Abstract
Poly (o-aminophenol-co-m-phenylenediamine) P(oAP-mPDA) as a copolymer is successfully synthesized by mechanochemical solid state polymerization (MCSSP) as a green and simple method. The mechanochemical solid state polymerization is achieved by a developed Mortar Grinder RM200 without using any solvents during the preparation process. The copolymer was also prepared by traditional interfacial polymerization method (IP). A comparison between the resulting copolymers of the two methods is conducted by various analyses including, Fourier transform infrared spectra (FTIR), ultra violet, visible spectra (UV-Vis), X-ray diffraction (XRD), scanning electron microscope (SEM), and thermogravimetric analysis (TGA). The analyses revealed that there is a good agreement between the two copolymers synthesized by MCSSP and IP method. The energy band gap was determined and found to be 2.19 eV and 2.09 eV for the copolymer synthesized by MCSSP method and the IP method, respectively. The investigated copolymers are located in the semiconductor material range and displayed a good thermal stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conjugated polymers have super physical and chemical properties such as lightweight, corrosion resistance, and flexibility of design. They have been widely applied in semiconductors as organic materials and photoelectronic fields, based on their electron-conjugated system [1,2,3,4,5].Various synthesizing methods of polyaniline or polyaniline derivatives have been applied for example, electropolymerization, interfacial polymerization, seeding polymerization, electrospinning, template free method and chemical oxidative polymerization method [6,7,8,9,10,11]. In comparison with the conventional synthesis methods, the solid-state synthesis route has many advantages including reduced pollution, rapid, simple, free hazard solvent, economically method and considered environmental friendly route [12,13,14,15]. By solid-state polymerization method, volatile organic solvents during polymerization are overcome [16]. The chemical synthesis of conducting polymers is attractive owing to its ability to fulfill the requirement of big quantities and ability to control the product properties by varying the reaction conditions [17,18,19,20]. Recently, polyorthoaminophenol as a hompolymer has been synthesized by Zoromba and Abdel-Aziz based on MCSSP method [16]. This work aims to polymerize ortho amino phenol with meta phenylene diamine as comonomers by MCSSP as an eco-friendly method (free organic solvents) and compare the product with that of the conventional standard interfacial polymerization method. The properties of the resulting P(oAP-mPDA) are investigated by comparative studies of FTIR, UV-vis, XRD, SEM, and the energy band gap (Eg) value.
Materials and methods
All chemicals were used without further purification. Ortho-amino phenol was purchased from Aldrich, ammonium persulphate, m. phenylene diamine, ethanol, and chloroform, dimethyl formamide were purchased from Merck.
Synthesis of o-aminophenol with m-pheylene diamine copolymer by mechanochemical solid polymerization
This copolymer was synthesized by ammonium persulphate as an oxidizing agent. Typically, 1.6 g o-aminophenol monomer and 1.6 g meta phenylene diamine and 4.1 g ammonium persulphate initiator in a solid state were mixed by a mechanical stirrer at 950 rpm for 10 min at room temperature. The resulting solid mixture was kept at room temperature for 4 h and then grinded by using a Grinder RM 200 for 10 min as shown in Fig. 1. The observed color spontaneously changed to grey at the beginning of the milling process. The resulting grey solid mixture from the grinding process was dispersed in 100 ml equimolar ratio of ethanol and distilled water (1:1) using magnetic stirrer with gentle heating in order to dissolve unreacted monomer, oligomer and excess initiator. Filtration process was carried out for the obtained copolymer. The solid retained on the filter paper (copolymer) was washed with distilled water several times followed by ethanol. The resulting copolymer (2.3 g) was assigned by P(oAP-mPDA)SP.
Synthesis of o-aminophenol, m-pheylenediamine copolymer by interfacial polymerization as traditional method
The two-phase polymerization was performed in 200 ml bottles at room temperature. The same amount, the ratio of the reactants and the initiator in the MCSSP method were applied in the interfacial polymerization method. OAP and mPDA monomers were dissolved in chloroform, ammonium persulphate as an initiator was dissolved in distilled water. Separately, ammonium persulphate (oxidizing agent) solution was added gently alongside of co-monomers mixture container. The copolymer formation was gradually observed between the organic and aqueous layers during the addition process. The entire aqueous phase was filled with copolymer homogeneously. The resulting copolymer was separated from the two phases and collected. Then it was washed several times with distilled water, followed by ethanol to remove unreacted monomer, oligomers and excess of the oxidizing agent. The resulting copolymer (2.5 g) was dried at 60 °C for 24 h and assigned by P(oAP- mPDA)IP.
Characterization
FT-IR analysis
The FT-IR spectra of the considered copolymers powder were recorded using World’s Smallest Laboratory FT-IR Spectrometer. The ALPHA in combination with Bruker Optics powerful OPUS/Mentor software and extensive infrared libraries provide easy-to- use turnkey solutions where unknown substances are identified in seconds.
UV-visible absorption spectra measurements
The UV–visible absorption spectra of prepared copolymer samples were measured using Shimadzu UV–visible spectrophotometer (M160 PC) at room temperature in the range of 200–800 nm. The copolymers was dissolved in dimethyl formamide (DMF) and DMF has been taken as a reference solvent as well during the measurement performance. The optical band gap (Eg) was measured from the absorption spectra data. Eg was calculated from Tauc’s relation for direct transitions [21, 22].
Thermal analysis
The thermal stability of the copolymers was recorded by thermogravimetric analyzer- TGA 4000 PerkinElmer. The thermal decomposition of the considered copolymer was applied under N2 atmosphere in the range of 30–750 °C at a heating rate of 10 °C/min.
X-ray diffraction
The X-ray diffraction spectra (XRD) of the copolymer samples was carried out by Burker D8 advanced XRD (x-ray diffractometer using the Cu-Kα radiation wavelength (λ = 1.54 Å). The scanning range was set from 5 to 80°. The operating conditions were 40, divengency slit: 1 mm, Ni filter, Lynx Eye one dimensional detector and detector slit 3 mm. The crystallinity analysis is calculated based on the peak area from EVA software.
SEM
The surface morphological study of the copolymers powders was carried out using scanning electron microscope (SEM), FE-SEM Zeiss Leo Supra 55. Samples were sputtered by a thin film of gold to improve the conductivity.
Results and discussion
FTIR spectra
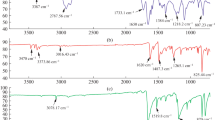
Figure 2 shows that FT-IR of ortho aminophenol and meta phenylene diamine copolymer prepared by mechanochemical solid state polymerization P(oAP-mPDA)SP and interfacial polymerization P(oAP- mPDA)IP. The two broads centered at 3423 and 3261 cm-1 indicate to the presence of secondary amino groups. This confirms the occurrence of polymerization and formation of the copolymer in both methods (MCSSP and IP) through –NH- linkage. The strong bands at 1587 and 1571 cm-1 can be attributed to C-N stretching vibrations for quinoid structure for P(oAP-mPDA)SP and P(oAP-mPDA)IP copolymers, respectively. The bands are located at 1498 and 1494 cm−1 can be attributed to C-N stretching vibrations for benzenoid structure for P(oAP-mPDA)SP and P(oAP-mPDA)IP copolymers, respectively. The absorption bands at 1297 and 1313 cm−1 for P(oAP-mPDA)SP and P(oAP-mPDA)IP indicate to C-O-C [23, 24] (etheric linkage) structure in the copolymer forming ladder structure in the copolymer either prepared by MCSSP or IP method. As can be seen from the FTIR analysis, the resulting bands from P(oAP-mPDA)SP is in a good agreement with the resulting band from for P(oAP-mPDA)IP.
UV-visible absorption spectra measurements
Figure 3 shows the Uv-visible absorption spectra measurements of P(oAP-mPDA)SP and P(oAP- mPDA)IP. Figure 3 reveals that there is a good agreement between the two main peaks from each copolymer. The investigated peaks for both copolymers are located at 293, and 501 nm. These peaks are characteristics for π -π* and π –polaron transition through the copolymer chains. The third peak which is a characteristic for polaron -π* is located at 346 nm for P(oAP- mPDA)SP and for P(oAP-mPDA)IP is located at 409 nm. At the third peak, Bathochromicaly red shift occurred from 346 nm in P(oAP-mPDA)SP to 409 nm in P(oAP-mPDA)IP. This can be attributed to that the nucleation process in the mother solution has more chance to takes place in the IP method of the copolymer than in the MCSSP method. This process gives P(oAP-mPDA)IP more ordered structure, longer chains, and more crosslinking compared to the P(oAP-mPDA)SP. The long chain increase the possibility of the presence of double bonds alternative with single bond (conjugated system) which leads to red shift [16].
Determination of optical band gap
The optical band gap (Eg) was measured from the absorption spectra data. Eg was calculated from Tauc’s relation for direct transitions [22]. The absorption coefficient (α) was calculated from the absorption spectra data by applying the relationships α = A/d, where d is the light path length. For allowed direct transitions, the optical band gap can be described by:
B is a parameter depends on the transition probability. Plotting (αhν)1/2 versus hν the direct transitions could be obtained by extrapolating the linear portion of the curve to (αhν)1/2 = 0. P(oAP-mPDA)SP has higher value of Eg than that of P(oAP-mPDA)IP as shown in Fig. 4. The optical band gaps (Eg) were estimated and it was found to be 2.19 and 2.09 eV for P(oAP-mPDA)SP and P(oAP-mPDA)IP respectively. The observed values of optical band gaps reveal that the investigated copolymer either synthesized by MCSSP method or IP method are located in the semiconductor materials range. The energy band gap value of P(oAP-mPDA)SP is higher than the energy band gap value of P(oAP-mPDA)IP, this is in a good agreement with the results reported in previous works [16]. On the other hand, the neutral polyaniline as a homopolymer has a band gap of 2.8 eV while doped polyaniline has an optical band gap of 2.21 eV. This means that polyaniline is showing strong absorption for visible light and for this reason some researchers used it in photocatalysis [25, 26]. The band gap energy of un-doped poly ortho phenylene diamine was found to be 2.25 eV [27]. The energy gap for polyaniline ortho aminophenol copolymer was found to be 2.35 eV [3]. Based on the calculated band gap values for the present investigated copolymers, they are located in the semiconductor materials range.
Thermogravimetric analysis (TGA)
The thermal decomposition of the copolymer was carried out on three stages. The first stage is within the range of 50–150 °C and it can be attributed to the evaporation of moisture from the copolymer. The second thermal stage within the range of 150–370 °C is due to thermal decomposition of the copolymer backbone into different number of chemical forms. The third stage is within the range 370–800 °C and it can be attributed to the final carbonization of the copolymer. This due to the thermal decomposition of the copolymer chains into the number of chemical forms [28, 29]. From TGA curves (Fig. 5), it can be noticed that P(oAP- mPDA)IP is more thermally stable than P(oAP-mPDA)SP, this can be attributed to the higher molecular weight of the copolymer synthesized by the IP method. The copolymer synthesized by the IP method has longer chains and more crosslinking than copolymer synthesized by solid state polymerization method. Both P(oAP-mPDA)SP and P(oAP-mPDA)IP have a good thermal stability (there is no decomposition before 370 °C) due to their rigid chain structure. Thus, the resulting copolymer could be useful for various applications by the engineering industry in different fields, including batteries [30, 31], solar cells [32, 33], sensors [34,35,36,37], playing roles in supercapacitors [38], electrochromic devices [39], and biomedical applications [40].
XRD analysis
Figure 6 shows the X-ray diffraction patterns of P(oAP-mPDA)SP and P(oAP-mPDA)IP. The diffraction patterns display 65.2% crystallinity of P(oAP-mPDA)IP and 17.7% crystallinity of P(oAP-mPDA)SP. The crystallinity value of P(oAP-mPDA)IP copolymer is smaller than that of polyortho-aminophenol homopolymer [16], while the crystallinity of P(oAP-mPDA)SP is differ than the crystallinity of P(oAP-mPDA)IP. The crystallinity difference between the two copolymers P(oAP-mPDA)IP and P(oAP-mPDA)SP may be attributed to that the chance for the molecules to be ordered in crystal structure in IP method is more than in MCSSP method. Copolymer synthesized by IP method exhibits strongest peaks at 6.8°, 10°, 18.5°, 24°, 25.4, 27.7° and 32 °. The copolymer synthesized by MCSSP method exhibits strongest peaks at 10°, 15.8°, 18.5°, 25.4 and 27.7°. 2θ = 25.4° is the characteristics of the van der Waals distances between stacks of phenylene rings through the copolymer chains [28, 41]. The crystallinity and orientation of conducting polymers have been interested, because more highly ordered systems can display a metallic conductive state (this in agreement with calculated optical band gap values as semiconductors materials) and may influence the anticorrosion performance [42].
Surface morphology
Figure 7a and b, shows the surface morphology of the resulting copolymers from the two methods and the images reveal that it is in the micro-scale. On the other hand, there is no similarity between the two SEM images. Therefore, surface morphology of the copolymer varies according to the preparation method. Figure 7b shows that SEM micrograph of P(oAP-mPDA)IP copolymer displays the sphere microparticles with different size.
Conclusions
Poly (ortho amino phenol –co- meta phenylene diamine) as a copolymer was successfully synthesized by mechano-chemical solid polymerization. An approach to a green method, the mechanochemical solid-state polymerization was achieved by using Mortar Grinder RM200 without using organic solvents during the preparation process. Poly (ortho amino phenol –co- meta phenylene diamine) copolymer was synthesized also by interfacial polymerization as a traditional method. Based on the analysis, there is a good agreement between the chemical structures of the copolymer synthesized by the MCSSP method and the IP method. The energy band gap was found to be 2.19 and 2.09 eV for the copolymers synthesized by the MCSSP method and the IP method, respectively. Copolymer synthesized by the MCSSP method displays a lower crystallinity than the copolymer synthesized by IP method. The copolymer prepared by either MCSSP or IP is located in the semiconductor materials range and they displayed a good thermal stability.
References
Salunkhe PH, Patil YS, Patil VB, Navale YH, Dhole IA, Ubale VP, Maldar NN, Ghanwat AA (2018) Synthesis and characterization of conjugated porous polyazomethines with excellent electrochemical energy storage performance. J Polym Res 25:147
Zoromba MS, Alghool S, Abdel-Hamid SMS, Bassyouni M, Abdel-Aziz MH (2017) Polymerization of aniline derivatives by K2Cr2O7 and production of Cr2O3 nanoparticles. Polym Adv Technol 28:842–848
Slimane AB, Al-Hossainy AF, Zoromba MS (2018) Synthesis and optoelectronic properties of conductive nanostructured poly (aniline-co-o-aminophenol) thin film. J Mater Sci Mater Electron 29:8431–8445
Hosny NM, Zoromba MS, Samir G, Alghool S (2016) Synthesis, structural and optical properties of nanoparticles derived from Cr doped polyanthranilic acid (CrPANA). J Mol Struct 1122:117–122
Li C, Li Y, Wang X, Zhang B, Chen Y (2015) Synthesis and photovoltaic properties of conjugated copolymers containing cyclopentadithiophene and two different electron-deficient moieties in the polymer backbone. J Polym Res 22:96
Zhang Z, Deng J, Wan M (2009) Highly crystalline and thin polyaniline nanofibers oxidized by ferric chloride. Mater Chem Phys 115:275–279
Chen CH, Ko CJ, Chuang CH, Mao CF, Liao WT, Hsieh CD (2017) Synthesis and characterization of polyaniline co-doped with nitric acid and dodecyl benzene sulfonic acid. J Polym Res 24:10
Jafari Y, Ghoreishi SM, Shabani-Nooshabadi M (2016) Electrochemical deposition and characterization of polyaniline-graphene nanocomposite films and its corrosion protection properties. J Polym Res 23:91
Pinto NJ, Ramos I, Rojas R, Wang PC, Johnson Jr AT (2008) Electric response of isolated electrospun polyaniline nanofibers to vapors of aliphatic alcohols. Sensors Actuators B Chem 129:621–627
Zoromba MS, Ismail MIM, Bassyouni M, Abdel-Aziz MH, Salah N, Alshahrie A, Memic A (2017) Fabrication and characterization of poly (aniline-co-o- anthranilic acid)/ magnetite nanocomposites and their application in wastewater treatment. Colloid Surface A: Physicochem Eng Aspects 520:121–130
Zhou CF, Du XS, Liu Z, Ringer SP, Mai YW (2009) Solid phase mechanochemical synthesis of polyaniline branched nanofibers. Synth Met 159:1302–1307
Hosny NM, Nowesser N, Al-Hussaini AS, Zoromba MS (2016) Doped copolymer of polyanthranilic acid and o-aminophenol (AA-co-OAP): synthesis, spectral characterization and the use of the doped copolymer as precursor of α-Fe2O3 nanoparticles. J Mol Struct 1106:479–484
Somboonsub B, Srisuwan S, Invernale MA, Thongyai S, Praserthdam P, Scola DA, Sotzing GA (2010) Comparison of the thermally stable conducting polymers PEDOT, PANi, and PPy using sulfonated poly (imide) templates. Polymer 51:4472–4476
Abdiryim T, Xiao-Gang Z, Jamal R (2005) Comparative studies of solid-state synthesized polyaniline doped with inorganic acid. Mater Chem Phys 15:367–372
Meng H, Perepichka DF, Bendikov M, Wudl F, Pan GZ, Yu W, Dong W, Brown S (2003) Solid-state synthesis of a conducting polythiophene via an unprecedented heterocyclic coupling reaction. J Am Chem Soc 125:15151–15162
Zoromba MS, Abdel-Aziz MH (2017) Ecofriendly method to synthesize poly (o-aminophenol) based on solid state polymerization and fabrication of nanostructured semiconductor thin film. Polymer 120:20–29
Basavaraja C, Veeranagouda Y, Lee K, Vishnuvardhan TK, Pierson R (2010) Synthesis and characterization of conducting polypyrrole-polymannuronate nanocomposites. J Polym Res 17:233–239
Mahmood WA, Azarian MH (2016) Sol-gel synthesis of polyaniline/zirconia composite conducting materials. J Polym Res 23:88
Li XG, Huang MR, Hua YM (2005) Facile synthesis of processible aminoquinoline/phenetidine copolymers and their pure semiconducting nanoparticles. Macromolecules 38:4211–4219
Li XG, Huang MR, Duan W, Yang YL (2002) Novel multifunctional polymers from aromatic diamines by oxidative polymerizations. Chem Rev 102:2925–3030
Gupta B, Prakash R (2012) Interfacial polymerization of polyanthranilic acid: morphology controlled synthesis. Macromol Chem Phys 213:1457–1464
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Ge and Si, Mater Res Phys Status Solidi B 15:627–637
Barbero C, Silber JJ, Sereno L (1989) Formation of a novel electroactive film by electropolymerization of ortho-aminophenol: study of its chemical structure and formation mechanism. Electropolymerization of analogous compounds. J Electroanal Chem 263:333–352
Tucceri R, Arnal PM, Scian AN (2012) Spectroscopic characterization of poly (ortho-aminophenol) film electrodes: a review article. J Spectroscopy 2013:1–26
Yu S, Xi M, Han K, Wang Z, Yang W, Zhu H (2010) Preparation and photoelectrocatalytic properties of polyaniline/layered manganese oxide self-assembled film. Thin Solid Films 519:357–361
Li J, Zhu L, Wu Y, Harima Y, Zhang A, Tang H (2006) Hybrid composites of conductive polyaniline and nanocrystalline titanium oxide prepared via self-assembling and graft polymerization. Polymer 47:7361–7367
Olgun U, Gülfen M (2014) Doping of poly (o-phenylenediamine): spectroscopy, voltammetry, conductivity and band gap energy. React Funct Polym 77:23–29
Thenmozhi G, Arockiasamy P, Santhi RJ (2014) Isomers of poly aminophenol: chemical synthesis, characterization, and its corrosion protection aspect on mild steel in 1 M HCl. Int J Electrochem 2014:1–11
Sayyah SM, El-Rabiey MM, El-Rehim SS, Azooz RE (2006) Electropolymerization kinetics of o-aminophenol and characterization of the obtained polymer films. J Appl Polym Sci 99:3093–3109
Dirlam PT, Glass RS, Char K, Pyun J (2017) The use of polymers in Li-S batteries: a review. J Polym Sci Part A: Polym Chem 55:1635–1668
Mobarak Y, Bassyouni M, Almutawa M (2013) Materials selection, synthesis, and dielectrical properties of PVC nanocomposites. Adv Mater Sci Eng 2013:1–6
Zoromba MS, Abdel-Aziz MH, Bassyouni M, Bahaitham H, Al-Hossainy AF (2018) Poly (o-phenylenediamine) thin film for organic solar cell applications. J Solid State Electrochem 22:3673–3687
Al-Hossainy AF, Thabet HK, Zoromba MS, Ibrahim A (2018) Facile synthesis and fabrication of a poly (ortho-anthranilic acid) emeraldine salt thin film for solar cell applications. New J Chem 42:10386–10395
Zoromba MS (2017) Novel and economic Acid-Base Indicator based on (p-toluidine) oligomer: synthesis; characterization; photoluminescence and Solvatochromism applications. Spectrochim Acta A: Mol Biomol Spectroscopy 187:61–67
Zoromba MS, Abdel-Aziz MH, Bassyouni M (2017) New microstructured chromium doped poly (p-toluidine) as a new acid–base indicator and precursor for chromic oxide nanostructured. Polym Adv Technol 28:1743–1749
Naveen MH, Gurudatt NG, Shim YB (2017) Applications of conducting polymer composites to electrochemical sensors: a review. Appl Mater Today 9:419–433
El-Ashtoukhy ESZ, Abdel-Aziz MH (2013) Removal of copper from aqueous solutions by cementation in a bubble column reactor fitted with horizontal screens. Int J Miner Process 121:65–69
Zoromba MS, Abdel-Aziz MH, Bassyouni M, Gutub S, Demko D, Abdelkader A (2017) Electrochemical activation of graphene at low temperature: the synthesis of three-dimensional nanoarchitectures for high performance supercapacitors and capacitive deionization. ACS Sustain Chem Eng 5:4573–4581
Wang H, Barrett M, Duane B, Gu J, Zenhausern F (2018) Materials and processing of polymer-based electrochromic devices. Mater Sci Eng B 228:167–174
Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S (2010) Applications of conducting polymers and their issues in biomedical engineering. J Royal Soc Interface 7:S559–S579
Xia H, Wang Q (2002) Ultrasonic irradiation: a novel approach to prepare conductive polyaniline/nanocrystalline titanium oxide composites. Chem Mater 14:2158–2165
Saravanan C, Palaniappan S, Chandezon F (2008) Synthesis of nanoporous conducting polyaniline using ternary surfactant. Mater Lett 62:882–885
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zoromba, M.S., Abdel-Aziz, M.H., Bassyouni, M. et al. Synthesis of o-aminophenol-m-phenylenediamine copolymer: an eco-friendly approach. J Polym Res 26, 34 (2019). https://doi.org/10.1007/s10965-019-1700-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1700-1