Abstract
In the present study, the influence of electrospinning parameters on poly(lactic acid) (PLA) nanofiber production and antimicrobial attitude of drug blending were investigated. The PLA concentrations (5 to 12% wt/v) were determined with solvent ratio variations of a triple solvent system (chloroform (CHL), dimethylformamide (DMF) and tetrahydrofuran (THF)). Flow rates were varied from 0.5 to 1 mL/h, while voltages were changed from 10 to 15 kV, and 10 kV voltage and 0.5 mL/h flow rate were selected as optimum electrospinning conditions. According to morphological investigations via scanning electron microscopy, the average PLA fiber diameters varied from 303 to 405 nm, for 5 and 8% wt/v PLA concentrations, respectively. The drug (ceftriaxone disodium) was blended into these concentrations and electrospun. Drug addition reduced the fiber diameters, and at 8% wt/v drug blended PLA concentrations homogeneous fiber distribution was obtained. Additionally, the antimicrobial attitude of drug blended PLA nanofibers was analyzed by using agar disc diffusion method and antimicrobial activity against Escherichia coli, and Listeria monocytogenes were observed.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, concern on achieving fibers ranging from micro to nanometer diameters by using the electrospinning method has been increased rapidly. It is one of the electrohydrodynamic methods with low cost, high production rate, extensive material applicability, and constant fiber quality [1,2,3]. Owing to this technique, a polymer solution electrospun into nanofiber that can be applied in the biomedical field like; medical prosthesis, drug release, wound dressing, and tissue scaffold [2,3,4,5,6]. Especially, for drug delivery applications two important circumstances as; high surface-to-volume ratio and high drug loading capacity of fibers can be obtained with electrospinning. Most of the studies focused on both arranging and understanding the delivery attitudes of the various drugs from antibiotics to anti-cancers inserted into numerous fibers. Also, the determination of antimicrobial behavior of these drug-loaded fibers is significant to prevent diseases caused by microorganisms/bacteria. Bacteria disease can be treated with using antibiotics but, types of antibiotics or application method selection are some of the crucial targets for drug delivery systems. [7,8,9,10,11].
When an effective polymer antibiotic delivery system is designed with electrospinning, the determination of suitable polymer-drug-solvent systems, the selection of drug-loading techniques, and the optimization of the process parameters must be handled in detail [12,13,14]. In biomedical and biocompatible field, generally biodegradable polymers are preferred. Among all, poly(lactic acid) (PLA) synthesized from renewable sources can be selected, owing to its enhanced characteristics on biocompatibility, and biodegradability [3, 5, 6, 12,13,14,15,16].
There are various drug-loading methods like; surface modification, blending, emulsion and coaxial electrospinning, etc. providing different fiber structure formations and drug-release mechanisms. The blending is one of the essential methods depending on dissolving or dispersing of the drug directly with the polymer solution before the electrospinning process [13, 16, 17]. The similarity of physicochemical properties between polymer and drug is preferable to achieve a better dissolution system. Therefore, the hydrophobic behavior of the PLA provides better release mechanisms with hydrophobic drugs [13, 17,18,19]. However, when incongruity between drug and polymer is observed, lowering the loading doses of the drug can provide a good dissolution [13, 20]. Also, the solubility of the drug determines the performance of the drug dispersion; a poor drug solubility may cause non-uniform drug distribution, while, high solubility may vitiate the drug performance [20].
Additionally, electrospinning parameters must be optimized to obtain uniform continuous fibers for drug delivery systems. These parameters are held into three categories as the solution, the environment, and the production. [3, 21,22,23,24,25,26,27,28,29,30]. The influence of solution parameters on PLA fiber production was investigated in various studies by using single or binary solvent systems. For instance, Casasola et al. [31] and Jahangir et al. [32] dissolved PLA in both single and binary-solvent systems to understand the fiber formation behavior, and they used various solvent types by changing the ratios. They obtained thinner PLA nanofibers for acetone (AC)/dimethylformamide (DMF) (60/40 v/v) binary solvent systems. Herrero et al. [22] also studied on electrospinning of PLA, polycaprolactone (PCL), and PLA/PCL nanofibers by using chloroform/methanol and dichloromethane (DCM)/DMF binary solvent solutions. Their results revealed that both the surface tension and the PLA fiber diameter increased within DCM/DMF more than chloroform (CHL)/methanol (MeOH) binary solutions. Also, Huang et al. [33] prepared the PLA solution by using a mixed solvent of CHL and AC. Rong et al. [34] dissolved PLA by using DCM and DMF as a binary solvent system. In all these studies, PLA production was done by using single or binary solvent solution systems. For drug delivery applications, additional solvents can be used after fiber production [35]. To our knowledge, for drug delivery, triple solvent systems were not directly applied for electrospinning of PLA fibers at the early stages of production.
Despite the solution parameter effect, electrospinning process parameters also played a great influence on PLA fiber formation and structure. Taylor [36] determined that the critical value of voltage must be 6 kV. When the voltage value was smaller than this critical value, the fiber formation became difficult. While, at very high voltage applications, the stability of the polymer jet advancing to the collector surface may disrupt and it may induce bead formation due to the increment of solution surface tension or charge density [19, 25, 27, 28]. Another important parameter of electrospinning is the flow rate of the polymer jet. A fast flow rate may prevent the nanofiber jet completely drying, and this leads to an increase in the fiber diameter. On the other hand, a very slow flow rate may induce beads formation. Additionally, ribbon-like defects and unspun droplets may occur at high or low flow rates. Therefore, the flow rate of the electrospinning process must be optimized to achieve a uniform and beadless electrospun nanofibers [16, 29, 33,34,35].
A sufficient drug delivery system can be obtained by the production of defect-free, continuous, and narrow-distributed diameter fibers [13, 15]. Additionally, the selection of an adequate polymer-drug-solvent system enhances the performance of the drug. Most of the researches dealt with only one parameter of electrospinning: the solution or the process to achieve homogeneous PLA fiber. Moreover, few studies addressed the influence of two or three parameters together [3, 8, 11, 28, 29]. Therefore, in the present study, both solution and process parameters were investigated in detail to obtain proper PLA production conditions. Instead of single or binary solvent systems, the triple solvent system (CHL, DMF, and THF) was used, and different PLA concentrations were attained by changing the solvent ratios. The process parameters were determined by changing both the voltage and the flow rate. After optimizing the electrospinning parameters, the drug (ceftriaxone disodium) was blended into PLA polymer solutions and electrospun. Additionally, antimicrobial analyses were done by using Escherichia coli, Bacillus cereus, Listeria monocytogenes, and Salmonella typhimurium bacteria to control the potential bacterial infections at the site of surgical/scaffold implant.
Materials and methods
Materials
Poly (lactic acid) (PLA 4060D, pellet shape) was obtained from Nature Works LLC. All the solvents were purchased from Merck and used without further purification. They were; chloroform (CHL, MA = 119.38 g/mol), N, N dimethylformamide (DMF, MA = 73.09 g/mol) and tetrahydrofuran (THF, MA = 72.11 g/mol). These solvents were selected due to their similar solubility parameters to PLA. Ceftriaxone disodium (C18H16N8 Na2O7S33.5H2O, MA = 661.60 g/mol, CAS 104376–79-6) salt is a Hemi (heptahydrate) - third-generation on cephalosporin antibiotic supplied from Sigma-Aldrich. It is soluble in water and various solvents from acetone to formamide [19].
Preparation and electrospinning of PLA nanofibers
The electrospinning process was carried out at 5, 8, and 12% wt/v PLA polymer concentrations by using triple solvent systems at different ratios. Firstly, PLA particles were dissolved in CHL, and then DMF and THF were added to the solution and stirred for 40 min. The amount of both PLA and CHL was kept constant, and the polymer concentrations were determined among the solvent ratio variations. The solvent ratios of DMF, THF and CHL were changed as: 2:2:1 v/v for 5% wt/v, 1:1:1 v/v for 8% wt/v and 0.5:0.5:1 v/v for 12% wt/v PLA polymer concentrations, respectively. Electrospinning processes studies were carried out at different voltages and flow rates. The voltages were changed from 10 to 15 kV, while the flow rates were varied from 0.5 to 1 mL/h for production of PLA nanofibers. All the fibers were collected on aluminum foil, and needle tip to collector distance was maintained as 15 cm. Ceftriaxone disodium (1% wt) was blended into PLA polymer solutions with 5 to 8% wt/v PLA concentrations without any surfactant addition and stirred for 50 min at room temperature. Drug blended PLA polymer solutions were electrospun at 10 kV voltage with a 0.5 mL/h flow rate. All electrospun fibers were dried under fume hood overnight to remove solvent residue before the tests and characterization.
Characterization
Morphological structures and fiber diameters of the produced fibers were investigated with scanning electron microscopy (SEM) (JSM 6335F - JEOL and JSM 6510LV – JEOL, Japan). 2 cm × 2 cm large aluminum foils were cut and stuck onto conductive carbon tapes. The surface of the samples was coated with platinum by a sputter coater (Polaron SC7640, Quorum Technologies, UK). The investigations were performed at 10 kV accelerating voltage. The fiber diameters and their size distributions were determined by using both the JEOL and Image J (U. S. National Institutes of Health, Bethesda, Maryland, USA) software.
The physical parameters of the polymer solutions such as viscosity and surface tension were measured by using a viscometer (DV-E, Brookfield AMETEK, USA), force tensiometer (Sigma 703D, Attension, Germany). All the measurements were repeated four times at ambient temperature (25 °C). All pieces of the pieces of equipment were calibrated prior to the measurements.
Antimicrobial analyzes
Agar disc diffusion assay test was used to evaluate antimicrobial activity. Media was prepared using nutrient agar poured into petri dishes and inoculated with bacteria from the broth using cotton swabs at 35 °C for 48 h. Sterile paper discs impregnated with the 30 μl volume of samples were placed into agar plate with a particular bacterium. After the incubated at 35 °C and 24 h, the inhibition zone around the discs was measured by using Image J software. In the experiments, Escherichia coli, Bacillus cereus, Listeria monocytogenes, and Salmonella typhimurium bacterial strains were used.
Statistical analysis
All the statistical analyses of the data were performed through ANOVA by using the GraphPad Prism version 8 software (GraphPad Software Inc., San Diego, CA, USA). The values were given as; means ± standard deviation (SD) and the statistical differences among them were analyzed by one-way ANOVA and Tukey’s multiple comparisons tests. In all cases, a value of P < 0.05 was considered statically significant.
Results and discussion
Effect of polymer concentration
Fiber diameter with a narrow size distribution, fiber uniformity, and continuity are some of the crucial factors required to obtain a successful drug delivery system. To achieve these targets, determination of the electrospinning conditions regarding the polymer type and the solvent systems are necessary [6, 17, 37].
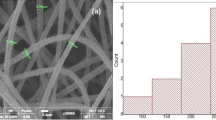
To figure out the effect of solution parameters on the fiber formation, PLA polymer solutions electrospun at 10 kV and 0.5 mL/h voltage and flow rate values, respectively. The PLA concentrations adjusted as 5, 8, and 12% wt/v by changing the ratios of triple solvent components (Table 1). In Fig. 1. the effect of PLA polymer concentration variations on morphologies, fiber diameters size with their distributions are given. The morphological features of the PLA fibers revealed that bead formation did not occur, and defect-free fibers were observed for all PLA solution concentrations. However, fiber diameters showed disparities and the average fiber diameters were measured as: 303.00 ± 69.74 nm for S1, 405.20 ± 125.60 nm for S2 and 589.40 ± 167.30 nm for S3 concentrations. The fiber diameter increased with the PLA concentration increment similar to Herrero et al. [38] and Huang et al. [33] results. These authors pointed out that the nanofiber diameter variations were related to the solution jet size and the polymer content in the jet. When the jet moved from a syringe to the metal collector, it may or may not let split to form fiber diameter differences. Even the split formation does not occur; one of the crucial parameters on fiber diameter will be the solution viscosity [14].
The solution viscosity values of the PLA nanofibers are presented in Table 1. The increment of PLA concentrations leads to an increase in solution viscosities from 94.40 ± 6.90 to 114.50 ± 8.60 mPa.s. Generally, an increment in the solution viscosity leads to an enhancement of fiber diameter [5, 39]. Also, Herrero et al. [38] and Huang et al. [33] demonstrated that the viscosity of a solution obtained by dissolving a solid polymer in a solvent is proportional to the polymer concentration, and high polymer concentration enlarges the diameter of the fiber. Our obtained results showed resemblance, and as the polymer concentration increase, the fiber diameter increases.
Additionally, the physical properties of the solvents such as; boiling point, viscosity, and surface tension effects not only the electrospinnability of the polymer and but also influences the application areas of the fibers. Casasola et al. [31] exhibited that chlorinated or fluorinated solvents are the commonly used solvent systems for PLA dissolution, and they emphasize using a less toxic solvent system is critical for medical applications especially, for tissue engineering issues. In the present study, PLA was dissolved in a triple solvent system known to have low toxicity. The boiling points of CHL, THF, and DMF are; 61, 66, and 153 °C, respectively [13]. Both CHL and THF have low boiling points, and during electrospinning, they can evaporate rapidly and may induce droplet formation or porous fiber structure occurrence. To prevent the occurrence of these types of failures, due to its high boiling point DMF, was added into the solution. Also, ceftriaxone disodium is less soluble in DMF [40]. The boiling point of the solvent influences not only the fiber formation but also fiber diameter. Wannatong et al. [41] found out that the fiber diameter decreases exponentially with the increase of the boiling point of the solvent. Additionally, when the ratio of DMF was decreased from 2 to 0.5 v/v, the PLA fiber diameters increased. The reason for this increment can be explained as; the solvent with a high boiling point evaporates slowly, and during the formation of the jet, the viscoelastic properties may cause a stretch by lowering the fiber diameter [7, 31]. The other crucial parameter influencing both the morphology and diameter of the PLA nanofibers is surface tension [31, 42].
In Table 1, the surface tension values increased slightly with the increment of polymer concentration. High surface tension may lead to an unstable jet formation or bead occurrence [31]. Unlike Fong et al. [42], surface tension increase enlarged the fiber diameters without bead formation. It is hard to generalize surface tension and the fiber morphology variations. However, it can be said that type of solvent determines the surface tension behavior [31].
In Fig. 1, the PLA fibers electrospun with different polymer concentrations showed cylindrical fiber formations. Additionally, both the average diameters and the standard deviation of the fibers increased with the increment of the PLA concentrations. To achieve a sufficient drug delivery system, a narrow size distribution of the fiber with a large specific surface area is preferable [13, 40, 43, 44]. Németh et al. [43] calculated the specific surface area (SSA) of the cylindrical fibers by measuring fiber diameters. They found out that the fiber diameter increase reduces the SSA value regarding the polydispersity index (PDI). We settled our results according to this approach, and we found that the SSA values of S1 and S2 PLA fibers were higher than S3. Therefore, further studies were carried out with S1 and S2 coded PLA polymer concentrations.
Effect of electrospinning process parameters
In the electrospinning process, a stabilized Taylor cone can be obtained by adjustment of flow rate and voltage. Generally, low flow rate values provide convenience to achieve a stable jet cone [29, 33]. However, Zargham et al. [45] emphasized that a low flow rate may induce small size droplet formation, and the jet may reduce owing to the drawing capability of electric field strength. A nonstable jet called receded jet can be formed inside the needle without appearing a droplet or cone. These receded jets can be incessantly replaced by cone, and large-sized nanofibers can be formed during the electrospinning. On the other hand, at high flow rates, aggregated fluid, and unspun droplets can occur. Moreover, the fibers on the collector can be taken without sufficient solvent evaporation that may induce defect formation. The studies are carried out by altering both the flow rate and the voltage values by keeping the capillary-collector distance constant. Table 2 and Fig. 2 exhibit the SEM images of PLA nanofibers electrospun at different flow rates as 0.5, and 1 mL/h, and voltages as 10, and 15 kV.
The defects like a bead or unspun droplets are not observed, and fibers formed like cylindrical shapes for all electrospinning conditions. The applied flow rate may influence the shapes of the initiating droplet and causes the fiber morphology variations. Also, the increment of the flow rate induces a decrease in the area density of the fibers.
In Table 2, the measured average diameters of the fibers concerning different flow rates, and PLA polymer concentrations, are given.
PLA fibers coded S1_A to S1_D, influenced from process parameter significantly (P < 0.05) higher than S2 coded fibers. When the voltages kept constant, and the flow rate varied from 0.5 to 1 mL/h, all the average fiber diameters increased. However, the increment of voltage from 10 to 15 kV induced a reduction in fiber diameters. The reason for this diameter decline can be explained as the increase of voltage may cause a stretch on polymer solution correlated with the charge repulsion in the polymer jet. According to Table 2 and Fig. 2, the lowest fiber diameter with small distribution was obtained for S1_C as 274.30 ± 54.63 nm. 8% wt/v PLA content fibers did not show a significant (P > 0.05) attitude with process parameters, and the average fiber diameters were measured around 400 nm for all conditions. To determine electrospinning process parameters, one-way ANOVA, and Tukey’s multiple comparisons tests were done (Fig. 3). The obtained results revealed that the significant variations were observed between S1_A vs. S1_B and S1_B vs. S1_C. Therefore, 10 kV and 0.5 mL/h, values were selected as the optimum electrospinning conditions.
Relation between average fiber diameters of PLA nanofibers electrospun at different process parameters. The differences were calculated by one-way ANOVA and Tukey’s multiple comparisons tests. Rel. Exp. indicates relative expression **P < 0.001, ***P < 0.0001; Error bars: Mean and SD of 100 measurements with duplicates for each measurement
Drug addition
Blending is one of the old drug delivery methods, and still the most preferred one, due to its low cost and easy application [46]. In this study, ceftriaxone disodium is selected as an antibiotic drug, and 1% wt drug was blended into 5 to 8% wt/v PLA concentration solutions without any surfactant addition. The physical behavior of drug blended PLA solution viscosities and surface tension values were measured and are given in Table 3. Both the viscosity and the surface tension values did not show significant (P > 0.05) variations with drug blended PLA solutions.
In Fig. 4, average fiber diameters of PLA and drug blended PLA nanofibers are given with their SEM images. Commonly, drug addition reduced the fiber diameter compared to non-drug fibers. Zeng et al. [19] obtained similar behavior about the diameter reduction, and they identify it with the decrease of surface tension. In the present study, surface tension showed not a direct reduction trend therefore it is hard to make comment on this diameter attitude.
In Fig. 5, SEM images, and fiber diameter distribution of the drug blended PLA nanofibers electrospun at different polymer concentrations are shown. The morphological investigation exhibited that, some undissolved drug particles were formed on the fiber surfaces of the drug blended 5% wt/v. PLA concentration (Fig. 5a). The reason for this particle occurrence may depend on the solubility and conformity of the drug in the drug-polymer-solvent system. Commonly, hydrophilic drugs encapsulated within hydrophilic polymers, while hydrophobic drugs showed better release performance in hydrophobic polymers [13]. PLA categorized in hydrophobic polymers, however, ceftriaxone disodium classified as a hydrophilic drug with the solubility order increasing from acetone to DMF [40]. During electrospinning, the solution jet elongated to form a fiber by removing the solvent immediately, and this may cause the remaining of the drug with PLA. Additionally, the amount of DMF was high in S1_D, and this may lead to an inadequate solution of the drug particles. However, at drug blended 8% wt/v. PLA concentration sufficient drug solubility was achieved without any drug particle occurrences (S2_D). Also, the relation between pure and drug blended PLA nanofibers were analyzed by one-way ANOVA and Tukey’s multiple comparisons tests (Fig. 6). S2 and S2_Drug fibers showed significantly (P < 0.05) higher dimeter variations than S1 and S1_Drug.
Relation between average fiber diameters of pure and drug blended PLA nanofibers. The differences were calculated by one-way ANOVA and Tukey’s multiple comparisons tests. Rel. Exp. indicates relative expression *P < 0.05, and **P < 0.001; Error bars: Mean and SD of 100 measurements with duplicates for each measurement
Antimicrobial activity
The antimicrobial performance of drug blended 8% wt/v PLA concentrations evaluated both qualitatively and quantitatively against model organisms by using the agar disc diffusion method. The antimicrobial test steps involved as; bacterial culture preparation, drug blended nanofibers connection with bacteria, and antimicrobial activity evaluation. The antimicrobial activities of non-drug and drug blended PLA nanofibers were investigated on Gram-positive; B. cereus and L. monocytogenes and Gram-negative; E. coli and S. typhi bacterial strains. Antimicrobial analyzes of the control group, PLA solvents, PLA and the drug blended PLA are shown in Fig. 7. Inhibition of microbial growth was not observed in the control group, solvents and PLA solution, whereas microbial inhibitions were observed for drug blended fibers. The inhibition zones were measured as: 28.33 ± 1.44, 7.84 ± 0.28, 23.16 ± 1.89, and 22.66 ± 0.76 mm, for E. coli, B. cereus, L. monocytogenes, and S. typhi, respectively.
In Fig. 8. Drug zone variations according to bacteria types are given. Drug blended PLA nanofibers showed antimicrobial activity against all of the bacteria. Especially, they had significantly (P < 0.0001) higher antimicrobial activity to Escherichia coli. The drug has a lethal effect by inhibiting cell wall synthesis in bacteria by binding to important target proteins used in the treatment of susceptible microorganisms infections.
Relation between Drug zone and drug blended PLA fibers treated with bacteria; Bacillus cereus, Salmonella Typhi, Listeria monocytogenes, and Escherichia coli and the agar plates. The differences were calculated by one-way ANOVA and Tukey’s multiple comparisons tests. Rel. Exp. indicates relative expression **P < 0.001, and***P < 0.0001; Error bars: Mean and SD of 4 measurements with duplicates for each measurement
Conclusion
This study exhibited the importance of electrospinning parameters on PLA fiber production for drug delivery. PLA nanofibers were produced by using the triple solvent system and the influence of both solution and production parameters were investigated in detail. PLA polymer concentrations were designated form 5 to 12% wt/v by solvent ratio variations of CHL, DMF and THF. The electrospinning processes parameters were investigated with small variations (the voltage from 10 to 15 kV; the flow rate from 0.5 and 1 mL/h). The optimum production conditions were achieved at 10 kV and 0.5 mL/h. At 5 and 8% wt/v PLA concentrations, the average nanofiber diameters were measured as 303.00 ± 69.74 and 405.20 ± 125.60 nm, respectively. Additionally, (1% wt) ceftriaxone disodium was blended into PLA solvents, and electrospun. The morphological investigations revealed that at 8% wt/v drug blended PLA concentrations, homogeneous fiber formation with good drug distribution was obtained. The antimicrobial attitude of 8% wt/v drug blended PLA nanofibers exhibited stronger antimicrobial activity against Escherichia coli.
References
Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X (2014) Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release 185:12–21
Chen C, Lv G, Pan C, Song M, Wu C, Guo D, Wang X, Chen B, Gu Z (2007) Poly(lactic acid) (PLA) based nanocomposites - a novel way of drug-releasing. Biomed Mater 2:L1–L4. https://doi.org/10.1088/1748-6041/2/4/L01
Bhardwaj N, Kundu SC (2010) Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 28:325–347
Song K, Wu Q, Qi Y, Kärki T (2017) Electrospun nanofibers with antimicrobial properties. In: Electrospun Nanofibers. Elsevier, pp 551–569.
Scaffaro R, Lopresti F, D’Arrigo M, Marino A, Nostro A (2018) Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl Microbiol Biotechnol 102:4171–4181. https://doi.org/10.1007/s00253-018-8879-7
Islam MS, Ang BC, Andriyana A, Afifi AM (2019) A review on fabrication of nanofibers via electrospinning and their applications. SN Appl Sci 1. https://doi.org/10.1007/s42452-019-1288-4
Li J, Feng X, Shi J, Liu T, Ding J (2018) Porous Polylactide film plus atorvastatin-loaded Thermogel as an efficient device for peritoneal adhesion prevention. ACS Omega 3:2715–2723. https://doi.org/10.1021/acsomega.8b00090
Wyrwa R, Otto K, Voigt S, Enkelmann A, Schnabelrauch M, Neubert T, Schneider G (2018) Electrospun mucosal wound dressings containing styptics for bleeding control. Mater Sci Eng C 93:419–428. https://doi.org/10.1016/j.msec.2018.07.066
Luo SH, Wu YC, Cao L, Wang QF, Chen SX, Hao ZF, Jing L, Wang ZY (2017) One-pot preparation of polylactic acid-ibuprofen conjugates and their performance characterization. Polym Chem 8:7009–7016. https://doi.org/10.1039/c7py01213f
Kenawy ER, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE (2002) Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release 81:57–64. https://doi.org/10.1016/S0168-3659(02)00041-X
Kenawy ER, Abdel-Hay FI, El-Newehy MH, Wnek GE (2009) Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater Chem Phys 113:296–302. https://doi.org/10.1016/j.matchemphys.2008.07.081
Pillay V, Dott C, Choonara YE, et al (2013) A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J Nanomater 2013:1–22. https://doi.org/10.1155/2013/789289
Zhang Q, Li Y, Lin ZYW et al (2017) Electrospun polymeric micro/nanofibrous scaffolds for long-term drug release and their biomedical applications. Drug Discov Today 22:1351–1366
Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63:2223–2253. https://doi.org/10.1016/S0266-3538(03)00178-7
Barkoula NM, Garkhail SK, Peijs T (2010) Effect of compounding and injection molding on the mechanical properties of flax fiber polypropylene composites. J Reinf Plast Compos 29:1366–1385. https://doi.org/10.1177/0731684409104465
Kaialy W, Emami P, Asare-Addo K, Shojaee S, Nokhodchi A (2014) Psyllium: a promising polymer for sustained release formulations in combination with HPMC polymers. Pharm Dev Technol 19:269–277. https://doi.org/10.3109/10837450.2013.775156
Bhattarai RS, Bachu RD, Boddu SHS, Bhaduri S (2019) Biomedical applications of electrospun nanofibers: drug and nanoparticle delivery. Pharmaceutics 11, 5:1–30. https://doi.org/10.3390/pharmaceutics11010005
Zeng J, Xu X, Chen X, Liang Q, Bian X, Yang L, Jing X (2003) Biodegradable electrospun fibers for drug delivery. J Control Release 92:227–231. https://doi.org/10.1016/S0168-3659(03)00372-9
Zeng J, Yang L, Liang Q, Zhang X, Guan H, Xu X, Chen X, Jing X (2005) Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J Control Release 105:43–51. https://doi.org/10.1016/j.jconrel.2005.02.024
Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M (2004) Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release 98:47–56. https://doi.org/10.1016/j.jconrel.2004.04.009
Haider A, Haider S, Kang IK (2018) A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J, Chem
Herrero-Herrero M, Gómez-Tejedor JA, Vallés-Lluch A (2018) PLA/PCL electrospun membranes of tailored fibres diameter as drug delivery systems. Eur Polym J 99:445–455. https://doi.org/10.1016/j.eurpolymj.2017.12.045
Sill TJ, von Recum HA (2008) Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006
Deitzel JM, Kosik W, McKnight SH et al (2001) Electrospinning of polymer nanofibers with specific surface chemistry. Polymer (Guildf) 43:1025–1029. https://doi.org/10.1016/S0032-3861(01)00594-8
Ramakrishna S, Mayer J, Wintermantel E, Leong KW (2001) Biomedical applications of polymer-composite materials: a review. Compos Sci Technol 61:1189–1224. https://doi.org/10.1016/S0266-3538(00)00241-4
Chong EJ, Phan TT, Lim IJ et al (2007) Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater 3:321–330. https://doi.org/10.1016/j.actbio.2007.01.002
Beachley V, Wen X (2010) Polymer nanofibrous structures: fabrication, biofunctionalization, and cell interactions. Prog Polym Sci 35:868–892
Wen MH, Cheng PW, Liao LJ, Chou HW, Wang CT (2013) Treatment outcomes of injection Laryngoplasty using cross-linked porcine collagen and hyaluronic acid. Otolaryngol Head Neck Surg 149:900–906
Qian YF, Su Y, Li XQ et al (2010) Electrospinning of polymethyl methacrylate nanofibres in different solvents. Iran Polym J (English Ed) 19:123–129
Mohammadian M, Haghi AK (2014) Systematic parameter study for nano-fiber fabrication via electrospinning process. Bulg Chem Commun 46:545–555
Casasola R, Thomas NL, Trybala A, Georgiadou S (2014) Electrospun poly lactic acid (PLA) fibres: effect of different solvent systems on fibre morphology and diameter. Polymer (Guildf) 55:4728–4737. https://doi.org/10.1016/j.polymer.2014.06.032
Jahangir MA, Rumi TM, Wahab A et al (2017) Poly lactic acid (PLA) Fibres: different solvent systems and their effect on fibre morphology and diameter. Am J Chem 2017:177–186. https://doi.org/10.5923/j.chemistry.20170706.01
Huang Y, Lu Y, Chen J (2016) Magnetic graphene oxide as a carrier for targeted delivery of chemotherapy drugs in cancer therapy. J Magn Magn Mater 427:34–40. https://doi.org/10.1016/j.jmmm.2016.10.042
Rong Z, Zeng W, Kuang Y, Zhang J, Liu X, Lu Y, Cheng X (2015) Enhanced bioactivity of osteoblast-like cells on poly(lactic acid)/poly(methyl methacrylate)/nano-hydroxyapatite scaffolds for bone tissue engineering. Fibers Polym 16:245–253. https://doi.org/10.1007/s12221-015-0245-0
Buschle-Diller G, Cooper J, Xie Z, Wu Y, Waldrup J, Ren X (2007) Release of antibiotics from electrospun bicomponent fibers. Cellulose 14:553–562. https://doi.org/10.1007/s10570-007-9183-3
Taylor G (1969) Electrically driven jets. Proc R Soc A Math Phys Eng Sci 313:453–475. https://doi.org/10.1098/rspa.1969.0205
Hadjiargyrou M, Chiu JB (2008) Enhanced composite electrospun nanofiber scaffolds for use in drug delivery. Expert Opin Drug Deliv 5:1093–1106
Herrero-Herrero M, Gómez-Tejedor JA, Vallés-Lluch A (2018) PLA/PCL electrospun membranes of tailored fibres diameter as drug delivery systems. Eur Polym J 99:445–455. https://doi.org/10.1016/j.eurpolymj.2017.12.045
Liu H, Ding X, Zhou G, Li P, Wei X, Fan Y (2013) Electrospinning of nanofibers for tissue engineering applications. J Nanomater 2013:1–11
Zhang C, Wang J, Wang Y (2005) Solubility of ceftriaxone disodium in acetone, methanol, ethanol, N,N,-dimethylformamide, and formamide between 278 and 318 K. J Chem Eng Data 50:1757–1760. https://doi.org/10.1021/je0501989
Wannatong L, Sirivat A, Supaphol P (2004) Effects of solvents on electrospun polymeric fibers: preliminary study on polystyrene. Polym Int 53:1851–1859. https://doi.org/10.1002/pi.1599
Fong H, Chun I, Reneker DH (1999) Beaded nanofibers formed during electrospinning. In: Polymer 40:4585–4592. https://doi.org/10.1016/S0032-3861(99)00068-3
Németh C, Gyarmati B, Gacs J, Salakhieva DV, Molnár K, Abdullin T, László K, Szilágyi A (2020) Fast dissolving nanofibrous matrices prepared by electrospinning of polyaspartamides. Eur Polym J 130:130. https://doi.org/10.1016/j.eurpolymj.2020.109624
Cui W, Li X, Zhu X, Yu G, Zhou S, Weng J (2006) Investigation of drug release and matrix degradation of electrospun poly(DL-lactide) fibers with paracetanol inoculation. Biomacromolecules 7:1623–1629. https://doi.org/10.1021/bm060057z
Zargham S, Bazgir S, Tavakoli A, Rashidi AS, Damerchely R (2012) The effect of flow rate on morphology and deposition area of electrospun nylon 6 Nanofiber. J Eng Fiber Fabr 7:155892501200700. https://doi.org/10.1177/155892501200700414
Akl MA, Ahmed MA, Ramadan A (2011) Validation of an HPLC-UV method for the determination of ceftriaxone sodium residues on stainless steel surface of pharmaceutical manufacturing equipments. J Pharm Biomed Anal 55:247–252. https://doi.org/10.1016/j.jpba.2011.01.020
Acknowledgments
The work has been supported by the Yildiz Technical University Scientific Research Projects Coordination Department. Project Number FDK-2019-3531.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duygulu, N.E., Ciftci, F. & Ustundag, C.B. Electrospun drug blended poly(lactic acid) (PLA) nanofibers and their antimicrobial activities. J Polym Res 27, 232 (2020). https://doi.org/10.1007/s10965-020-02215-0
Published:
DOI: https://doi.org/10.1007/s10965-020-02215-0