Abstract
The hydrophobic TiO2/lignin based carbon nanofibers (TiO2@CFs) composite with high photocatalytic efficiency and excellent cycle performance is successfully prepared by the method of electrospinning and thermo-treatment. The results of FTIR, SEM and EDS analyses show that the TiO2 nano-particles are uniformly and firmly coated on the surface of CFs. Due to good hydrophobicity and high electrical conductivity of CFs, such unique properties endow TiO2@CFs composites with enhanced light energy utilization efficiency and photocatalytic efficiency because of their floatability on the solution, high adsorptive capacity of MB, and low recombination of photo-generated electrons and holes. Compared with the commercial TiO2 powder, the degradation rate toward MB of TiO2@CFs is improved about 2.62 and 3.02 times at 30 and 15 min, respectively, under static state and xenon lamp irradiation. The degradation rate of TiO2@CFs toward MB reaches 62.77% within 15 min under static state, and further increase to 87.28% after 30 min. In addition, the removal rate of MB can be obtained up to 91.5% even after four cycles at a stirring speed of 200 RPM. Therefore, these unique material structures make it become a promising photocatalytic material.

The TiO2/lignin lased carbon nanofibers composite with excellent photocatalytic performance, reusability and durability was prepared from a green and low-cost biomass materials of lignin by using the simple method of electrospining.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methylene blue (MB), as an aromatic heterocyclic compound, has been widely used in the field of aquatic fungicide, biological stain, and fabric dye [1, 2]. About 15% used MB is annually discharged into the river or underground water without any treatment, causing serious water pollution and damage to aquatic ecosystem [3, 4]. Currently, the common treatment method of wastewater is that various adsorbents is used to adsorb harmful components, such as aerogels, activated carbon materials and other large specific surface area materials [5,6,7]. However, the raw materials of these adsorbents either are chemically unstable or come from fossil fuels, thus the relatively low adsorption capacity leads to the limited practical application. As a new type of nanomaterial, lignin-based carbon nanofibers (CFs) which are widely used in the treatment of organic pollutants have the advantages of low cost, reproducibility, high specific surface area, excellent chemical stability, mechanical properties [8,9,10,11,12,13]. Although CFs are considered as effective absorbent for organic dyes and toxic gases, the absorbed pollutants just accumulate on the surface of the CFs rather than degrade into harmless substances, which causes secondary pollution in subsequent process. In addition, as the amount of the adsorption increases, the adsorption performance of the adsorbent material gradually decreases until it completely loses at adsorption-desorption equilibrium. Against this backdrop, a green technology of photocatalysis has been greatly developed and is quickly applied in the field of environmental treatment. Under the illumination of the light source, photocatalyst rapidly generates the photo-generated electrons and holes, which can degrade the organic pollutants to form H2O, CO2 and other small molecular compounds without any loss of itself [14,15,16,17]. Among various photocatalysts, titanium dioxide (TiO2) is considered to has broad application prospects due to its superior performance, including chemical stability, low-cost, environmentally friendly and the like [18,19,20,21]. However, low photocatalytic efficiency and rapid recombination between photo-generated electrons and holes have become bottlenecks restricting its further development. Moreover, the photocatalysis of TiO2 usually occurs under ultraviolet light, which occupies only a small part in natural light [22, 23]. These factors have seriously affected the photocatalytic efficiency and application ranges of TiO2 to some extent. Therefore, generating as much photo-generated electrons and holes as possible under natural light and preventing their recombination is a key factor for improving the photocatalytic efficiency of TiO2.
Combining existing photocatalysts with highly conductive materials or adsorbent materials is a viable strategy for preparing suitable photocatalysts compared to the exploration of a new photocatalyst [24,25,26,27]. Due to favorable electron transfer ability of the highly conductive materials, the photo-gene rated electrons on the conductive band of photocatalysts can be rapidly transferred to the highly conductive materials, which greatly reduces the occurrence of recombination reaction of photo-generated electrons and holes. Recently, the combination of photocatalysts with a noble metal or carbon materials has received increasing attention due to the excellent inhibiting effect on recombination of photo-generated electrons and holes. Compared with the exorbitant price of noble metals (Ag, Pt, and Cu) [28,29,30], utilizing carbon based composite materials is a facile and effective strategy to improve the photocatalytic properties of photocatalysts [31,32,33]. At present, some researchers have synthesized some carbon-based composite photocatalysts, such as Bi2MoO6/reduced graphene oxide composite aerogel [34] and TiO2/carbon nanotube layered nanocomposites [35]. However, these carbon-based composite photocatalysts have complicated preparation process and high energy consumption, which are still difficult to be commercialized in the practical applications [36,37,38]. In addition, the photocatalytic performance of the photocatalyst is affected by the dye concentration. The method of combining the photocatalyst with the adsorbent material will be a good solution [39, 40], which will not only solve the problem of limited adsorption capacity of the adsorbent but also improve the photocatalytic efficiency of the photocatalyst. The combination of the adsorbent material and photocatalyst shows a lot of advantages of decreasing the agglomeration of powdered photocatalyst that is easy to recycle and increase active sites.
Inspired by the above ideas, a novel three-dimensional network structure photocatalytic material of the TiO2/lignin-based carbon nanofibers composite (TiO2@CFs) was successfully prepared through electrospinning followed by carbonization process in an inert gas (N2) atmosphere. This material can be used as a new type of photocatalytic material since it combines the advantages of outstanding electrical conductivity, high adsorption of CFs and the high photocatalytic activity of TiO2. It has been demonstrated that this material has increased the charge separation of TiO2 and improved the photocatalytic efficiency, reusability and durability of TiO2. The obtained TiO2@CFs display a good three-dimensional topography, high hydrophobicity, excellent photocatalytic properties and cycling stability. The degradation rate of TiO2@CFs with the stirring rate of 200 RPM toward MB reaches 100% within 21 min and as high as 91.5% retention rate of MB removal is obtained even through four cycles. Thereby, this study provides a novel approach for the preparation of low-cost, high-efficiency organic dye photocatalysts using biomass materials (lignin).
Material and methods
Materials
Reed was provided by Yueyang Forest & paper (Yueyang, China). Polyoxyethylene powder (PEO, P101345, average Mv: ~2 × 106 g mol−1) and TiO2 nano-particles (T104949, particle size: 10–25 nm, anatase, Hydrophilic) were obtained from Aladdin Reagent Co., Ltd. (Shanghai, China), Ethanol, N, N-dimethylformamide (DMF), and hydrochloric acid were obtained by Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Methylene blue (MB) was purchased from Sigma Aldrich (St. Louis, MO, USA). All reagents were analytically pure in this study.
Lignin preparation
80 g of reed was extracted using 640 ml ethanol solution with a 50/50 volume ratio of ethanol/water at 175 °C in a 1 L stainless steel reactor (PL1–00 type, Xianyang Testing Equipment Co., Ltd., China) and the reaction time was 90 min. After the reaction was completed, it was naturally cooled to room temperature, the mixture was filtered using a nylon filter paper. Then the solid was washed with a 50/50 volume ratio of ethanol/water. The obtained filtrate was concentrated to 200 mL at 50 °C using a rotary evaporator (RV10 control, IKA Co., Germany). The concentrated mixture was then added to 1 L dilute hydrochloric acid solution having a pH of 2 and is vigorously stirred until it settled naturally. After 24 h, the mixture was filtered through a glass microfiber filter and the solid was washed with deionized water. Finally, lyophilization was carried out at −50 °C for 96 h to obtain lignin using a freeze dryer from Virtis, USA.
The extraction yield of lignin from reed is calculated from Eq. (1) as follow:
Where, m is the weight of obtained lignin. M is the initial weight of reed.
Synthesis of TiO2@CFs composites
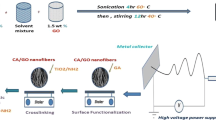
The preparation process of TiO2@CF composite material adopts a combination of electrospinning and carbonization, as shown in Fig. 1. 0.2 g PEO was dissolved in 14 g DMF at 60 °C for 12 h, and then 3.8 g of lignin was added to the above solution. The mixture solution was stirred with 600 rpm at 40 °C for 24 h to ensure the complete dissolution. At the same time, 2 g of TiO2 was added to the above mixture solution under vigorous agitation. The total solid content of the spinning solution in this study was 30 wt% and the ratio of lignin to PEO was 95:5. The solution was then transferred to an electrospinning machine (TL-Pro, Tongli Co. China) for spinning using a 20 G needles. The distance between the collector and the tip was 20 cm and the feed rate was set at 1.0 mL h−1. Applied voltage was kept at 20 KV (−5 KV, +15 KV) constantly. The nanofibers were collected with a copper net (200 mesh) wrapped collector and dried overnight at 60 °C under vacuum. For comparison, pure nanofibers were prepared with similar process without addition of TiO2. Finally, the TiO2@CFs and pure carbon nanofibers (CFs) were obtained by a carbonization process at 600 °C with the heating rate of 5 °C min−1 under N2 atmosphere.
Characterization
The morphology of samples was characterized by scanning electron microscopy (JSM 7800F, Japan, SEM). Gold powder was sprayed on the surface of the sample before observation. The energy dispersive spectrometry (EDS) of samples was performed on an Oxford X-Max 50 spectrometer without any treatment. Transmission electron microscopy (TEM) images were characterized by a JEM-2100(UHR) TEM instrument (JEOL Co., Japan). The infrared spectrum was measured by a Fourier infrared spectrometer from American PE Company, and the spectral range was from 400 to 4000 cm−1. Molecular weight of each sample was analyzed by Gel Permeation Chromatography (GPC) with differential refraction detector (type 2414). The sample (1 mg) was dissolved in 1 mL tetrahydrofuran (THF). The quantitative 13C NMR of lignin was recorded on a bruker avance 400 MHz spectrometer. The crystalline phases were measured using an X-ray diffraction (Shimadzu XRD-7000S, XRD) with Cu Kα radiation (30 kV, 80 mA, λ = 0.1542 nm). The pore size distribution of samples was calculated by ASAP 2020 PLUS HD88 analyser (Micromeritics Co., USA).
Photocatalytic activity testing
Photocatalytic activities of TiO2@CFs and TiO2 were characterized by the degradation of MB aqueous solution (20 mg L−1) under PLS-SXE300/300UV xenon lamp (Perfect Light Co., China, the total light power is 50 W) irradiation with the stirring rate of 200 rpm or static state. The wavelength of the light source was in the range of from 320 to 780 nm. Photocatalytic activity testing was carried out in a 100 mL photoreactor containing 50 mg catalyst and 50 mL MB solution. About 2 mL liquid was taken from the MB solution at 3 min intervals to measure the change in absorbance of MB with an UV–visible spectrometer. And the concentration of MB solution was calculated according to the absorbance and MB standard curve (Fig. S1). In order to examine the durability and reusability of TiO2@CFs, four consecutive photocatalytic cycles of MB were tested under the same condition. After the end of each cycle, TiO2@CFs, CFs and TiO2 were removed from MB solution and dried at 50 °C for 24 h under vacuum in the dark room to remove water. These obtained catalysts after four cycles were named as TiO2@CFs*, CFs*, TiO2*, respectively. The proportion of degradation of MB (Pd) is calculated from Eq. (2) as follow:
Where, c is the concentration of MB solution for each irradiated time. c0 is the initial concentration of dye in solution.
Results and discussion
Characterizations
The extraction yield of lignin from reed is 14.98 wt%. To understand the detailed features of the prepared lignin, the GPC, FTIR and 13C NMR characterizations were carried out, and the corresponding results are shown in Fig. S2. As show in Fig. S2(a), the molecular weights (Mw) and polydispersity index (PDI) of original lignin are 3296 g mol−1 and 2.15, respectively. Fig. S2(b) shows the FTIR spectrum of lignin. A typical broad and intense peak appeared at 3200–3600 cm−1 is assigned to the O-H stretching vibration. The peaks at 2920 and 2849 cm−1 are associated to methylene C-H asymmetric stretching and symmetric stretching vibration, respectively. The peak at about 1740 cm−1 is attributed to -C=O stretching vibration. The typical absorbance peaks at the range of 1400 to 1600 cm−1 are assigned to the aromatic skeletal vibrations. The quantitative 13C NMR analysis was also performed to character the chemical structure of lignin, The corresponding spectrum is shown in Fig. S2(c). No signal appears between δ 102 and 90 ppm, indicating the inexistence of carbohydrates in the lignin. For quantitative analysis, the region from δ 160 to 102 ppm is selected as criterion. The main chemical shift assignments of lignin are given in Table S1. The region from δ 160 to 140 ppm is attributed to oxygenated aromatic carbons, and the region from δ 140 to 123 ppm is attributed to aromatic carbon-carbon structures. The aromatic methine carbons and beta-O-4 bonds of lignin were characterized at δ 123–103 ppm and δ 64–58 ppm, respectively. All these indicate that the extracted lignin has high purity.
The FTIR spectra of TiO2@CFs, CFs, TiO2@CFs*, and CFs* are shown in Fig. 2. A strong and broad absorption peak appears at 3665 cm−1 in the spectrum of TiO2@CFs, which corresponds to Ti-OH stretching vibration [41], revealing that TiO2 tightly bonds with the carbon fiber through chemical bond. The vibration peak intensity of Ti-OH in TiO2@CFs* is hardly changed indicating there is still a strong interaction between TiO2 and CFs in the catalyst even after four cycles. It means that the fibrous catalyst can be reused at least four times without significant change in composition. The absorption band at 3346 cm−1 corresponds to O-H stretching of hydroxyl groups in catalyst. The peaks at about 1608, 1515, and 1422 cm−1 are attributed to the aromatic skeletal vibrations of MB [42]. Compared with the FTIR spectrum of CFs*, the corresponding vibrations of MB disappear in both the TiO2@CFs and TiO2@CFs* spectra, which indicates the prepared TiO2@CFs possesses outstanding photocatalytic degradation capacity towards MB even after four cycles. After four cycles, new stretching vibration peaks of the C-H (1335 cm−1), C=N (1243 cm−1) and C-S (1138 and 1068 cm−1) appear in the FTIR spectra of CFs* demonstrate that the existence of MB in CFs* [43, 44]. This illustrates that CFs only adsorbed in the MB without degradation. However, these characteristic absorption peaks of MB were not detected in TiO2@CFs* which means the MB is absorbed in TiO2@CFs that were timely degraded, which will contribute to the improvement of catalyst service life and catalytic efficiency.
The XRD patterns of the CFs, TiO2, and TiO2@CFs are shown in Fig. 3. Two relatively diffraction peaks at about 24o and 43o in CFs are attributed to disordered carbonaceous structure (the crystallographic plane of 002) and graphite crystals structure (100), respectively [45]. Unique peaks at 2θ = 25.3o, 37.8o, 48.1o, 53.9o, 55.1o and 62.7o can be observed in both TiO2 and TiO2@CFs, which are empirically assigned to (101), (004), (200), (105), (211), and (204) reflections of anatase crystals, respectively [46, 47]. These results suggest that the lattice type of TiO2 isn’t changed during carbonization process. In addition, the disappearance of the peak at 43o in TiO2@CFs demonstrates that the addition of TiO2 might restrain the formation of graphitized structure, thereby increasing the adsorption active sites.
The morphology of CFs is the critical factor to affect the adsorptive and photocatalystic properties of the TiO2@CFs catalyst. The SEM images of CFs are shown in Fig. 4a. Relatively smooth, uniform, and randomly-ordered electrospun CFs are obtained from lignin/PEO blend solutions without TiO2. In contrast, uniformly distributed beads (TiO2 nano-particles) appear in the continuous TiO2@CFs prepared by the lignin/PEO blend solutions with TiO2 (as shows in Fig. 4b). The diameter distributions and averages of carbon nanofibers are shown in Fig. S3. The diameter of CFs was measured as 235 ± 32 nm, which is greater than that of TiO2@CFs with 207 ± 35 nm. This may be attributed to the addition of TiO2 that prevents the crosslinking between lignin and PEO, which can further decrease the viscosity of spinning solution and result in thinner diameter carbon nanofibers. Partial fragmentation occurs in CFs* (as shows in Fig. 4c), which may be explained by the destructive effect of the adsorbed MB on CFs. However, a continuous fibrous morphology appears in the TiO2@CFs* even after four cycles which may be attributed to the excellent degradability of TiO2 towards MB, and thus ensuring CFs possess its original performance. Careful comparison of morphology between TiO2@CFs and TiO2@CFs* displays that the distribution and quantity of the beads on fiber are little changed after four photocatalytic cycles, which reveals the TiO2 nano-particles tightly bond with the fibers. In order to further investigate the binding form of bead on the fibers, the micro-morphology of TiO2@CFs was characterized by TEM, the results are shown in Fig. 4e–f. As can be seen from Fig. 4f, these beads are not only adhered to the surface of the CF, but also tightly wrapped by carbon shell to form a semi-embedded structure. Such unique micro-structure endows the TiO2 beads with excellent fiber-bonding ability during the photocatalytic process, which ensures the reusability and durability of TiO2@CFs in the photocatalytic application.
The distribution of TiO2 on the surface of CFs is crucial for its photocatalytic performance. Although the results of SEM in Fig. 4 have clearly indicated that TiO2 is evenly distributed on the surface of CF, the distribution of TiO2 on the CF surface can be more intuitively observed by the EDS test. The EDS mapping of TiO2@CFs as shown in Fig. 5a. Well-dispersed blue dots (titanium element) demonstrate that TiO2 is uniformly distributed on the surface of CFs. To confirm the content of titanium, surface EDS energy spectrum analysis is performed on TiO2@CFs and the results are shown in Fig. 5b. The elemental content of carbon, hydrogen, oxygen, and titanium in the TiO2@CFs are 76.46, 1.42, 13.94, and 8.18 wt%, respectively. As high as 13.65 wt% deposition of TiO2 on CFs can be calculated from the content of titanium. These uniformly distributed and large attached amount of TiO2 are very important for photocatalyst to obtain excellent photocatalytic efficiency.
As shown in Fig. S4, two broad peaks at about 1350 and 1580 cm−1 can be attributed to the D band and G band, respectively. Additionally, four relatively weak peaks appeared at 143, 392, 519 and 642 cm−1 are corresponded to the characteristic peaks of TiO2. The R value calculated from the intensity ratio of G to D band represents the carbonization degrees of the carbon material. The calculated R values of the prepared TiO2@CFs and CFs are 1.10 and 0.83, respectively. Significantly high degree of graphitization in the TiO2@CFs may be attributed to the effective formation of graphitized structure in the presence of TiO2.
The photocatalytic ability of the catalyst is also affected by the wetting properties of TiO2@CFs. The hydrophobicity of catalyst is beneficial to improve the photocatalytic efficiency due to its good self-cleaning ability to dyeing sewage and its small molecular compound [48]. In hydrophilic photocatalytic materials, although the organic dyes can be efficiently decomposed by photocatalysts, the small molecules and inorganic nano-particles are difficult to remove from the surface of catalyst and further affects the absorption of light energy. The static water contact angle of TiO2 is only 46o (as shows in Fig. 6), such hydrophilicity greatly limits the catalytic efficiency in dyeing sewage. To overcome the limitation of self-cleaning, a hydrophobic TiO2@CFs with the water contact angle of 130o was constructed. Because of the Lotus effect on the surface of catalyst, small molecules and inorganic nano-particles on the surface of photocatalysts will be taken along by water stream. Thus, other organic contaminant (MB) can be timely contacted with the photocatalyst and decomposed by photocatalysts (TiO2@CFs) under the irradiation of xenon lamp, resulting in improvement of the photocatalytic efficiency. Water contact angle of each material after 4 cycles is show in Fig. S5. The static water contact angles of TiO2*, TiO2@CFs*, and CFs* are 44o, 126o and 142o, respectively. Compared with the contact angle before cycles, the contact angles of TiO2* and TiO2@CFs* drop slightly after 4 cycles. However, the contact angle of CFs* increases from 136o to 142o, which may be attributed to the improvement of the surface roughness derived from the partial fragmentation in CFs* fiber. Wenzel-cassie model reveals the larger surface roughness for hydrophobic materials and the higher contact angle.
The high-resolution transmission electron microscopy (HRTEM) image of each catalyst before and after four cycles is illustrate in Fig. 7. Both the pure TiO2 nano-particles and TiO2@CFs have the perfect lattice, as shown in Fig. 7a–b. The average d-spacing distance of the TiO2 lattice was measured to be 0.35 nm, corresponding to the (101) planes of TiO2 [49]. However, after four photocatalysis cycles, the lattice image of pure TiO2 nano-particles became blurred (Fig. 7c). This is induced from the deactivation of a catalyst due to the deposition of contaminants after repeated cycles. Interestingly, clear lattice image of TiO2 exhibits in TiO2@CFs* (Fig. 7d), indicating that the catalysts still have some photocatalytic activity. This maybe attributes to the quick migration of the electrons on the conductive band from TiO2 to the carbon fibers, which greatly reduces the recombination rate of electrons and holes pair, and thus improving the cycle life of TiO2@CFs.
Photocatalytic performance
The photocatalytic activities of CFs, TiO2, TiO2@CFs on the degradation of MB with the stirring rate of 200 rpm are shown in Fig. 8 and Table S2. The absorbance between 400 and 800 nm corresponds to the characteristic peak of MB. After 30 min of xenon lamp irradiation, the absorbance peak at 664 nm in CFs is reduced by roughly half (Fig. 8a). By contrast, the corresponding absorption peak decreases dramatically in the presence of TiO2@CFs (Fig. 8c) and the color of the MB solution changes from blue to colorless rapidly, showing an excellent level of MB photodegradation and is much better than that of the commercial anatase TiO2 (Fig. 8b). This may be because the combination of TiO2 and CFs can improve carrier lifetime and charge separation, thereby increasing photocatalytic activity. The percentages of MB degradation of at different period are shown in Fig. 8d. The MB degradation efficiencies of pristine CFs and TiO2 under xenon lamp irradiation for 30 min are 61.26% and 91.04%, respectively. Almost 100% efficient photocatalytic activity is observed in the TiO2@CFs at only 24 min irradiation. Furthermore, it is found that the degradation percentage of commercial anatase TiO2 is merely 69.90% after 15 min irradiation, while the corresponding degradation efficiency of TiO2@CFs reaches 94.54%. The high value is even higher than the degradation rate (91.04%) of commercial titanium dioxide at 30 min, i.e. the combination of TiO2 and CFs greatly improves the photocatalytic efficiency of TiO2. For comparison, some photocatalytic performances of the materials prepared in our laboratory and in literatures are summarized in Table 1. These values are larger than that of recent reports. Obviously, the effective degradability of MB and degradation time of our catalyst are significantly superior to that of other literatures.
The photocatalytic performances under static condition were also investigated to simulate the actual situation of factory wastewater treatment. The comparison of MB degradation among CFs, TiO2, and TiO2@CFs are shown in Fig. 9 and Table S2. It is clearly that obvious difference of catalytic capacities for MB existed in catalysts. The absorption peaks of CFs and TiO2 at 664 nm are slightly reduced in Fig. 9a–b. However, TiO2@CFs exhibit higher catalytic capacities for MB, which may be explained by the existence of large number of adsorption sites and photocatalytic active sites on the surface of the TiO2@CFs. As shown in Fig. 9d, the reductions of MB in the CFs and TiO2 under 30 min xenon lamp irradiation are less than 20 and 35%, respectively. In contrast, TiO2@CFs photocatalyst exhibits higher photocatalytic activity of 87.28% under the same condition (30 min irradiation). To quantitatively analyse the kinetics behaviors of the MB degradation, the pseudo-first-order reaction kinetics model was used to fit the photodegradation reaction kinetics of the CFs, TiO2, and TiO2@CFs, the results are shown in Fig. 9e. The rate constant of TiO2@CFs reaches 0.06973 min−1, which is about 9.7 and 5.5 times of CFs (0.00717 min−1) and pure TiO2 (0.01274 min−1) respectively. Such significant enhancement of photocatalytic efficiency of TiO2@CFs indicats that there is a synergistic effect on photodegradation of MB between TiO2 and CFs.
Reusability and durability
The cycling performance and stability of the catalyst are the key factors to influence the practical application. Therefore, the reusability and durability of various photocatalysts were investigated. The MB degradation efficient of CFs, TiO2, and TiO2@CFs after four cycle adsorptions under the xenon lamp irradiation is shown in Fig. 10. The MB removal rate of pure TiO2 dramatically decreases from 91.6% to 1.3% after four cycles, which declines nearly 70 times. It may be related to the inactivation of TiO2 and is consistent with the TEM image (Fig. 7c). The same phenomenon existed in CFs and the corresponding MB removal rate reduces from 61.4% to 3.0%. These results suggest that pure CFs or TiO2 has a poor scavenging ability for MB and this ability will decrease as the amount of adsorption increases. Compared with TiO2 or CFs, TiO2@CFs composite exhibits higher MB removal rate after multiple adsorption. As high as 91.5% retention rate of MB removal is obtained in the TiO2@CFs composite even through four cycles, illustrating excellent reusability and durability. Compared with the traditional powder catalysts, TiO2@CFs has many advantages of good catalytic efficiency, high recovery, good stability and so on.
Mechanisms of improved photocatalytic activity
The schematic diagram of the improved photocatalytic of TiO2@CFs composite is shown in Fig. 11. In this process, the photocatalytic consists of three processes (Fig. 11-I) including (a) absorption of MB molecules by CFs; (b) light energy capture by TiO2@CFs composite and (c) the decomposition of MB molecules by photocatalyst with the help of charge transfer reaction. In this model, the CFs not only serve as a carrier for TiO2 photocatalyst, but also provide an adsorption drive force to quickly aggregate MB molecules. The existence of many micropores & mesoporous in TiO2@CFs (Fig. 11-II) results in high MB concentration around the surface of photocatalyst of TiO2. Meanwhile, TiO2 photocatalyst looks like a pair of ‘scissors’, which will break the absorbed MB molecular chains and degrade. Therefore, the combination of TiO2 and CFs displays an excellent synergistic effect on the photocatalytic efficiency of MB. In addition, the photo-generated electrons and holes generated in TiO2 under xenon lamp irradiation reacts with H2O to produce strong oxidizing •O2 and •OH radicals. The resulting •O2 and •OH radicals further react with MB to form H2O, CO2 and other small molecular compounds, such as Cl−, NH4+, SO42−, and NO3−. Due to excellent electrical conductivity of CFs, the photo-generated electrons on the conductive band of TiO2 rapidly transfers to the CFs, greatly decreasing the recombination reaction of photo-generated electrons and holes. The hydrophobic surface of TiO2@CFs also reduces the loss of TiO2 in solution, which enhances the reusability and durability of photocatalyst. Furthermore, the TiO2@CFs composite has the advantage of floating on the surface of solution due to its loose structure, which can greatly improve the utilization of light energy (Fig. 11-III). Therefore, the combined effects of the above factors improve the photocatalytic efficiency and cycle life of TiO2@CFs composites.
Conclusion
In conclusion, the superior photocatalytic performance of TiO2@CFs derived from a renewable lignin have been prepared by a simple process of thermo-treatment after electrospinning. In TiO2@CFs composite, CFs are not only used as carriers of TiO2 photocatalyst and provided more electron transfer channels to increase the charge separation, but also increased the adsorption capacity of TiO2@CFs composites on account of the existence of micro-pores in CFs. Therefore, compared with the conventional photocatalyst, TiO2@CFs composites show the advantages of good reusability, strong durability and faster MB degradation rate. In addition, the use of tweezers for the recycle of TiO2@CFs composites is very convenient compared to powdered photocatalysts. The degradation rate of MB over TiO2@CFs composite is about 1.10 and 1.35 times in dynamic degradation experiment as high as the commercial TiO2 powder when it is exposed to xenon lamp irradiation for 30 and 15 min, respectively. And further increases to 2.63 and 3.02 times as high as commercial TiO2 powder in static degradation experiment. Furthermore, due to the low-density characteristic and the property of hydrophobic, the materials of TiO2@CFs are able to float on water, which greatly improve the utilization of light energy. As a result, the produced TiO2@CFs composites have been demonstrated that they have important application prospects in the field of high-efficiency photocatalysts in the future, especially in static water treatment process.
References
Nasrullah A, Khan H, Khan AS, Man Z, Muhammad N, Khan MI, El-Salam NMA (2015) Potential biosorbent derived from Calligonum polygonoides for removal of methylene blue dye from aqueous solution. Sci World J 2015:1–11
Martino EA, Winterton D, Nardelli P, Pasin L, Calabro MG, Bove T, Fanelli G, Zangrillo A, Landoni G (2016) The blue coma: the role of methylene blue in unexplained coma after cardiac surgery. J Cardiothorac Vasc Anesth 30:423–427
Betancourt-Buitrago LA, Vasquez C, Veitia L, Ossa-Echeverry O, Rodriguez-Vallejo J, Barraza-Burgos J, Marriaga-Cabrales N, Machuca-Martinez F (2017) An approach to utilize the artificial high power LED UV-A radiation in photoreactors for the degradation of methylene blue. Photochem Photobiol Sci 16:79–85
Houas HLA, Ksibi M, Elaloui E, Guillard C, Herrmann J (2001) Photocatalytic degradation pathway of methylene blue in water. Appl Catal B Environ 31:145–157
Tang B, Chen H, He Y, Wang Z, Zhang J, Wang J (2017) Influence from defects of three-dimensional graphene network on photocatalytic performance of composite photocatalyst. Compos Sci Technol 150:54–64
Prathap A, Sureshan KM (2017) Organogelator-cellulose composite for practical and eco-friendly marine oil spill recovery. Angew Chem Int Ed 56:9045–9049
Dong Y, Lin H, Jin Q, Li L, Wang D, Zhou D, Qu F (2013) Synthesis of mesoporous carbon fibers with a high adsorption capacity for bulky dye molecules. J Mater Chem A 1:7391–7398
Beck RJ, Zhao Y, Fong H, Menkhaus TJ (2017) Electrospun lignin carbon nanofiber membranes with large pores for highly efficient adsorptive water treatment applications. J Water Process Eng 16:240–248
Jiao Y, Wan C, Li J (2016) Synthesis of carbon fiber aerogel from natural bamboo fiber and its application as a green high-efficiency and recyclable adsorbent. Mater Des 107:26–32
Collins MN, Nechifor M, Tanasa F, Zanoaga M, McLoughlin A, Strozyk MA, Culebras M, Teaca CA (2019) Valorization of lignin in polymer and composite systems for advanced engineering applications - a review. Int J Biol Macromol 131:828–849
Dai Z, Shi XJ, Liu H, Li HM, Han Y, Zhou JH (2018) High-strength lignin-based carbon fibers via a low-energy method. RSC Adv 8:1218–1224
Kai D, Tan MJ, Chee PL, Chua YK, Yap YL, Loh XJ (2016) Towards lignin-based functional materials in a sustainable world. Green Chem 18:1175–1200
Thakur VK, Thakur MK (2015) Recent advances in green hydrogels from lignin: a review. Int J Biol Macromol 72:834–847
Sharma S, Dutta V, Singh P, Raizada P, Rahmani-Sani A, Hosseini-Bandegharaei A, Thakur VK (2019) Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: a review. J Clean Prod 228:755–769
Heitmann AP, Rocha IC, Pereira IM, Oliveira LCA, Patrício PSO (2019) Nanoparticles of niobium oxyhydroxide incorporated in different polymers for photocatalytic degradation of dye. J Polym Res 26:159
Ossoss KM, Hassan MER, Al-Hussaini AS (2019) Novel Fe2O3@PANI-o-PDA core-shell nanocomposites for photocatalytic degradation of aromatic dyes. J Polym Res 26:199
Regonini D, Bowen CR, Jaroenworaluck A, Stevens R (2013) A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater Sci Eng R 74:377–406
Xing Z, Zhang J, Cui J, Yin J, Zhao T, Kuang J, Xiu Z, Wan N, Zhou W (2018) Recent advances in floating TiO2-based photocatalysts for environmental application. Appl Catal B Environ 225:452–467
Zhao Y, Wang Y, Xiao G, Su H (2019) Fabrication of biomaterial/TiO2 composite photocatalysts for the selective removal of trace environmental pollutants. Chin J Chem Eng 6:1416–1428
Khalid NR, Majid A, Tahir MB, Niaz NA, Khalid S (2017) Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: a review. Ceram Int 43:14552–14571
UDDIN MJ, CESANO F, Scarano D, Bonino F, Agostini G, Spoto G, Bordiga S, Zecchina A (2008) Cotton textile fibres coated by Au/TiO2 films : synthesis, characterization and self cleaning properties. J Photochem Photobiol A 199:64–72
Zhang J, Zhang Y, Lei Y, Pan C (2011) Photocatalytic and degradation mechanisms of anatase TiO2: a HRTEM study. Catal Sci Technol 1:273–278
Augugliaro V, Bellardita M, Loddo V, Palmisano G, Palmisano L, Yurdakal S (2012) Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J Photochem Photobiol C 13:224–245
Sánchez-Rodríguez D, Mendez Medrano MG, Remita H, Escobar-Barrios V (2018) Photocatalytic properties of BiOCl-TiO2 composites for phenol photodegradation. J Environ Chem Eng 6:1601–1612
Justh N, Mikula GJ, Bakos LP, Nagy B, Laszlo K, Parditka B, Erdelyi Z, Takáts V, Mizsei J, Szilagyi IM (2019) Photocatalytic properties of TiO2@polymer and TiO2@carbon aerogel composites prepared by atomic layer deposition. Carbon 147:476–482
Ortelli S, Blosi M, Albonetti S, Vaccari A, Dondi M, Costa AL (2014) TiO2, based nano-photocatalysis immobilized on cellulose substrates. J Photochem Photobiol A 176:58–64
Noreen Z, Khalid NR, Abbasi R, Javed S, Ahmad I, Bokhari H (2019) Visible light sensitive Ag/TiO2/graphene composite as a potential coating material for control of campylobacter jejuni. Mater Sci Eng C-Mater 98:125–133
Jayaraman J, Pavadai N, Venugopal T, Ramaiyan R (2019) Interfacial charge-transfer in Cu-TiO2-HBDPPIN-Ag film and AIEE-active chemosensor. J Photochem Photobiol A 377:318–338
Sanzone G, Zimbone M, Cacciato G, Ruffino F, Carles R, Privitera V, Grimaldi MG (2018) Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattice Microst 123:394–402
Natarajan S, Lakshmi DS, Thiagarajan V, Mrudula P, Chandrasekaran N, Mukherjee A (2018) Antifouling and anti-algal effects of chitosan nanocomposite (TiO2/Ag) and pristine (TiO2 and Ag) films on marine microalgae Dunaliella salina. J Environ Chem Eng 6:6870–6880
Kusiak-Nejman E, Morawski AW (2019) TiO2/graphene-based nanocomposites for water treatment: a brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl Catal B Environ 253:179–186
Wang T, Tang T, Gao Y, Chen Q, Zhang Z, Bian H (2019) Hydrothermal preparation of Ag-TiO2-reduced graphene oxide ternary microspheres structure composite for enhancing photocatalytic activity. Phys E 112:128–136
Sohail M, Xue H, Jiao Q, Li H, Khan K, Wang S, Feng C, Zhao Y (2018) Synthesis of well-dispersed TiO2/CNTs@CoFe2O4 nanocomposites and their photocatalytic properties. Mater Res Bull 101:83–89
Liu X, Wang J, Dong Y, Li H, Xia Y, Wang H (2018) One-step synthesis of Bi2MoO6/reduced graphene oxide aerogel composite with enhanced adsorption and photocatalytic degradation performance for methylene blue. Mater Sci Semicond Process 88:214–223
Djokic VR, Marinkovic AD, Ersen O, Uskokovic PS, Petrovic RD, Radmilovic VR, Janackovic DT (2014) The dependence of the photocatalytic activity of TiO2/carbon nanotubes nanocomposites on the modification of the carbon nanotubes. Ceram Int 40:4009–4018
Jiang F, Yu Y, Feng A, Song L (2018) Effects of ammonia on graphene preparation via microwave assisted intercalation exfoliation method. Ceram Int 44:12763–12766
Torrisi L, Cutroneo M, Havranek V, Silipigni L, Fazio B, Fazio M, Di Marco G, Stassi A, Torrisi A (2019) Self-supporting graphene oxide films preparation and characterization methods. Vacuum 160:1–11
Eltayeb NE, Khan A (2019) Design and preparation of a new and novel Nanocomposite with CNTs and its sensor applications. J Mater Res Technol 8:2238–2246
Yang X, Ma J, Ling J, Li N, Wang D, Yue F, Xu S (2018) Cellulose acetate-based SiO2/TiO2 hybrid microsphere composite aerogel films for water-in-oil emulsion separation. Appl Surf Sci 435:609–616
Ng HKM, Leo CP (2019) The coherence between TiO2 nanoparticles and microfibrillated cellulose in thin film for enhanced dispersal and photodegradation of dye. Prog Org Coat 132:70–75
Jung SM, Grange P (2004) Characterization of the surface hydroxyl properties of sepiolite and Ti(OH)4 and investigation of new properties generated over physical mixture of Ti(OH)4–sepiolite. Appl Surf Sci 221:167–177
Ren W, Pan X, Wang G, Cheng W, Liu Y (2016) Dodecylated lignin-g-PLA for effective toughening of PLA. Green Chem 18:5008–5014
Akkoz Y, Coskun R, Delibas A (2019) Preparation and characterization of sulphonated bio-adsorbent from waste hawthorn kernel for dye (MB) removal. J Mol Liq 287:110988
Ahamad T, Naushad M, Eldesoky GE, Al-Saeedi SI, Nafady A, Al-Kadhi NS, Al-Muhtaseb AAH, Khan AA, Khan A (2019) Effective and fast adsorptive removal of toxic cationic dye (MB) from aqueous medium using amino-functionalized magnetic multiwall carbon nanotubes. J Mol Liq 282:154–161
Huang L, Key J, Shen PK (2019) Boosting the volumetric energy of supercapacitors using polytetrafluoroethylene pyrolysis gas. J Power Sources 414:76–85
Devikala S, Kamaraj P, Arthanareeswari M (2018) A.C. conductivity studies of PMMA/TiO2 composites. Mater Today Proc 5:8678–8682
Suphankij S, Mekprasart W, Pecharapa W (2013) Photocatalytic of N-doped TiO2 Nanofibers prepared by electrospinning. Energy Procedia 34:751–756
Zhu C, Fu Y, Liu C, Liu Y, Hu L, Liu J, Bello I, Li H, Liu N, Guo S, Huang H, Lifshitz Y, Lee ST, Kang Z (2017) Carbon dots as fillers inducing healing/self-healing and anticorrosion properties in polymers. Adv Mater 29:1701399
Yin ZW, Betzler SB, Sheng T, Zhang Q, Peng X, Shangguan J, Bustillo KC, Li JT, Sun SG, Zheng H (2019) Visualization of facet-dependent pseudo-photocatalytic behavior of TiO2 nanorods for water splitting using in situ liquid cell TEM. Nano Energy 62:507–512
Tho NT, Mai NTT, Van NT, Phat BD, Hieu LV, Thi CM, Viet PV (2019) Direct synthesis of reduced graphene oxide/TiO2 nanotubes composite from graphite oxide as a high-efficiency Visible-light-driven photocatalyst. J Nanosci Nanotechnol 19:5195–5204
Sim LC, Leong KH, Ibrahim S, Saravanan P (2014) Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electron mobility and visible-light-driven photocatalytic performance. J Mater Chem A 2:5315–5322
Nguyen-Phan TD, Pham VH, Shin EW, Pham HD, Kim S, Chung JS, Kim EJ, Hur SH (2011) The role of grapheme oxide content on the adsorption-enhanced photocatalysis of titanium dioxide/graphene oxide composites. Chem Eng J 170:226–232
Kim SP, Choi HC (2014) Photocatalytic degradation of methylene blue in presence of graphene oxide/TiO2 nanocomposites. Bull Kor Chem Soc 35:2660–2664
Lee JS, You KH, Park CB (2012) Highly photoactive, low bandgap TiO2 nanoparticles wrapped by grapheme. Adv Mater 24:1084–1088
Zhang H, Lv X, Li Y, Wang Y, Li J (2010) P25-graphene composite as a high performance photocatalyst. ACS Nano 4:380–386
Guo J, Zhu S, Chen Z, Li Y, Yu Z, Liu Q, Li J, Feng C, Zhang D (2011) Sonochemical synthesis of TiO2 nanoparticles on graphene for use as photocatalyst. Ultrason Sonochem 18:1082–1090
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51773167, 21706208 and 51573147); the Natural Science Foundation of Shaanxi Province (2018JM5036); the Science and technology plan of Xi’an (2019217814GXRC014CG015-GxyD14.7).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Electrospining is a promising method for photocatalyst composite synthesis
• CFs would efficiently enhance the photocatalytic efficiency of photocatalysts
• The floating TiO2@CFs composite greatly improve the utilization of light energy
• The tightly combination of TiO2 and CFs increase the durability of photocatalysts.
Electronic supplementary material
ESM 1
(DOCX 8714 kb)
Rights and permissions
About this article
Cite this article
Dai, Z., Ren, P., Cao, Q. et al. Synthesis of TiO2@lignin based carbon nanofibers composite materials with highly efficient photocatalytic to methylene blue dye. J Polym Res 27, 108 (2020). https://doi.org/10.1007/s10965-020-02068-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02068-7