Abstract
A new aromatic diamine 4-(3′,5′-diamino benzoyl amido)-3-pentadecyl anisole; (V) containing pendant pentadecyl substituted methoxyphenyl moiety was synthesized and characterized by FT-IR, NMR (1H and 13C) and mass spectrometry. New aromatic polyamides were prepared from the diamine (V) and two aromatic diacid chlorides, Isophthaloyl chloride (IPC), Terphthaloyl chloride (TPC) using low temperature solution polycondensation method. Inherent viscosities of the polyamides were in the range 0.30 to 0.52 dL/g inN,N,Dimethylacetamide(DMAc) indicating moderate to high molecular weight of polyamides. The resulting polyamides were soluble in N-Methyl Pyrrolidone(NMP), N, N, Dimethyl formamide (DMF), N, N, Dimethyl acetamide (DMAc), N, N, Dimethyl sulphoxide(DMSO) and m-cresol. These polyamides showed relatively lower glass transition temperatures which were in the range 196-249 °C; Probably due to pendant alkyl substituted anisole structure which could impart internal plasticization effect. Thermal stability with T10 weight loss temperature in the range of 359-387 °C under nitrogen atmosphere illustrated good thermal stability. XRD of all polyamides showed that polyamides are amorphous in morphology; which is well reflected in good solubility of these polyamides in polar aprotic solvents named above. Thus incorporation of pendant methoxyphenylunit with pentadecyl substituent brought improvement in their solubility and processable properties by lowering Tgvalues; without any significant deterioration of their thermal stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyamides are characterized by their excellent thermal and mechanical properties, good flame resistance and electrical properties and good chemical resistance. The major factors that account for low solubility of aromatic polyamides due to interlectual molecular order, chain stiffness provided by high density of aromatic rings and strong interchain attractive forces mainly hydrogen bonding, that enhance effective molecular packing. The fabrication most of unsubstituted aromatic polyamides is difficult as result of their high softening temperature and their insolubility in common organic solvents.
Therefore, many efforts have been focused to enhance the solubility and processability [1,2,3,4] of polymer without affecting thermal stability.In this topic we report long aliphatic chain containing pentadecyl unit via meta-oriented diamine with pendant methoxyphenylene 1, 3-catenated diamine moiety containing amide linkage provide kinks to the polymer backbone.
The purpose of this study is to show that the solubility and processability can be imparted to polymer by using appropriate diamine that having kink aromatic structure; wherein bulky pendant group have been introduced [5, 6]. It is part of our extensive research in this area to develop new polymers incorporating connecting group like ether, amide, phenyl, pendant pentadecyl groups, from renewable natural resources; namely cardanol, a byproduct of cashew industry. Aromatic polyamides are a class of high temperature resistant [7,8,9] polymers with good chemical resistance and thermal stability, low flammability, and very good mechanical properties [10,11,12, 13]. However, high melting and glass transition temperature of aromatic polyamides have limited solubility in organic solvents. So these aromatic polyamides are difficult to process these problems are caused by strong-intermolecular hydrogen bonding of amide function, which cause chain stiffness. Worldwide efforts have been devoted to improve the solubility and processability of thermally stable polymers.
This section deals with the synthesis of a new series of polyamides containing m-catenated amide-aromatic linkages in main polyamide chains with pendant long alkyl pentadecyl substituted methoxyphenyl group via amide link to enhance processability of polymer without significant affect on thermal stability.
This section deals with the synthesis of new aromatic diamine 4-(3′,5′,diamino benzoyl amido)-3-pentadecyl anisole (V) starting from 3-pentadecyl phenol and 3,5-dinitro benzoic acid. The new aromatic diamine was characterized by physical constant, FT-IR, NMR (1H,13C) spectroscopy and was used to synthesize polyamides using low temperature solution polymerization method. These polyamides were characterized by the inherent viscosity, solubility, FT-IR, TGA, DSC and XRD, to illustrate structure property relationship.
Experimental
Materials
Cammercially available 3-pentadecyl phenol, dimethyl sulphate, Terephthaloyl chloride (TPC) and Isophthaloyl chloride (IPC) (all Aldrich make), 3,5, dinitro benzoic acid, phosphorous pentachloride (sd fine make), were used as received.
Diamine synthesis
The new aromatic diamine 4-(3′,5,diaminobenzoylamido)-3-pentadecyl anisole (V) was synthesized in several steps; as below.
- a)
4-Amino-3-pentadecyl-anisole (III):
- i)
4-Nitro-3-pentadecyl phenol (I)
3-Pentadecyl phenol (20 g, 0.065 mol) was dissolved in chloroform (75 mL) and (5.3 g, 1.2 mmol) of fuming nitric acid (sp.gr.1.5) was added to it dropwise with stirring and cooling in an ice bath maintained at 5-10 °C. The reaction mixture was stirred for another 20 min at 10 °C; then poured into water, the organic layer was separated by separating funnel. Chloroform solvent was removed by distillation using water aspirator, the residual mass solidified on cooling. After filteration and drying 23 g of red orange solid mixture of isomeric nitro phenols was obtained. It was crystallized 150 mL of petroleum ether (60-80 °C) to get about 10 g of light tan powder of 4-nitro-3-pentadecyl phenol (I). This on futherrecrystalization from the petroleum ether gave the purified (I). Yield 8.0 g (71%), m.p 70-71 °C.
Analysis found: C 72.45%, H 10.33%, N 4.07%,
Analysis Calculated for C21H35NO3: C 72%, H 10.05%, N 4.02% .
- ii)
4-Nitro-3-pentadecyl anisole (II)
In one neck round bottom flask equipped with magnetic stirrer. a mixture of 4-nitro-3-pentadecyl phenol (3.63 g 10 mmol), in dichloromethane (50 mL), sodium hydroxide (0.7g15mmol)) in water (50 mL),; the alkylating agent dimethyl sulphate (2.5 mL) (20-30 mmol), and phase transfer catalyst benzyl triethylammonium chloride (0.1 g, 1 mmol), was agitated with stirring at room temperature for 12 h. The organic layer was then separated and the aqueous layer was extracted twice with dichloromethane. The organic extract was washed with 2 M aq. ammonia solution to remove residual dimethyl sulphate, the solvent was evaporated and the solid 4-nitro-3-pentadecyl anisole (II) was purified by crystalization from methanol. Yield 2.9 g (85%), m.p. 49 °C.
1H NMR (in CDCl3, ppm) 0.96 (q, 3H), 1.45 (t, 26 H), 2.5 (t, 2H), 3.8 (s, 3H), 6.7 (d, 1H), 7.9 (d, 1H), 7.2 (s, 1H), 7.9 (d, 1H).
- iii)
4-Amino-3-pentadecyl anisole (III)
In three neck round bottom flask (250 mL) equipped with magnetic stirrer, reflux condenser, calcium chloride guard tube, were placed 4-nitro-3-pentadecyl anisole 3.63 g (0.01 mol), 10% Pd/C (0.2 g), ethanol (50 mL). The reaction mixture was heated to 80 °C and hydrazine hydrate (8 mL), was added dropwise stirring and heating the reaction mixture at 80 °C was continued for 8 h. Reaction mixture was cooled to room temperature and poured into the ice cold water to precipitate solid (III), which was filtered, washed with plenty of water, and crystalized from ethanol. Yield 2.4 g, (80%), m.p. 44 °C. 1H NMR (in CDCl3) (ppm), 0.96 (t, 3Hd), 1.45 (t, 2Hc), 2.5 (t, 2Hb), 3.5 (s, 2Ha), 3.8 (s, 3Hf), 6.6 (dd,1Hh), 6.7 (dd, 2Hg), 7.2 (s, 1He).
- b)
3, 5-Dinitro benzoyl chloride:
In one neck round bottom flask: equiped with magnetic stirrer, calcium chloride guard tube, reflux condenser were Placed 30 g (0.141 mol) 3,5-dinitro benzoic acid and 33 g of phosphorous pentachloride and heated the reaction mixture in an oil bath at 120-130 °C for 75 min. Byproduct phosphourous oxytrichloride (POCl3) was removed by vacuum distillation (80 mm/hg), at 110 °C. The residual 3, 5-dinitro benzoyl chloride solidifies on cooling to brown mass, the yield is quanititative. It was recrystalized from carbon tetrachloride. Yield 25 g (77%) m.p. 67-68 °C.
- c)
4-(3′,5′- Dinitro benzoyl amido)- 3-pentadecyl anisole (IV)
In three neck round bottom flask were placed 3.33 g (0.01 mol), of 4-amino 3-pentadecyl-anisole (III) dissolved in 25 mL N-methyl pyrrolidine. The reaction mixture was cooled at 0 °C. 2.31 g (0.01 mol) of 3,5 dinitro benzoyl chloride, was added to reaction mixture and mixture was stirred for 1 h. Then 3 mL triethyl amine, as proton acceptor, was added; reaction stirred at 0 °C for 2 h. Stirring was continued at room temperature for 12 h. Viscous mixture was poured into cold water to precipitate (IV); which was filtered,washed with water and dried. Dry product was, recrystalized from ethanol. Yield 4.6 g; 90%, m.p. 180 °C.
- d)
4-(3′,5′- Diamino benzoyl amido)- 3-pentadecyl anisole (V)
Mixture of 5.27 g (0.01 mol) 4-(3′,5′-dinitro benzoyl amido)- 3-pentadecyl anisol (IV), 10% Pd/C, 0.2 g; 60 mL ethanol were placed in three neck round bottom flask fitted with reflux condenser. The reaction mixture was heated at temperatue 80 °C, and 10 mL hydrazine hydrate, was added dropwise. Reaction mixture was stirred at 80 °C for 16 h; and then filtered throughwhatmann paper to remove Pd/C. The filterate was poured with stirrer into ice water to form precipitate; which was filtered, dried and recrystalized from methanol. Yield 3.8 g 80%, m.p. 150 °C (Fig. 1).
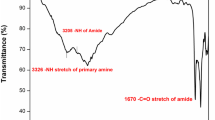
FT-IR spectrum (Fig. 2) showed characteristics absorption bands at 3395 (-NH stretching), 1668 (amide, C=O stretching), and 1597 (amide CO-NH deformation), ether linkage absorption observed at 1265 cm−1 (Fig. 3).
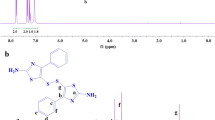
1H NMR spectrum of (V) (in CDCl3, ppm) solvent showed peak at 0.96 (t,3Hg), 1.1 (m, 26 He), 2.5 (t, 2Hd), 3.82 (s, 3H), 5.5 (broad peak, -NH2) 6.6 ppm (broad peak, amide –NH), 6.67 (s, 1Ha), 6.7 (dd, 1Hb), 6.8 (dd,1Hc), and 7.2 (s, 2Hi).13C NMR spectrum of (V); Fig. 4 showed the peak at 149 ppm is assigned to carbon directly attached to –NH2; 157 ppm is due to carbon attached to –O- group, and various aromatic phenyl rings carbon signals at 110, 111, 128, 136, 137. Carbonyl carbon of amide C8 signal appeared at 157 ppm. The 13C DEPT NMR of (V); Fig. 5 (in CDCl3, ppm) shows methylene carbon of pentadecyl chain at 14 (C7), 22 (C6), 29 (C5), 31 (C4). The signal for methoxy group carbon appeared at 55 ppm. Mass spectrum of (V) in (Fig. 6) showed (M + 1) peak as srong base peak at 468 indicating molecular formula weight of 467 for diamine (V), corresponding to molecular formula; C29H45N3O2 .
Polymerization
To 100 mL three neck round bottom flask were added 0.467 g (0.001 mol), 4-(3′,5′- dinitro benzoyl amido)-3-pentadecyl anisole, 3 mL NMP solvent and the solution was stirred for 10 min at room temperature and then cooled to 0 °C. To this solution solid isophthaloyl chloride 0.203 g (0.001 mol), was added in small portions with stirring. The stirring was continued for 1 h at 0 °C and then 0.36 mL triethylamine, was added to the reaction mixture stirring was continued for 2 h. The reaction mixture was allowed to attain room temperature at which it was stirred for 12 h. The content were then poured into 500 mL of methanol. The precipitated polymer was separated by filtration, washed with distilled water, methanol, and then dried under vacuum at 70 °C for 24 h. The yield of the polymer was 0.575 g (96%). Other co-polyamides were also prepared by a similar procedure and their yields and viscosity values are given in Table 1.
Result and discussion
Diamine synthesis
The new aromatic diamine 4-(3′,5′- diamino benzoyl amido)- 3-pentadecyl anisole (V) was synthesized in six steps (Scheme 1). In three step 4-amino-3-pentadecyl anisole (III) was synthesized from 3-pentadecyl phenol as starting material. In fourth step 3,5-dinitrobenzoyl chloride; prepared from 3,5-dinitro benzoic acid; was reacted with 4-amino-3-pentadecyl anisole (III) NMP solvent at room temperature to produce amide linked obtain new aromatic dinitro compounds (IV); In sixth step, (IV) was reduced Catalytically to diamine (V).
It was recrystallized from ethanol. Yield 80%.
Characterization of diamine (V)
The chemical structure of aromatic diamine (V) was Characterized by FT-IR, NMR (1H,13C) and mass spectrum. As per the Scheme 1 synthesized 4-amino-3-pentadecyl anisole (III) was characterized by 1H NMR as shown in Fig. 1.
Polymer synthesis
New aromatic polyamides were synthesized by low temperature solution polymerization methodfrom aromatic diamine (V) with aromatic dicarboxylic acid chloride (IPC/TPC). The results on yield and inherent viscosity of polyamides are presented in Table 1. All the polyamides PA-1 to PA-5 showed inherent viscosity in the range of 0.38–0.52 (dL/g) (Scheme 2).
Polymer characterization
These polyamides PA-1 to PA-5 had the inherent viscosities in the range of 0.30 to 0.52 dL/g, this indicating the formation of moderate molecular weight of polymers.
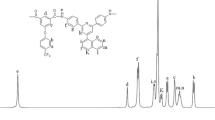
The formation of polyamide was confirmed by infrared spectroscopy. The FT-IR spectrum of PA-1(Fig. 7) showed characteristics amide absorption bands at 1600–1667 (amide C=O stretching), 3250–3300 (-NH stretching), showed characteristic absorptions for aromatic ether linkage at 1265 cm−1.
Polymer solubility
The solubility of polyamides PA-1 to PA-5 was tested in various organic solvents and the results are given in Table 2. A 3% solution of polymer in solvent was taken as criterion for solubility. It was observed that polymers were soluble in aprotic polar solvents such as NMP, DMF, DMSO, DMAC and m-cresol. The solubility of these polyamides may be due to presence of pendant long aliphatic chain substituent containing methoxyphenyl moiety via amide linkage to the chain of polymer. Polyamides were partially soluble in chloroform and dichloromethane, long pendant group discounts polarity and endows with its solubility low boiling point solvent, DCM and chloroform.
Thermal properties
Thermal stability for polyamides PA-1 to PA-5 was determined by thermogravimetric analysis under nitrogen atmosphere at heating rate of 10 °C/min. The TGA curves of polyamides are shown in (Fig. 8). Initial decomposition temperature (Ti) and Temperature at 10% weight loss (T10) were determined from thermograms and the data is given in Table 3. Initial decomposition temperature of polyamides is well above 320 °C; in the range of 320 to 364 °C. maximum weight loss temperature (TMax) and 10% weight loss at temperature (T10) were in the range 401 to 418 °C and 359 to 387 °C, respectively. The char yield at 900 °C 7 to 12. These parameter indicated good thermal stability of these polymers.
DSC was used to evaluate the thermal transitions of the polyamides PA-1 to PA-5. The influence of the residual solvent or the absorbed moisture and history of thermal annealing is sometimes observed in the first heating scan of DSC. The polyamides were heated under nitrogen at a heating rate of 20 °C/min; The Tg values of the polyamides (Fig. 9) and results are summarized in Table 3. The glass transition temperature of polyamides PA-1 to PA-5 were in the range of 196 to 249 °C, depending on the structure of the aromatic diamine (V) component, interchain amide (-CO-NH) strong interaction and m/p catenation due to aromatic acid structure. The flexibility of polymer reduces by increase chain stiffness and close packing structure. m-Orientation linkage in the backbone of polymers imparts relatively low Tg as compared to p-oriented aryl-amide units. Polyamide PA-5, containing rigid p-phenylene unit derived from TPC could not clearly show transition of Tg; where as PA-4 containing higher proportion of TPC derived structure showed higher Tg at 249 °C. Polyamide PA-1 obtained from IPC showed low Tg of 200 °C; due to m-catenated polyamides. In general polyamides derived from showed Tg in the range of 196 to 207 °C for IPC contents and pendant methoxyphenyl group having bulky long pentadecyl substituent.
The wide angle X-Ray diffraction studies of the polyamides were performed with powdered polymers and the XRD patterns of PA-1 to PA-5 are shown in (Fig.10). Broad amorphous halos were observed in wide angle X-ray diffraction of all polyamides in the range of 2θ = 15 to 300. All the polyamides have amorphous nature due to presence of pendant methoxyphenyl group; long aliphatic pentadecyl chain and 1, 3 oriented aromatic diamine into the backbone of polymer which may have disrupted the chain regularity and packing. The amorphous nature of polyamides reflects in good solubility of polyamides in polar aprotic solvents.
Summary and conclusion
-
1.
Synthesis for new aromatic diamine containing pendant methoxyphenyl moiety with pentadecyl substituent via amide linkage namely 4-(3′,5′-diamino benzoyl amido)-3-pentadecyl anisole was successfully performed and characterized by FT-IR,1H and 13C NMR, spectroscopy.
-
2.
A series of Polyamides was synthesized by low temperature solution polymerization of new aromatic diamine 4-(3′,5′-diamino benzoyl amido)-3-pentadecyl anisole with two aromatic diacid chlorides, Isophthaloyl chloride (IPC), Terephthaloyl chloride (TPC). Inherent viscosities were in the range of 0.30 to 0.52 dL/g in DMAc.
-
3.
Polyamides were soluble in aprotic polar solvents such as NMP, DMAc, DMSO, DMF and m-cresol due to the m-catenated polyamide structure derived from new diamine (V) and due to pendant methoxyphenyl moiety containing long aliphatic pentadecyl substituent in the chain of polymer disrupted the chain regularity, and close packing structure of polymers.
-
4.
Thermogravimetric Analysis of polymer shows initial weight loss at temperatures in the range of 320 to 364 °C indicating good thermally stability of these polyamides.
-
5.
The glass transition temperature (Tg) were determined by Differential scanning Calorimetry (DSC) and values were in the range of 196 to 249 °C.
-
6.
XRD of all the polyamides have amorphous nature.
References
Tagle LH, Terraza CA, Leiva A, Devilat F (2008) Synthesis, characterization, and thermal studies of polyamides and polyimides containing two silarylene units in the main chain. J Appl Polym Sci 110:2424–2431
Ghaemy M, Nasr F, Alizadeh R, Taghavi M (2012) Synthesis and characterization of novel photoactive, thermally stable, and organosoluble polymers based on carbazole and imidazole derivatives in the main-chain. J Macromol Res 20:614–622
Ghaemy M, Behmadi H, Alizadeh R (2009) Synthesis of organosoluble polyamides with bulky triaryl imidazole pendent group. Chin Chem Lett 20:961–964
Yamazaki N, Higashi F, Kawataba J (1974) Studies on reactions of the N-phosphonium salts of pyridines. XI. Preparation of polypeptides and polyamides by means of triarylphosphites in pyridine. J Polym Sci A Polym Chem 12:2149
Espeso JF, de la Campa JG, Lozano AE, De Abajo A (2000) Synthesis and characterization of new soluble aromatic polyamides based on 4-(1-adamantyl)-1, 3-bis(4-aminophenoxy)benzene. J Polym Sci A Polym Chem 38:1014–1023
Hsiao SH, Chang YH (2004) New soluble aromatic polyamides containing ether linkages and laterally attached p-terphenyls. Eur Polym J 40:1749–1757
Ferreiro JJ, De la Campa JG, Lozano AE, De Abajo J, Preston J (2007) Synthesis and evaluation of properties of novel poly(benzimidazole-amide)s. J Polym Sci Part A Polym Chem 45:4671–4683
Liu XL, Wu D, Sun R, Yu LM, Jiang JW, Sheng SR (2013) Synthesis and properties of novel soluble fluorinated polyamides containing pyridine and sulfone moieties. J Fluor Chem 154:16–22
Maji S, Sen SK, Dasgupta B, Chatterjee S, Banerjee S (2009) Synthesis and characterization of new poly(ether amide)s based on a new cardo monomer. J Polym Adv Tech 20:384–392
Wu SC, Shu CF (2003) Synthesis and properties of soluble aromatic polyamides derived from 2,2′-bis(4-carboxyphenoxy)-9,9′-spirobifluorene. J Polym Sci Part A Polym Chem 41:1160–1166
Matsuo S (1994) Synthesis and properties of poly(arylene ether phenyl-s-triazine)s. J Polym Intern 32:2093
Debaditya B, Venkat P, Banerjee S (2015) Highly gas permeable polyamides based on substituted Triphenylamine. J Macromolecules 48:4541
Salunkhe PH, Patil YS, Patil VB, Nawale YH, Dhole IA, Ubale VP, Maldar NN, Ghanwat AA (2018) Synthesis and characterization of conjugated porous polyazomethines with excellent electrochemical energy storage performance. J Polym Res 25:147
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tamboli, A.B., Maldar, N.N. Soluble aromatic polyamides containing pendant pentadecyl substituted methoxy phenyl unit. J Polym Res 26, 139 (2019). https://doi.org/10.1007/s10965-019-1799-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1799-0