Abstract
Comb-like polycarbonate superplasticizers (PCEs) are widely used as a cement additive, and obtaining high-performance PCEs has become a hot topic. PCEs are usually kind of random polymers obtained by free radical solution polymerization, and thus it is hard to clarify the relationship between the properties of PCE and its structure. In this work, the block copolymers PCEs with the clear structure were synthesized by the reversible addition-fragmentation chain transfer (RAFT) polymerization. The relationship between the structure of PCE and its dispersion properties in cement was systematically discussed based on the flowability data of the cement paste with PCEs and the adsorption data of PCEs on the cement particles. Effects of the monomer ratio and the molecular weight on the adsorption and dispersion ability of PCEs in cement paste were studied. The results showed that the adsorption capacity and dispersion properties of PCEs in cement paste are related to the structural parameters of the polymer molecules. PCEs exhibited the optimal dispersion properties when the ratio of the monomers was 6:1 and the molecular weight was no greater than PCE-8 (Mn = 53,450, Mw = 62,358).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid development of modern construction technology puts forward higher requirements on the performance of cement [1]. The most effective methods to improve the strength of the cement and enhance the final properties were the addition cement additives [2]. Compared with other cement additives, comb-like polycarbonate superplasticizers (PCEs) have better performance on water-reducing, slump maintenance ability and versatility of cement [3]. Therefore, PCEs have been more and more widely used in high-performance cement [4].
PCEs molecule is consist of a main chain with carboxyl groups and polyethylene oxide side chains, just like a comb. Their composition of monomer molar ratio, the type and number of side chains are flexibility factors [5,6,7]. Therefore, the chemical structure of PCEs could be redesigned or modified to meet the performance requirements of different cements so that the cement-based materials can obtain favorable properties [4, 8]. The efficiency of PCEs is largely dependent on their molecular structure [9]. It is still one of the hot topics to research the relationship between the PCEs structure and the performance of PCEs in cement-based materials [10, 11]. However, PCEs are generally synthesized by the free radical aqueous solution polymerization method and thus they are random copolymer, in which the monomers are randomly arranged in the molecular structure. Even with the same monomers and polymerization initiator, PCEs copolymer with a clear molecular structure cannot be obtained by conventional synthetic techniques. Therefore, such random structures of copolymers make it difficult to specifically adjust the PCE molecular structure to obtain better dispersion properties.
Controlled free-radical polymerization is a mature technology which has been extensively studied [12], such as reversible addition-fragmentation chain transfer (RAFT) polymerization and atomic transfer radical (ATRP) polymerization, etc. Controlled free-radical polymerization could be used to prepare well-defined block copolymers with controlled molecular weight, definite molecular structure and narrow molecular weight distribution [13, 14]. Compared with other controlled free-radical polymerization methods, RAFT polymerization has a wide range of monomer adaptability [15, 16]. Many monomers such as acrylate, styrene and vinyl monomers can be applied in this kind of polymerization [17]. RAFT polymerization provide an excellent opportunity for the development of PCEs with clear structures [12]. Some researchers pay attention to the problems in the structure of PCEs and provide ideas for further research on high-performance PCEs [16, 18]. For example, Ezzat et. al. [16] synthesized polymer with controlled properties by RAFT polymerization and studied the effect of the charge type and side-chain length of the polymer on the adsorption, rheology and dispersion capabilities of the cement paste. Yu et. al [18]. synthesized block polycarboxylate superplasticizers as cement additive through RAFT polymerization and studied the effect of monomer mole ratio on polymer properties. However, in these reports, PCEs were just synthesized by RAFT polymerization but not purified. More over the relationship between polymer molecular weight and performance has not been further studied.
In this work, isoprenyl oxy polyethylene glycol ether (TPEG, with Mw = 2400) and monomer acrylic acid (AA) were used to synthesize block PCEs by RAFT polymerization. By gradually adding reaction monomers, separating and purifying the products of each step, PCEs with clear molecular structure are finally obtained. The relationship between PCEs molecular structure and their performance in cement was established by studying the influence of monomer ratio and molecular weight on PCEs dispersion performance, which is conducive to the synthesis of high-performance PCEs molecules. Such comb-like polymers exhibit the optimal dispersion property when the ratio of n(AA)/n(TPEG) was 6:1 and the molecular weight was no greater than PCE-8 (Mn = 53,450, Mw = 62,358).
Materials and methods
Materials
The cement used in the experiments was 42.5-grade ordinary cement supplied by Shandong Shanshui Cement Group Co., Ltd. Its chemical and mineral compositions were shown in Table 1. TPEG was obtained from Shandong Zhuoxing Chemical Co., Ltd. AA, analytical grade, was purchased from Tianjin Damao Chemical Reagent Factory. The initiator 2,2-azobisisobutyronitrile (AIBN) was supplied by Shanghai Aladdin Reagent, and it was recrystallized twice from methanol before use. The 2-dodecylsculfanyl carbothioyl-sulfanyl-propanoic acid was prepared in the laboratory as a chain transfer agent (CTA) [19].
RAFT polymerization of block type PCEs
In this study, AA was used as monomer A and polyether macromonomer TPEG was used as monomer B. The AB-type block PCEs were synthesized by RAFT polymerization. Firstly, n-propanol as solvent was added in a glass flask with a thermometer and magnetic stirrer. Monomer TPEG, CTA, and initiator AIBN were added to the glass flask according to the proportion. Then glass three-way piston with a balloon was mounted on the glass reaction instrument, and the glass tee was connected to the vacuum pump. Then the vacuum pump was started and the glass tee was adjusted to create a vacuum in the glass flask. The glass flask with internal reactants has been frozen to remove as much oxygen as possible. The reaction mixture was subsequently degassed by three freeze–vacuum–thaw cycles. Finally, the mixture was stirred in a 70 °C water bath for 24 h. Then, reaction products were precipitated by cold petroleum ether (PET). The reaction products were dialyzed with a dialysis bag with interception of molecular weight 10,000 for 48 h, and then the solution in the dialysis bag was freeze-dried to obtain the macromolecular chain transfer agent (PTPEG).
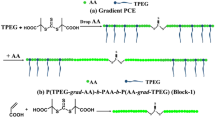
The PTPEG can adjust the polymerization of the monomer. The AA, PTPEG and AIBN were added into solvent n-propanol according to the proportion and the reaction device was operated in the same freezing vacuum as the above. The reaction mixture was reacted at the same way and was precipitated by PET to obtain block polymers. The PCEs dialyzed with a dialysis bag with interception of molecular weight 20,000 for 48 h, and then the solution in the dialysis bag was freeze-dried to obtain PCEs. The synthetic route is described in Fig. 1.
To investigate the effects of the monomer ratio and molecular weight on the dispersion property of cement, two routes were designed as follows: (1) PCEs were synthesized with equivalent molecular weight but different monomer ratio, namely PCE-1 to PCE-6;(2) PCEs were synthesized with same monomer ratio but different molecular weight, namely PCE-7, PCE-8, and PCE-9, and all the experiments in this study were shown in Table 2.
Measurement
Fourier transform infrared spectroscopy (FTIR)
The absorption spectra of the samples were obtained by FTIR spectrometer (Bruker Vertex 70). The test samples were prepared as potassium bromide (KBr) pellets and the spectra of samples were recorded at a resolution of 4 cm−1.
1H nuclear magnetic resonance spectroscopy (1HNMR)
1HNMR spectra of PCEs were obtained by an Avance III 400 MHz NMR spectrometer (Bruker, Faellanden, Switzerland) in deuterium hydrogen oxide at room temperature.
Gel permeation chromatography
The PCEs were characterized by PL-GPC50 gel chromatography, and the molecular mass was measured. The PCEs samples were analyzed with water as the mobile phase at a flow rate of 1 mL/min. The concentration of the sample solution is 5 mg/ml, and the sample solution is filtered with a 0.45 µm filter membrane.
Dispersion of cement paste
The fluidity of cement paste represents the dispersion ability of PCEs on cement paste. The cement paste fluidity was measured according to Chinese standard GB/T8077-2012 “Methods for Testing Uniformity of Concrete Admixture” by the Standardization Administration of the People’s Republic of China. The cement pastes with the water-cement ratio (w/c) of 0.5 were prepared at 20 ± 1 °C. The PCEs were added into the water with the range from 0.16% to 0.23% (weight percent of cement). When the fresh cement pastes were prepared, they were immediately poured into a mini-slump cone (the height of 60 mm, the upper diameter of 36 mm, and the bottom diameter of 60 mm). Then the cone was vertically removed, and the average spread diameter of the pastes was measured. Each cement sample with PCEs was tested three times, and the average was calculated as the final fluidity value.
Adsorption amounts of PCEs
The adsorption capacity of PCEs molecular on cement pastes was determined by the depletion method. Different amounts of dispersant samples were added to cement (w/c = 0.57). Cement paste was centrifuged at 8500 rpm for 7 min to obtain the liquid supernatant. Then liquid supernatant was filtered with a 0.45 μm membrane. Organic carbon content was measured by the total organic carbon (TOC, Shimadzu TOC-5000A, Japan). The adsorption amount of PCEs on the surface of cement can be calculated based on the change of PCEs amount in the aqueous phase before and after mix cement.
Mortar tests
The effects of PCEs on mechanical properties of cement were tested according to Chinese Standard (GB/T 17,671–1999). The mortar specimens were prepared with Cement: Sand = 1:3 and the amount of PCEs was 0.16% of cement. The mortar specimens were used to adjust water consumption to maintain the mortar flow at 180 ± 5 mm. The mortar specimens were molded at 40 mm × 40 mm × 160 mm. The flexural and compressive strength of specimens were measured on of 3 days, 7 days, and 28 days by the electric folding tester (KZJ-500) and compression-testing machine (WHY-2000). A group with three samples was tested. Finally, averages of flexural and compressive strength were calculated.
Results and discussions
Structure characterization of PCEs
Figure 2 shows the FTIR spectra of TPEG, AA and PCE-7, which were analysed according to reported literatures [5, 8, 12].
In the spectrum of AA, two recognizable peaks at 1726 cm−1 and 1633 cm−1 were attributed to a carbonyl group (-C = O) and unsaturated double bond (-C = C-). In the spectrum of TPEG, the stretching vibrations of ether linkage (-C–O–C-) were evidenced by the peak at 1101 cm−1. As seen in Fig. 2, the spectrum of PCE-7 showed that PCE-7 contained carboxyl groups such as ether group -C–O–C- and carboxyl group –C = O. Moreover, the absorption peaks of the unsaturated double bond disappeared in the spectrum of PCE-7, which further confirmed the successful preparation of PCEs by RAFT polymerization. Besides other PCEs synthesis steps are consistent with PCE-7, so the spectra of FTIR verify the introduction of characteristic groups into the macromolecules, which was in accordance with the expected structure.
As shown in Fig. 3, the presence of peak at 2.21 ppm in PTPEG indicates that the monomer TPEG has been successfully synthesized with CTA. And the peak at 2.29 ppm in PCE-7 indicates that the monomer AA has been successfully introduced into PTPEG molecule during the polymerization process.
In order to investigate the effect of the molecular weight on the performance of the product in cement, four products (PCE-4, PCE-7, PCE-8, and PCE-9) with different molecular weights were designed and synthesized. The designed molecular weights (d-Mn) were listed in Table 3. According to the molecular formula of PCEs shown in Fig. 1, the product was polymerized from TPEG and AA with the ratio of n(AA)/n(TPEG) as 6:1. By controlling the feeding amount of TPEG and AA, PCEs with different molecular weights can be obtained. The real molecular weight (Mn) of the product was measured and listed in Table 3. Results showed that Mn of four products is consistent with d-Mn, indicating that the target product with definite structure can be successfully obtained by the designed synthetic route.
The molecular weight test results of PCE-4, PCE-7, PCE-8, and PCE-9 were shown in Table3. The polydispersity index represents the dispersion of the molecular weight of the polymer. The value is closer to 1, which means that the molecular weight distribution of the polymer is narrower. Polydispersity index is distributed between 1.05 and 1.35 that is numerically similar to the result reported by Ezzat et al. [16]. Figure 4 shows that the molecular weight distribution curves of PCE-4, PCE-7, PCE-8 and PCE-9 showed one peak which proved that the different molecular weight of PCEs were controlled.
Dispersion performance
In the early hydration of cement, the cement particles encapsulated a large number of water molecules so that water needs to be added to achieve the working ability. The high water consumption would weaken the final strength of cement [8]. When the cement was mixed with water, the aluminate mineral (C3A) firstly occurred hydration reaction and its surface was positively charged [20,21,22]. The surface of cement particles in the early stage of cement hydration also presented positive electricity. Thus, PCEs with carboxyl groups could be adsorbed on cement particles by electrostatic interactions [23, 24]. In addition, PCEs with carboxyl groups can chelate with Ca2+ in the surface of the silicate, which also help the polymers be adsorbed on the cement particles [25]. As shown in Fig. 5, when PCEs were added into cement paste, the main chain with carboxyl groups were absorbed on the surface of cement particles and the polyethylene oxide side chains break the flocculent structure of cement particles by the steric hindrance. Both the relative ratios of carboxyl groups to side chains and the molecular weight affect the dispersion ability of PCEs in cement. PCEs can disperse cement particles and release water molecules wrapped by cement particles to reduce the viscosity of cement paste, thus the cement paste with PCEs has better workability than that without PCEs with the same water-cement ratio [26].
The fluidity of cement paste represents the PCEs’ dispersion ability on cement particles. The obvious change of fluidity value was observed when PCEs were incorporated to cement pastes (w/c = 0.5) at a dosage of 0.23% (weight percent of PCEs content to cement). The experimental results of PCE-1 to PCE-6 are shown in Fig. 6.
Referring to the fluidity of cement paste with PCE-1 to PCE-6, the dispersion properties of PCEs with different monomer ratios were discussed. As the initial fluidity data are shown in Fig. 6, the fluidity of cement paste improved significantly when PCEs were introduced. The fluidity of the blank samples without PCEs was 185 mm and the sample with PCE-4 achieved the maximum value of fluidity at 295 mm.
In the monomer composition of PCE-1, PCE-2, PCE-3 and PCE-4, the ratio of AA/TPEG is gradually increasing. PCEs with more carboxyl groups is easier to be adsorbed on cement particles. With the increase of carboxyl ratio, the dispersibility of PCE-1, PCE-2, PCE-3 and PCE-4 to cement particles improved in turn. However when the ratio of AA monomer in PCEs molecular continuously increased, such as PCE-5 and PCE-6, the dispersibility of PCEs to cement particles decreased conversely, which may be due to the excessive number of carboxyl groups that would cause molecular chain curling [27]. The coiled molecular chain encapsulated the adsorption sites of PCEs, which caused the corresponding decrease of the polymer adsorption on the cement particle surface. Furthermore, the proportion of side chains providing spatial steric hindrance decreased when the proportion of AA monomer was too high, thus the dispersion property of PCE in cement particles was reduced. Therefore, there is an optimal ratio of monomers for the comb-like polymer molecule, which is the most important for dispersing cement particles[8]. The cement paste fluidity showed that PCE-4 with a monomer ratio of 6:1 exhibits a higher dispersion efficiency in cement compared with other PCEs. In addition to the ratio of PCEs carboxyl groups to side chains, the molecular weight of PCEs also has a great influence on the dispersion ability of PCEs on cement particles.
Then, the PCE-7, PCE-8 and PCE-9 with different molecular weights were synthesized by RAFT polymerization with the monomer ratio of 6:1. PCE-4, PCE-7, PCE-8 and PCE-9 were incorporated to cement pastes (w/c = 0.5), respectively, at a dosage of 0.16% which keep the fluidity of cement paste within a reasonable range. The initial fluidity results are shown Fig. 7.
In Fig. 7, the fluidity of cement paste with PCEs was higher than of the blank cement paste (185 mm). The initial fluidity value of PCE-8 was 295 mm and the fluidity values of PCE-4, PCE-7 and PCE-9 were lower correspondingly. With the increase of molecular weight, the fluidity of PCE-4, PCE-7 and PCE-8 was gradually increasing. PCE-8 had more carboxyl groups and side chains than PCE-7 and PCE-4, which is conducive to the adsorption of PCE-8 molecules on cement particles to disperse cement particles. When the PCEs molecular weight continues to increase to that of PCE-9, the fluidity of cement paste decreased to 275 mm. Although PCE-9 has a larger molecular weight and relatively more side chains. However, PCE-9 has long macromolecular chains which are prone to intertwine and wrap the carboxyl groups and further reduce the absorbable points. The long macromolecular chains can be adsorbed on different cement particles at the same time, which hindered the dispersion of cement and led to the agglomeration of cement particles [25], the dispersibility of PCE-9 for cement particles was descended. It could be inferred from these data that the monomer ratio and molecular weight of PCEs are largely responsible for the dispersibility difference of PCEs in cement.
Adsorption behaviors of cement
In PCEs-added cement, PCEs molecules can disperse cement particles. Firstly, the polymer molecules could be adsorbed on cement particles by carboxyl groups. The second, the side chains of polymer will disperse cement particles by steric hindrance effect. Adsorption behaviour of cement is one of the most effective measures to measure cement interaction [28]. The adsorption curves are shown in Fig. 8.
The adsorption curves of PCE-1 to PCE-6 (at a dosage of 0.23%, limited 120 min) in the cement paste (w/c = 0.57) are displayed in Fig. 8(a). The adsorption amounts on the cement particles with different PCEs increased with time until the stage of adsorption saturation. In Fig. 8(a), the maximum adsorption amount is PCE-4, which was contributed to that PCE-4 molecule carried more carboxyl groups than PCE-1, PCE-2 and PCE-3. Although PCE-5 and PCE-6 have more carboxyl groups than PCE-4, the effect of polymer chain entanglement shielding carboxyl groups is stronger than that of carboxyl groups anchoring cement particles. The effective adsorption sites of PCE-5 and PCE-6 on cement particles reduced [27]. The adsorption curves of PCE-4, PCE-7, PCE-8 and PCE-9 (at a dosage of 0.16%, limited 120 min) in the cement paste (w/c = 0.57) are displayed in Fig. 8 (b). The adsorption amounts increased with time until the stage of adsorption saturation. The PCE-8 was easily absorbed on cement particles, and the adsorption ability of PCE-7 and PCE-4 decreased sequentially. Compared with PCE -8 molecule, the content of carboxyl groups in the PCE-4 and PCE-7 molecules is reduced, which also reduces the effective adsorption of the PCE-4 and PCE-7 molecules on the cement particles. Compared with PCE-8, the adsorption amount of PCE-9 was reduced. PCE-9 molecule with long main chains move slowly and could adsorbed on the surface of many cement particles simultaneously, which resulted in agglomerating of cement particles [29]. It is also worth recalling that the trend of adsorption capacity of PCEs corresponds to that of cement fluidity data mentioned above, which is also consistent with other studies reported, such as Qian’s [8] study, pointing out that the dispersion effect of PCEs to cement particles is well connected to the amount of polymer that adsorbed on cement particles. These results show that the synthesized PCE-8 with proper monomer ratio and molecular weight can exhibit high working efficiency, verifying the contribution of regulating structural parameters to the improvement in flow performances of cement paste.
Mechanical properties
The strength of mortar is affected by the amount of water mixed to mortar. The great effect of dispersibility on mortar applied by PCEs contributed to the outstanding flexural and compressive strength of mortar products [11]. The flexural and compressive resistance of mortar specimens were tested, under the same initial fluidity and the results are shown in Table 4. The mortar specimens were prepared with PCEs at a dosage of 0.16%. The water consumption for mortar specimens was adjusted to achieve the mortar flow at 180 ± 5 mm.
In Table 4, the flexural and compressive strength of mortar specimens with PCEs was significantly higher than that of the blank mortar specimens without PCEs. When the same fluidity is achieved as that of the blank mortar specimens, the amount of water used for achieving the same fluidity reduced when PCEs were introduced into mortar specimens. Because the water-cement ratio of mortar mixed with PCEs decreased, the strength of hardened mortar at each age increased. In addition, cement mortar samples containing PCE-8 exhibited higher flexural and compressive strength than that of the other samples. It can be attributed to the more excellent dispersion ability of PCE-8 in cement compared with other PCEs.
Conclusions
In this work, PCEs with defined molecular structures were synthesized by RAFT polymerization. The effects of monomer ratio and molecule weight on the dispersion and adsorption properties of PCEs were obtained.
1. In terms of the monomer ratio of PCEs, when the monomer ratio of PCEs is lower than 6:1, the dispersion and adsorption properties of PCEs are increase with the increase of the monomer ratio. However, when the monomer ratio of PCEs is greater than 6:1, the synergistic effect of the adsorption of PCEs molecules on cement particles and the steric hindrance is reduced, resulting in the effect of dispersion property in cement particles was reduced.
2. In terms of molecular weight of PCEs, when the molecular weight is lower than PCE-8 (Mn = 53,450, Mw = 62,358), the dispersion property of PCEs is increase with the growth of the PCEs molecular weight. When the molecular weight of PCEs is greater than PCE-8, the adsorption and dispersion properties of PCEs decreased with the growth of PCEs molecular weight.
References
Ng S, Justnes H (2016) Influence of plasticizers on the rheology and early heat of hydration of blended cements with high content of fly ash. Cement Concr Compos 65:41–54. https://doi.org/10.1016/j.cemconcomp.2015.10.005
Plank J, Li H, Ilg M, Pickelmann J, Eisenreich W, Yao Y, Wang Z (2016) A microstructural analysis of isoprenol ether-based polycarboxylates and the impact of structural motifs on the dispersing effectiveness. Cem Concr Res 84:20–29. https://doi.org/10.1016/j.cemconres.2016.02.010
Ren Q, Zou H, Liang M, Wang Y, Wang J (2014) Preparation and characterization of amphoteric polycarboxylate and the hydration mechanism study used in portland cement. RSC Advances 4:44018–44025. https://doi.org/10.1039/C4RA05542J
Yamada K, Takahashi T, Hanehara S, Matsuhisa M (2000) Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem Concr Res 30:197–207. https://doi.org/10.1016/S0008-8846(99)00230-6
Li Y, Yang C, Zhang Y, Zheng J, Guo H, Lu M (2014) Study on dispersion, adsorption and flow retaining behaviors of cement mortars with TPEG-type polyether kind polycarboxylate superplasticizers. Constr Build Mater 64:324–332. https://doi.org/10.1016/j.conbuildmat.2014.04.050
Dalas F, Pourchet S, Nonat A, Rinaldi D, Sabio S, Mosquet M (2015) Fluidizing efficiency of comb-like superplasticizers: The effect of the anionic function, the side chain length and the grafting degree. Cem Concr Res 71:115–123. https://doi.org/10.1016/j.cemconres.2015.02.001
Wen X, Feng L, Hu d, Wang K, Zhang Z, (2019) Effect of side-chain length in polycarboxylic superplasticizer on the early-age performance of cement-based materials. Constr Build Mater 211:26–32. https://doi.org/10.1016/j.conbuildmat.2019.03.124
Qian S, Yao Y, Wang Z, Cui S, Liu X, Jiang H, Guo Z, Lai G, Xu Q, Guan J (2018) Synthesis, characterization and working mechanism of a novel polycarboxylate superplasticizer for concrete possessing reduced viscosity. Constr Build Mater 169:452–461. https://doi.org/10.1016/j.conbuildmat.2018.02.212
Ferrari L, Kaufmann J, Winnefeld F, Plank J (2010) Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J Colloid Interface Sci 347(1):15–24. https://doi.org/10.1016/j.jcis.2010.03.005
Stecher J, Plank J (2019) Novel concrete superplasticizers based on phosphate esters. Cem Concr Res 119:36–43. https://doi.org/10.1016/j.cemconres.2019.01.006
Lin X, Liao B, Zhang J, Li S, Huang J, Pang H (2019) Synthesis and characterization of high-performance cross-linked polycarboxylate superplasticizers. Constr Build Mater 210:162–171. https://doi.org/10.1016/j.conbuildmat.2019.03.185
Li G, Xu A, Geng B, Yang S, Wu G, Zhang S (2014) Synthesis and characterization of fluorinated diblock copolymer of 2,2,2-trifluoroethyl methacrylate and methyl methacrylate based on RAFT polymerzation. J Fluorine Chem 165:132–137. https://doi.org/10.1016/j.jfluchem.2014.06.029
Barsbay M, Güven O (2018) Nanostructuring of polymers by controlling of ionizing radiation-induced free radical polymerization, copolymerization, grafting and crosslinking by RAFT mechanism. Radiat Phys Chem 169:107816. https://doi.org/10.1016/j.radphyschem.2018.04.009
Parkatzidis K, Wang HS, Truong NP, Anastasaki A (2020) Recent Developments and Future Challenges in Controlled Radical Polymerization: A 2020 Update. Chem 6:1575–1588. https://doi.org/10.1016/j.chempr.2020.06.014
Abreu CMR, Fonseca AC, Rocha NMP, Guthrie JT, Serra AC, Coelho JFJ (2018) Poly(vinyl chloride): current status and future perspectives via reversible deactivation radical polymerization methods. Prog Polym Sci 87:34–69. https://doi.org/10.1016/j.progpolymsci.2018.06.007
Ezzat M, Xu X, El Cheikh K, Lesage K, Hoogenboom R, De Schutter G (2019) Structure-property relationships for polycarboxylate ether superplasticizers by means of RAFT polymerization. J Colloid Interface Sci 553:788–797. https://doi.org/10.1016/j.jcis.2019.06.088
Pafiti K, Patrickios C, Abetz C, Abetz V (2013) High-Molecular-Weight Symmetrical Multiblock Copolymers: Synthesis by RAFT Polymerization and Characterization. J Polym Sci, Part A: Polym Chem 51:4957–4965. https://doi.org/10.1002/pola.26936
Yu B, Zeng Z, Ren Q, Chen Y, Liang M, Zou H (2016) Study on the performance of polycarboxylate-based superplasticizers synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization. J Mol Struct 1120:171–179. https://doi.org/10.1016/j.molstruc.2016.05.035
Mi X, Zhang X, Ding M, Zhang M, Pei M (2020) Structure and properties of polycarboxylic acid dispersants synthesized by RAFT method. Polym Adv Technol 1–9. https://doi.org/10.1002/pat.5160
Dalas F, Pourchet S, Rinaldi D, Nonat A, Sabio S, Mosquet M (2015) Modification of the rate of formation and surface area of ettringite by polycarboxylate ether superplasticizers during early C3A–CaSO4 hydration. Cem Concr Res 69:105–113. https://doi.org/10.1016/j.cemconres.2014.12.007
Plank J, Dai Z, Zouaoui N (2008) Novel hybrid materials obtained by intercalation of organic comb polymers into Ca–Al–LDH. J Phys Chem Solids 69:1048–1051. https://doi.org/10.1016/j.jpcs.2007.10.042
Li PP, Yu QL, Brouwers HJH (2017) Effect of PCE-type superplasticizer on early-age behaviour of ultra-high performance concrete (UHPC). Constr Build Mater 153:740–750. https://doi.org/10.1016/j.conbuildmat.2017.07.145
Plank J, Sachsenhauser B (2009) Experimental determination of the effective anionic charge density of polycarboxylate superplasticizers in cement pore solution. Cem Concr Res 39:1–5. https://doi.org/10.1016/j.cemconres.2008.09.001
Zhang Y, Kong X, Lu Z, Lu Z, Hou S (2015) Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem Concr Res 67:184–196. https://doi.org/10.1016/j.cemconres.2014.10.004
Stecher J, Plank J (2020) Adsorbed layer thickness of polycarboxylate and polyphosphate superplasticizers on polystyrene nanoparticles measured via dynamic light scattering. J Colloid Interface Sci 562:204–212. https://doi.org/10.1016/j.jcis.2019.11.108
Plank J, Sakai E, Miao CW, Yu C, Hong JX (2015) Chemical admixtures — Chemistry, applications and their impact on concrete microstructure and durability. Cem Concr Res 78:81–99. https://doi.org/10.1016/j.cemconres.2015.05.016
Sha S, Wang M, Shi C, Xiao Y (2020) Influence of the structures of polycarboxylate superplasticizer on its performance in cement-based materials-A review. Constr Build Mater 233:117257. https://doi.org/10.1016/j.conbuildmat.2019.117257
Tan H, Zou F, Ma B, Liu M, Li X, Jian S (2016) Effect of sodium tripolyphosphate on adsorbing behavior of polycarboxylate superplasticizer. Constr Build Mater 126:617–623. https://doi.org/10.1016/j.conbuildmat.2016.09.077
Kong F, Pan L, Wang C, Zhang D, Xu N (2016) Effects of polycarboxylate superplasticizers with different molecular structure on the hydration behavior of cement paste. Constr Build Mater 105:545–553. https://doi.org/10.1016/j.conbuildmat.2015.12.178
Funding
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant NO 51672106 and 51778269), and the Key Research and Development Program of Shandong Province (2019GSF110002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, M., Zhang, X., Mi, X. et al. Preparation and dispersion properties of polycarbonate superplasticizers based on RAFT polymerization. J Polym Res 28, 105 (2021). https://doi.org/10.1007/s10965-021-02464-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02464-7