, K-4 and K-4/r-PS-3 blends. The storage modulus of a material is the ability of the material to store the deformation ability, which resembles the elastomeric behavior of the sample and, it is similar to the young’s modulus which predicts the stiffness behavior of the material. The storage modulus seems to be lower for r-ABS, it could be cross verified with the TGA graph as the thermal stability of r-ABS also seems to be lower than all other polymers and blends. The effect of temperature on the storage modulus of all the samples starts showing after 90°C. The plastic soften immediately after 90°C and the molecular motions of the polymeric blend increases beyond this temperature.
At 130°C, the recycled polymers and its blends completely loses its storage modulus which means that, the polymer does not withstand any stress after this temperature. The recycled polymers consists of polymeric chain segments of different lengths, wherein the shorter chains initially starts moving as the temperature is increased. The whole polymeric molecule starts vibrating only when it achieves the glass transition temperature or above. It was noted that the maximum stress of r-ABS is 1200 MPa, whereas for r-PS and r-HIPs is 1300 MPa and 1500 MPa respectively. The maximum stress absorbed was highest for r-HIPS, may be due to the presence of polybutadiene phase present in them, and this was also evidenced by mechanical properties of r-HIPS. The thermal stability of r-PS was higher as compared to other polymers, the reason for higher thermal stability may be due to its structural configuration which was also shown in the TGA graph of r-PS. The storage modulus of the blends K-4 and K-4/r-PS-3 lie between the thermogram of r-HIPS and r-PS which is well supported by TGA analysis as reported in the earlier section.
The glass transition temperatures obtained from tan delta curve of the recycled samples are represented in Table S4. The Tg of r-ABS is 118°C, r-HIPS is 115°C, and that of K-4 blend is 120°C, representing same trend as mentioned in DSC results with higher value as compared to the DSC results. Similar results also obtained by the experimental work done by Brennan et al. has performed the DMTA on ABS and HIPS and the Tg of ABS and HIPS recorded were 94°C and 104°C [17]. It was observed that the glass transition temperature obtained from DMA is higher than the Tg observed in DSC. Similarily, Cella et al. has also reported about the increase in Tg of polystyrene in DMA as compared to DSC in his work [36]. This may be due to the dynamic mechanical load on the samples under thermal heating condition, wherein DSC experience only thermal transition under nitrogen atmosphere.
Morphological analysis of binary and ternary blends
The impact fractured morphology of K-4 and K-4/r-PS-3 blend was imaged using scanning electron microscopy (SEM) technique. The samples were etched using potassium chromate for 30 to 60 min, in order to etch the butadiene content present in the surface. Figure 9 shows the SEM image of the blend K-4, and the etched surface clearly shows that the butadiene content was well dispersed throughout the polymer surface. The good distribution of PB phase was evidenced by the presence of voids on all over the polymer surface. The etched PB phase etched out was not regular in its size, which implies that the domain size is larger leading to low interfacial adhesion between the polymeric phases. Denise et al. had reported that the impact strength of ABS and HIPS is dependent on the shape and size of the domains which should be in the range of 0.3 to 3 µm and spherical in shape [23]. These domain sizes and shapes were observed in virgin polymers. In our case, the majorities of PB domains were of diameter greater than 3 µm and are mostly large due to accumulation of the particles during recycling or elongated due to fact of incomplete blending of the dispersed phase. The elongated voids may also be due to the etching of PB phases which are closer to each other as shown in Fig. 9.
De Souza et al. had reported that the SEM image of ABS/HIPS 50:50 showed a coarse morphology, which ultimately decreased the mechanical property of the blend and it was also concluded in their work that the polymers ABS and HIPS did not make a homogenous blend [18]. But in the current study, SEM images indicate coarse structure with improved impact strength maintaining the tensile strength. The formation of bubbles was discussed by Arnold et al. and Taurino et al. representing the processing method and the presence of flame retardants for the formation of bubbles [28, 32]. In our case, the blends were processed with the help of a batch mixer initially, and it is grinded and further processed in a micro injection moulding, hence the bubbles were avoided in our material. In general nodules are formed as a sign of incompatibility and it was observed in ABS/HIPS blends experimented by Denise et al. [23]. As observed in the morphology of the binary blends, the ternary blend also doesn’t show any nodules in its surface, this again evidences the compatibility effect between r-ABS, r-HIPS and r-PS. Didier et al. had worked on impurities of PS and ABS, on HIPS, and they observed that there were small nodules of PS in HIPS of diameter less than 1 µm and also stated that PS is compatible with HIPS [29]. ABS started forming nodules with a diameter ranging 1–3 µm and this shows the lack of miscibility of ABS with HIPS, the partial miscibility of ABS with HIPS was also observed by Brennan et al. [17]. Jaidev et al. had mentioned the formation of nodules or pebbles in their work where they blended r-PVC with r-HIPS and the same was not formed when r-PVS was blended with r-ABS, which showed that the later forms a homogenous blend [13]. In our case as we do not observe any nodules in the ternary blends, which suggest that the blend formed is a homogenous blend with better compatibility to each other. The SEM image also indicates the absence of cracks on the fractured surface giving stronger justification towards our claim. The compatibility between the polymers is mainly dependent on the source of recovery of the polymers and its segregation methodology.
It was observed that some of the particles present in the etched area were SAN domains and they were surrounded by PB phase in PS matrix. It was discussed by Arnold et al. that on lower concentration of ABS, SAN domains tend to entrap the PB domains within them [28]. But, in our case it could be observed that some SAN domains appear in between the etched surfaces, which means that they were surrounded by PB phase which were, etched out in chromic acid solution. It was also discussed by Arnold et al., that in a blend of 25 wt % of ABS in HIPS, the domains were majorly composed of SAN [28]. Some PB phase which are in bond with SAN matrix are present inside these domains, and the PB phase which are outside these domains, are those in which the bonds between the SAN matrix were fragmented due to ageing or thermo-mechanical recycling. Didier et al. has observed some cracks in HIPS/ABS blends with minimum quantity of ABS, the same was not observed while blending the same quantity of PS in HIPS, which shows that PS has better miscibility than ABS [29]. In our case, we do not observe any cracks in the fractured surface and hence we can conclude that the polymers recovered from keyboards are compatible with each other. Yamila et al. had discussed that in their work, the blend ratio HIPS/ABS (80:20) shows a slight reduction in young’s modulus and this reduction in modulus value wasn’t expected [16]. It was explained that the additives and rubber particles were replaced during the mechanical recycling process, and it leads to encapsulation of additives by rubber particles, which increases the impact strength along with the elongation at break property thereby reducing the tensile strength. A similar increase in property was also observed in our blend ratio K-4 (r-ABS/r-HIPS 30:70) and it is due to the redistribution of rubbery phases during recycling and this phenomenon shows that the mechanical property is totally dependent on the rubber phase present in the material. Further, absence of sharp edges supports our claims on impact performance.
Figure 10 shows the SEM micrographs of the ternary blend examined at 20 µm resolutions. Some of the different fillers like calcium carbonate, silica, carbon black, talc and titanium di oxide are common in styrenic plastics, and these fillers have a typical particle shape. Yamila et al. had discussed that ABS has comparatively brittle behavior as compared to HIPS and this was evidenced by sharper fractured edges in its morphology [16]. PS is also a brittle polymer, but the morphology of K-4 and K-4/r-PS-3 blends shows the PB phase all over the polymer, representing better impact strength of the blended polymers.
Some of the researchers like Chen et al. and Piorkowska et al. had observed debonding of particles from the impact fractured surface in PS/ABS blend which leads to the formation of holes in the fractured surface which was not observed in the current study [27, 37]. Further the current study also represents salami structures at the etched surface which were more deepen due to the removal of PB phase. These salami structures were also observed by Yamila et al. [16]. The whole etched surface shows merkerel pattern when observed under higher magnification and the same was also claimed by Porkowska et al. in their blended systems of PS/HIPS and PS/PB/HIPS [37].
Conclusion
In the current work, we have recovered the polymers ABS, HIPS and PS from keyboard casings and sorted them according to their RIC codes. The individual polymers were initially analyzed under FTIR-ATR and mechanical analysis such as tensile and impact tests. The primary aim of our work was to improve the impact strength of the polymers recovered from similar products and hence a ternary blend was formulated with ABS, HIPS and PS. The ABS and HIPS were initially blended together in different ratios, and all the samples were analyzed for tensile and impact tests. It was observed that the blend ratio K-4 (r-ABS/r-HIPS; 30:70) is the optimized blend ratio as it has the highest impact strength of 42 J/m. Further, r-PS was added with K-4 blend in different ratios, and the samples from all the ratios were tested under impact and tensile analysis. The result of impact test showed that the blend K-4/r-PS-3 (30:70) has the highest impact strength of 66 J/m. The optimized binary and ternary blends were further subjected to thermal, morphological and flame analysis. The thermal analysis showed slight improvement in thermal stability after the addition of PS. A slight reduction in the tensile property of the blend K-4/r-PS-3 was observed. The morphology of the impact fractured blend showed that the PB phase was distributed all over the polymer, which evidences about the homogeneity and improvement in impact properties of the blends. Hence, the formulated ternary blend has higher impact strength and improved thermal stability as compared to the individual polymers. In general, PS is a hard and brittle material, and it was seen that the ternary blend with PS as the major phase has a brittle to ductile transition in its characteristics due to intercalation of PB phase. This study suggests the encouragement of product based recycling for better homogeneity of the blends and to increase the efficiency of recycling.
Data availability
The entire machine generated data for thermal and mechanical tests are available for future references.
References
Thakur S, Verma A, Sharma B et al (2018) Recent developments in recycling of polystyrene based plastics. Curr Opin Green Sustain Chem 13:32–38
Singh N, Hui D, Singh R et al (2017) Recycling of plastic solid waste: A state of art review and future applications. Compos Part B Eng 115:409–422
Rahimifard S, Coates G, Staikos T et al (2009) Barriers, drivers and challenges for sustainable product recovery and recycling. Int J Sustain Eng 2:80–90
Jung LB, Bartel TJ (1999) Computer take-back and recycling: An economic analysis for used consumer equipment. J Electron Manuf 9:67–77
Lin C, hsu, Wen L, Tsai Y mi, (2010) Applying decision-making tools to national e-waste recycling policy: An example of Analytic Hierarchy Process. Waste Manag 30:863–869
Schlummer M, Mäurer A, Leitner T, Spruzina W (2006) Report: Recycling of flame-retarded plastics from waste electric and electronic equipment (WEEE). Waste Manag Res 24:573–583
Stenvall E, Tostar S, Boldizar A et al (2013) An analysis of the composition and metal contamination of plastics from waste electrical and electronic equipment (WEEE). Waste Manag 33:915–922
Datta J, Kopczyńska P (2016) From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit Rev Environ Sci Technol 46:905–946
Hamad K, Kaseem M, Deri F (2012) Preparation and Characterization of Binary and Ternary Blends with Poly(Lactic Acid), Polystyrene, and Acrylonitrile-Butadiene-Styrene. J Biomater Nanobiotechnol 03:405–412
Grigore ME (2017) Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2:1–11
Vollmer I, Jenks MJF, Roelands MCP et al (2020) Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew Chemie - Int Ed 59:15402–15423
Scaffaro R, Botta L, Di Benedetto G (2012) Physical properties of virgin-recycled ABS blends: Effect of post-consumer content and of reprocessing cycles. Eur Polym J 48:637–648
Jaidev K, Suresh SS, Gohatre OK et al (2020) Development of recycled blends based on cables and wires with plastic cabinets: An effective solution for value addition of hazardous waste plastics. Waste Manag Res 38:312–321
Sarath P, Biswal M, Mohanty S, Nayak SK (2018) Effect of silicone rubber based impact modifier on mechanical and flammability properties of plastics recovered from waste mobile phones. J Clean Prod 171:209–219
Wang J, Li Y, Song J et al (2015) Recycling of acrylonitrile-butadiene-styrene (ABS) copolymers from waste electrical and electronic equipment (WEEE), through using an epoxy-based chain extender. Polym Degrad Stab 112:167–174
Vazquez YV, Barbosa SE (2016) Recycling of mixed plastic waste from electrical and electronic equipment. Added value by compatibilization Waste Manag 53:196–203
Brennan LB, Isaac DH, Arnold JC (2002) Recycling of Acrylonitrile – Butadiene – Styrene and High- Impact Polystyrene from Waste Computer Equipment. J Appl Polym Sci 86:572–578
De Souza AMC, Cucchiara MG, Ereio AV (2016) ABS/HIPS blends obtained from WEEE: Influence of processing conditions and composition. J Appl Polym Sci 133:1–7
Hirayama D, Saron C (2015) Characterisation of recycled acrylonitrile-butadiene-styrene and high-impact polystyrene from waste computer equipment in Brazil. Waste Manag Res 33:543–549
Vilaplana F, Ribes-Greus A, Karlsson S (2006) Degradation of recycled high-impact polystyrene. Simulation by reprocessing and thermo-oxidation. Polym Degrad Stab 91:2163–2170
Oksman K, Lindberg H, Holmgren A (1998) The nature and location of SEBS–MA compatibilizer in polyethylene–wood flour composites. J Appl Polym Sci 69:201–209
Munteanu SB, Vasile C (2005) Spectral and thermal characterization of styrene- butadiene copolymer with different architectures 7:3135–3148
Hirayama D, Saron C (2018) Morphologic and mechanical properties of blends from recycled acrylonitrile-butadiene-styrene and high-impact polystyrene. Polymer (Guildf) 135:271–278
Tarantili PA, Mitsakaki AN, Petoussi MA (2010) Processing and properties of engineering plastics recycled from waste electrical and electronic equipment (WEEE). Polym Degrad Stab 95:405–410
Tiganis BE, Burn LS, Davis P, Hill AJ (2002) Thermal degradation of acrylonitrile – butadiene – styrene ( ABS ) blends. 76:425–434.
Kolawole EG, Agboola MO (1982) Environmental degradation of some polymer blends. Blends of polystyrene with acrylonitrile butadiene styrene, poly(vinyl chloride), and polybutadiene and blends of polybutadiene with poly(vinyl chloride). J Appl Polym Sci 27:2317–2335
Chen B, Evans JRG (2011) Mechanical properties of polymer-blend nanocomposites with organoclays: Polystyrene/ABS and high impact polystyrene/ABS. J Polym Sci Part B Polym Phys 49:443–454
Arnold JC, Watson T, Alston S et al (2010) The use of FTIR mapping to assess phase distribution in mixed and recycled WEEE plastics. Polym Test 29:459–470
Perrin D, Mantaux O, Ienny P, et al (2016) Influence of impurities on the performances of HIPS recycled from Waste Electric and Electronic Equipment (WEEE). Waste Manag. 1–8.
Hamad K, Kaseem M, Deri F (2013) Recycling of waste from polymer materials: An overview of the recent works. Polym Degrad Stab 98:2801–2812
Hedenqvist MS, Gedde UW (1999) Parameters affecting the determination of transport kinetics data in highly swelling polymers above T g 40:2381–2393
Taurino R, Pozzi P, Zanasi T (2010) Facile characterization of polymer fractions from waste electrical and electronic equipment (WEEE) for mechanical recycling. Waste Manag 30:2601–2607
Kim JK, Kang CK (1995) Basic studies on recycling of ABS resin. Polym Plast Technol Eng 34:875–890
Siregar JP, Salit MS, Zaki M, Rahman A (2011) Thermogravimetric Analysis ( TGA ) and Differential Scanning Calometric ( DSC ) Analysis of Pineapple Leaf Fibre ( PALF ) Reinforced High Impact Polystyrene ( HIPS ) Composites. 19:161–170.
Suzuki M, Wilkie CA (1995) The thermal degradation of acrylonitrile-butadiene-styrene terpolymei as studied by TGA/FTIR. Polym Degrad Stab 47:217–221
Cella RF, Mumbach GD, Andrade KL et al (2018) Polystyrene recycling processes by dissolution in ethyl acetate 46208:1–7
Piorkowska E, Argon AS, Cohen RE (1993) Izod impact strength of polystyrene-based blends containing low molecular weight polybutadiene. Polymer (Guildf) 34:4435–4444
Acknowledgement
The authors sincerely acknowledge the financial assistance provided by Department of Science and Technology, Government of India for undertaking the study.
The authors also thank Mr. Omdeo K Gohatre and Dr. Sunil S Suresh for technical assistance.
Funding
The financial assistance was provided by Department of Science and Technology, Government of India under the grant number DST/TSG/WM/2015/466-G.
Author information
Authors and Affiliations
Contributions
The work has been equally contributed and reviewed by all the authors before submission to the journal.
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
K, J., Biswal, M., Mohanty, S. et al. Compatibility effect of r-ABS/r-HIPS/r-PS blend recovered from waste keyboard plastics: evaluation of mechanical, thermal and morphological performance. J Polym Res 28, 129 (2021). https://doi.org/10.1007/s10965-021-02481-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02481-6