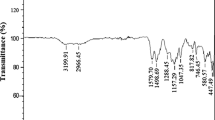
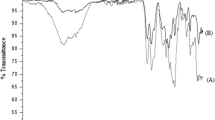
Abstract
Poly(aniline-co-(m-aminobenzoic acid)) was synthesized electrochemically at graphite electrode under galvanostatic conditions. Aqueous electrolyte for synthesis was consisted of HCl and different amount of aniline and m-aminobenzoic acid. The presence of the meta positioned carboxylic group in m-aminobenzoic acid influenced higher co-polymerization potential, different morphology and electrochemical behavior of copolymers compared to polyaniline. Electrochemical activity is achieved by proton exchange in neutral environment that can result in a faster charge/discharge process, which is in the case of PANI limited by slow anion exchange, making this material promising for consideration in super-capacitors and in biological system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyaniline (PANI), apart from being the eldest intrinsically conducting polymer (ICP), is probably the most investigated owing to a low cost monomer, existence of different oxidation states, electrical conductivity, reversible electrochemistry and optical activity. These properties are promising for commercialization in various fields such as: electrochemical power sources, supercapacitors, electrochromic devices, light emitting diodes, sensors and biosensors, protective coatings, nanotechnology etc. [1,2,3,4]. Despite mentioned characteristics, practical application of PANI is, similarly to other ICP, limited by low solubility and processability. Another limitation of PANI is lost of electrochemical activity and conductivity at pH ˃ 3, which is particularly important for application in the biological systems, in the field of supercapacitors, electrochemical power sources etc. These limitation initiated research in the field of self-doped PANI [5, 6]. Self-doped PANI has structure, in which negatively charged functional groups covalently bonded to a polymer chain, have role of an intramolecular dopant, thus compensating positive charge on nitrogen in the polymer chain by replacing dopant anions from the auxiliary solution. The structural modification by introduction of inner anions as dopants alters many properties of self-doped PANI comparing to PANI itself, such are: lower molecular weights, increased solubility lower DC conductivity and electrochemical activity extended to higher pH [6,7,8,9,10,11,12,13].
Synthesis of self-doped PANI can be achieved both by chemical and electrochemical procedures. Chemical synthesis can be performed by either copolymerization of aniline along with substituted aniline or by homopolymerization of substituted aniline bearing negatively charged groups attached to ortho or meta position. Amaya et al. recently reported phosponic acid as self-doping anion attached to polyaniline backbone, moreover they have polymerized both 2-metoxyaniline-5phosponic acid 2-metoxyaniline-5phosponic acid monoethyl ester and investigated its characteristics [14]. Chemical synthesis is usually performed by oxidative polymerization using common oxidants, such is ammonium peroxodisulfate [15]. For example, Yang et al. reported the formation of nanofibrous self-doped polyaniline obtained by self-assembly method in which aniline was copolymerized with o-aminobenzenesulfonic acid that had acted both as self-doping monomer and surfactant [16]. Since introduction of variety of functional groups with steric and inductive effects may bring difficulties to the polymerization process, controlled postpolymerization treatments were also applied. Barbero et al. considered various post-polymerization reactions such are: nucleophilic addition, coupling of diazonium salts and electrophilic aromatic substitution. They also pointed out the differences of electrochemical and spectroscopic behavior introduced by added functionalities [17]. Generally, electrochemical polymerization has certain advantages. The desired ICP is obtained at anode upon application of the positive potential hence; the usage of oxidants is avoided, leading to increased purity of the polymer. On the other hand, the polymer is directly deposited onto electrode and further electrochemical characterization is facilitated. Potential controlled techniques are practically always used for electrochemical synthesis of self-doped PANI mostly on the inert anodes from the acidic electrolytes containing aniline and its derivate bearing self-doping functional groups. The most common technique is cyclic voltammetry [12, 18,19,20,21,22]. Potetiostatic technique was also applied. For example, Mazeikiene et al. potentistatically deposited self-doped polyaniline on the gold from sulfuric acid electrolyte containing aniline and m-aminobenzoic acid [23]. Ghenaatian et al. obtained nanostructured self-doped polyaniline from hydrochloric electrolyte containing same monomers onto stainless steel electrode. Other, less common potential controlled techniques refers to reverse pulse voltammetry or multi-potential step method. For example, Ghenaatian et al. applied series of potential pulses with specified duration and decrement between the pulses in order to obtain nanostructures of self-doped polyaniline from acidic solution of aniline and m-aminobenzoic acid on platinum electrode [24] Similarly, Bian et al. performed electro-co-polymerization of aniline and m-benzenesulfonic acid on functionalized carbon cloths by multipoltential step procedure in which they imposed the working electrode ten times to two potential steps during specified time and duration [25]. ICP can be electrochemicaly synthetized using galvanostatic technique which refers to formation of the polymer at constant rate (current density). The polymer film, obtained at the end of the polymerization is in doped, i.e. conductive form. Galvanostatic technique offers control of the polymer film thickness by estimation of the time of the polymerization process. Owing the simplicity, galvanostatic technique is also suitable for practical application. Since there are no data on application of galvanostatic technique in the electrochemical co-polymerization of aniline and its derivates with self-doping anionic groups, the purpose of this work was an attempt to galvanostatily co-polymerize aniline in the presence of different amount of m-aminobemzoic acid in order to obtained poly(aniline-co-(m-aminobenzoic acid)) (PAMBA) and to characterize its self-doping features.
Experimental procedure
Electrochemical synthesis
Electrochemical synthesis of PANI and PAMBA on graphite electrode was performed galvanostativally at constant current density of 1.0 mA cm−2 from aqueous electrolyte of 1.0 mol dm−3 HCl (p.a. Merck) with the total monomer concentration of 0.10 mol dm−3 and different molar ratio of aniline and m-aminobenzoic acid. Prior to use aniline (p.a. Aldrich) was distilled in argon atmosphere, while m-aminobenzoic acid (p.a. Aldrich) was used as received.
Before electrochemical synthesis, the working electrode, cylindrically shaped graphite (A = 0.5 cm2) inserted in a teflon holder, was first mechanically polished with emery papers (2/0, 3/0 and 4/0, respectively), and then with polishing alumina (0.5 μm, Banner Scientific Ltd.) on polishing cloths (Buehler Ltd.). The traces of the polishing alumina were removed from the electrode surface in ethanol ultrasonically during 5 min.
Characterization
UV-Vis spectroscopy measurements recorded with Uni SPEC 2, were used for characterization of electrochemically synthesized PANI and PAMBA with different molar ratio of aniline and m-aminobenzoic acid. The spectra of electrochemically synthesized PANI and PAMBA were taken upon dissolution in N-Metil-2-pirolidon (HPLC grade, Merck). The spectra were collected at 0.2 nm resolution and the results were presented as relative absorbance taking absorbance related to Q peak (electron excitation from the highest occupied molecular orbital, HOMO, of the benzenoid rings to the lowest unoccupied molecular orbital, LUMO of the quinonoide rings) as unit.
SEM micrographs of electrochemically synthesized PANI and PAMBA with different molar ratio of aniline and m-amninobenzoic acid were taken at 20 kV using field emission scanning electron microscope SEM MIRA 3 TESCAN.
Electrochemical characterization of PANI and PAMBA electrodes was performed by cyclic voltammetry in 0.5 mol dm−3 HCl and in phosphate buffer solution (PBS), 0.1 M at pH 7, with scan rate of 20 mV s−1. The following procedure was applied prior to cyclic voltammetry experiments. After electrochemical synthesis of PANI and PAMBA, electrodes were first galvanostatically discharged with 1 mA cm −2 to the potential of −0.6 V, rinsed in bidistilled water and transferred to another electrochemical cell, before cyclic voltammetry was initiated, electrodes had been held at −0.5 V during 600 s in order to be fully discharged (dedoped), after which experiments were performed to anodic potential limit of 1.0 V.
Electrochemical cells and instrumentation
All electrochemical experiments were carried out at ambient temperature (23±1 °C) in a standard three compartment electrochemical cell. Saturated calomel electrode (SCE) served as reference electrode, while Pt wire was used as counter electrode. The experiments were performed using potentiostat/galvanostat SP-300 BioLogic Science Instruments, connected to a PC.
Results and discussion
Electrochemical synthesis of polyaniline and poly(aniline-co-(m-aminobenzoic acid))
Electrochemical synthesis of aniline and copolymerization of aniline and m-aminobenzoic acid (with overall monomer concentration of 0.1 mol dm−3) at graphite electrode from 1.0 mol dm−3 HCl, at constant current desnsity of 1.0 mA cm−2, during 1000 s is given in Fig. 1.
As it can be seen in Fig. 1. electrochemical polymerization of anilne occurred with fast increase of the potential followed by the potential plateau. With aniline in the electrolyte, current density was high enough to enable potential required for formation of high concentration of radical cations in the vicinity of the electrode, after which dimmers and oligomers are formed. The established potential plateau of 0.7 V (SCE) refers to the growth of polyaniline film on the electrode by further adding of aniline monomers through chain propagation [26].
Galvanostatic curves of copolymerization of aniline and m-aminobenzoic acid with different ratio of monomers are characterized by initial time, often referred as “induction period” [26] with potential increasing from 0.3 to 0,7 V (SCE), during which different oxidation forms and oligomers [22] are likely to be formed, followed by the potential plateau. As seen, induction time was longer, while the potential of the plateau was higher for the higher content of m- aminobenzoic acid in the electrolyte. It can be concluded that copolymerization of aniline and m-aminobenzoic acid was slower compared to polymerization of aniline and that the rate of the copolymerization decreased with increase of the molar ratio of m-aminobenzoic acid.
As expected, the presence of the m-aminobenzoic acid monomer had an impact on the polymerization process. Meta positioned carboxylic group with its electron withdrawing effect, withdraws electron density from the aromatic structure making it less reactive towards electrophilic substitution. Therefore, polymerization process is slower meaning that both higher potentials and longer induction times were required for the oxidation of m-aminobenzoic acid monomer and the co-polymerization process.
Generally established mechanism of the electrochemical polymerization of aniline refers to formation of the aniline cation radicals by oxidation of the aniline monomer [27, 28]. Further steps involve coupling of the radicals and formation of dimers, lately oligomers, by the proton elimination and rearomatization. Dimers and oligomers are anodicaly oxidized together with aniline. The propagation of the chain and formation of the polymer is achieved by coupling of the oligomer and aniline cation radicals. Formation of cation radical by oxidizing of m-aminobenzoic acid on anode is similar to formation of aniline cation radical, as presented schematically on Fig. 2, although several factors should be considered. Amino group attached to benzene ring in aniline monomer, being an electron donor, with its free electron pair strongly activates electrophilic attack directing it at ortho and para positions leading to further coupling of the radicals predominately at p-p positions. On the other hand, carboxylic group in m-amonobenzoic acid, being a metha director deactivates cation radical due to electron withdrawing effect making it less reactive, therefore polymerization is slower i.e. higher potentials are required for the electropolymerization process. Since in m-aminobenzoic acid two groups are attached to benzene ring, strongly activating amino group and moderately deactivating carboxylic group, the effect of the strongly activated group is expected to win out, therefore directing ortho and para positions, as given in Fig. 2. The effect of carboxylic group as withdrawing, is expected to be less pronounced at ortho position (with respect to amino group), this position also has lower structural hindrance. Therefore coupling of two m-aminobenzoic acid cation radicals probably occurs at o-o positions and coupling of aniline and m-aminobenzoic acid probably occurs at p-o positions leading to differences in the polymerization process, morphology etc.
Morphology
SEM micrographs of PANI and PAMBA, obtained by electrochemical synthesis on graphite electrode from aqueous acidic electrolyte containing aniline and different aniline and m-aminobenzoic monomer ratio, are given in Fig. 3.
As it can be seen from Fig. 3, all samples had uniformly deposited polymers, i.e. copolymers. Electrochemically formed polyaniline had a porous nanosized fibrous network with open structure characteristic for doping by highly hydrated small sized chloride ion [29].
The presence of m-aminobenzoic acid, having impact on the electropolymerization process also exhibited influence on the morphology of the PAMBA copolymers comparing to those of PANI. With increase of the molar ratio of m-aminobenzoic acid, fibers tend to bind and twist and the structure tends to be less open. Copolymers are “internally” i.e. self doped by carboxylic ion from m-aminobenzoic acid, as being less hydrated the structure of the copolymers is expected to be less open.
UV-visible spectroscopy
Figure 4 shows UV-vis absorbance spectra of PANI and PAMBA obtained with different aniline and m-aminobenzoic acid molar ratio. After galvanostatic electrochemical synthesis polymers are obtained in the doped state, but since the spectra were taken upon dissolving in N-metil pyrrolidone, the given spectra represent polymers in their dedoped forms.
It is well known that PANI can exist in different oxidation form, depending on the degree of oxidation [30] given schematically on Fig. 5.
Completely reduced form refers to the state of leucoemerladine base, LB (x = 0), fully oxidized state is perningraniline base PB, (x = 1), while half reduced state stands for emeraldine base EB (x = 0.5). R refers to any substituent group, while when R is equal to H, the given formula stands to “pure” polyaniline. Actually the exact degree of oxidation is unknown, but de Albuquerque et al. showed that the position of the absorption peak related to molecular transition in EB (Q peak) shifted to the lower wavelengths, as the oxidation degree was increased [30, 31]. As it can be seen in Fig. 4, the absorbance spectra of PANI exhibited maximum at 630 nm, often reported as Q peak, assigned to electron excitation from the highest occupied molecular orbital, HOMO, of the benzenoid rings to the lowest unoccupied molecular orbital, LUMO of the quinonoide rings [32]. As the molar ratio of m-aminobenzoic acid increased, the absorption maximum was shifted to lower wavelengths of 622 nm, 595 nm and 548 nm, for aniline/m-aminobenzoic acid ratio of: 3:1, 2:1 and 1:1, respectively. This shift is a consequence of increased oxidation degree of the copolymers, which is in accordance with the fact that higher ratio of m-aminobenzoic acid, required higher potentials for the electropolymerization process. On the other hand, the second adsorption maximum at ~ 330 nm, (B peak) related to π → π* transition of the benzene ring electrons; can be seen in all given spectra. B peak is mostly related to the intra-chain interactions, therefore its position was not affected by the oxidation degree of polyaniline.
As demonstrated by Yang et al. the shift of the wave length of Q, Q-QEB (where QEB, refers to the position in EB peak in PANI) can be correlated to the oxidation level of polyaniline [33].
Summarized data from the Fig. 4 used for the estimation of the oxidation level, x, of PANI and PAMBA, according to Yang et al. are given in Table 1 [33].
Electrochemical characterization
In order to investigate electrochemical behavior and degradation in acidic aqueous electrolyte of PANI and PAMBA obtained electrochemically, sixty cyclic voltammograms were performed with anodic potential limit of 1.0 V, and results are shown in Fig. 6. While second and sixtieth voltammograms extracted from Fig. 6 are given together in Fig. 7.
Second and sixtieth cyclic voltammograms of PANI and PAMBA obtained with different aniline and m-aminobenzoic acid molar ratio, (as marked on figure) extracted from the Fig. 6
It can be seen that cyclic voltammograms of PANI and PAMBA obtained with molar ratio of aniline/m-aminobenzoic acid 3:1 and 2:1, have similar shape with lower values of the peak currents, probably due to decrease of the conductivity. First anodic peak positioned at potential of ~ 0.24 V could be connected to transformation of leucoemeraldine base to emeraldine salt ES, while second peak at potential ~ 0.7 V refers to further oxidation and transformation of ES to perningraniline salt PS, as schematically given in Fig. 8. Not well defined peaks, around 0.5 V, stands to appearance of PANI degradation products [26].
After around 30 cycles for polyaniline and 20 cycles for PAMBA with molar ratio of aniline/m-aminobenzoic acid 3:1 and 2:1, a peak related to existence of PS is lost while broad peak related to degradation appeared. The increase of the charge related to degradation upon further cycling is observed. On the other hand, cyclic voltammograms of PAMBA with equal molar ratio of aniline/m-aminobenzoic acid are characterized by one well defined anodic peak at 0.40 V, and not well defined peak at ~ 0.5 V related to appearance of the degradation products (Fig. 6d). The reverse scan of the cyclic voltammograms is also characterized by two peaks related to mentioned electrochemical process.
The difference in the shape of cyclyc voltammograms for PAMBA with equal ratio of aniline and m-aminobenzoic acid might be related to a different mechanism of the redox process. Since it is possible that cations can also participate in the redox process in acidic media [11]. Thus, the oxidation process of PAMBA with equal molar ratio of aniline and m-aminobenzoic acid can be related to participation of protons, which can be expelled from the copolymer during oxidation process together by insertion of anions, in this case chloride, according to:
Where y refers to degree of doping, defined as ratio between the number of charges in the polymer and the number of monomer units.
As suggested by Salavagione et al. the carboxylic group might form cyclic species with the positive charge from nitrogen in NH, that can even facilitate deprotonation [17, 34]. In order to investigate electrochemical activity in neutral electrolytes of PANI and PAMBA obtained electrochemically with different molar ration of aniline and m-aminobenzoic acid, cyclic voltammograms were recorded in phosphate buffer at pH 7, and results are presented in Fig. 9.
As it can be seen from Fig. 9, low electrochemical activity of PANI and PAMBA obtained with molar ratio of aniline/m-aminobenzoic acid of 3:1 and 2:1, was observed in phosphate buffer solution at pH 7. On the other hand, copolymer with equal molar ratio of aniline/m-aminobenzoic acid exhibited electrochemical activity, although the value of current was lower comparing to the acidic electrolyte. Cyclic voltammogram is characterized by one peak positioned at around 0,2 V (SCE), which can be related to oxidation of copolymer according to reaction (1), the lower value of the peak potential, comparing to those in acidic solution is reasonable, since protons are expelled during the oxidation.
The results suggest that PAMBA copolymer with equal molar ratio of aniline and m-aminobenzoic acid can be considered as promising material in neutral environment such is required in biological systems. On the other hand electrochemical activity in less acidic environments is promising characteristic for application as cathodic material in Zn batteries since the dissolution of Zn can be avoided. Moreover, the fact that electrochemical activity is achieved by proton exchange that can result in a faster charge/discharge process, which is the case of polyaniline limited by slow anion exchange, seem to be promising for consideration in super-capacitors.
Conclusions
Electrochemical co-polymerizations of polyaniline and m-aminobenoic acid was successfully achieved using constant current density (galvanostatically). It was observed that amount of m-aminobemzoic acid monomer in the electrolyte, had impact on the polymerization process leading to higher potentials, morphology and electrochemical activity. The increase of the co-polymerization potential comparing to the potential of aniline polymerization can be related to aggravation of the polymerization process due to meta positioned electron withdrawing carboxylic group. On the other higher polymerization potentials of the co-polymerization process resulted in higher oxidation level which was confirmed by UV-vis spectroscopy. PAMBA copolymer with equal molar ratio of aniline and m-aminobenzoic acid can be considered as promising material in neutral environment, due to its electrochemical activity probably related to proton exchange process contrary to parent polymer PANI, whose electrochemical activity is observed exclusively in acidic electrolytes and limited by anion exchange.
References
Bezbradica D, Jugović B, Gvozdenović M, Jakovetić S, Knežević-Jugović Z (2011) Electrochemically synthesized polyaniline as support for lipase immobilization. J Mol Catal B Enzym 70:55–60
Jugović B, Gvozdenović M, Stevanović J, Trišović T, Grgur B (2009) Characterization of electrochemically synthesized PANI on graphite electrode for potential use in electrochemical power sources. Mater Chem Phys 114:939–942
Mahmood WAK, Azarian MH (2016) Sol-gel synthesis of polyaniline/zirconia composite conducting materials. J Polym Res 23:88
Chen C-H, Ko C-J, Chuang C-H, Mao C-F, Liao W-T, Hsieh C-D (2017) Synthesis and characterization of polyaniline co-doped with nitric acid and dodecyl benzene sulfonic acid. J Polym Res 24:10
Deore BA, Yu I, Freund MS (2004) A switchable self-doped polyaniline: interconversion between self-doped and non-self-doped forms. J Am Chem Soc 126:52–53
Malinauskas A (2004) Self-doped polyanilines. J Power Sources 126:214–220
Tarver J, Yoo JE, Dennes TJ, Schwartz J, Loo YL (2009) Polymer acid doped polyaniline is electrochemically stable beyond pH 9. Chem Mater 21:280–286
Mažeikienė R, Tomkutė V, Kuodis Z, Niaura G, Malinauskas A (2007) Raman spectroelectrochemical study of polyaniline and sulfonated polyaniline in solutions of different pH. Vib Spectrosc 44:201–208
Chen S-A, Hwang G-W (1994) Synthesis of water-soluble self-acid-doped Polyaniline. J Am Chem Soc 116:7939–7940
Mažeikiene R, Niaura G, Malinauskas A (2003) Voltammetric study of the redox processes of self-doped sulfonated polyaniline. Synth Met 139:89–94
Mello RMQ, Varela H, Maranha SLDA, Ticianelli EA, Torresi RM (2001) Comparisons of charge compensation process in aqueous media of polyaniline and self-doped polyanilines. Synth Met 122:321–327
Brett C, Thiemann C (2002) Conducting polymers from aminobenzoic acids and aminobenzenesulphonic acids : influence of pH on electrochemical behaviour. J Electroanal Chem 539:215–222
Chen S, Hwang G (1996) Structure characterization of self-acid-doped sulfonic acid ring-substituted Polyaniline in its aqueous solutions and as solid film. Macromolecules 9297:3950–3955
Abe Y, Amaya T, Inada Y, Hirao T (2014) Characterization of self-doped conducting polyanilines bearing phosphonic acid and phosphonic acid monoester. Synth Met 197:240–245
Min Y, Liu Y, Poojari Y, Wu JC, Hildreth BE, Rosol TJ, Epstein AJ (2014) Self-doped polyaniline-based interdigitated electrodes for electrical stimulation of osteoblast cell lines. Synth Met 198:308–313
Yang CH, Chih YK, Cheng HE, Chen CH (2005) Nanofibers of self-doped polyaniline. Polymer (Guildf) 46:10688–10698
Barbero C, Salavagione HJ, Acevedo DF, Grumelli DE, Garay F, Planes G, Morales GM, Miras MC (2004) Novel synthetic methods to produce functionalized conducting polymers I. Polyanilines. Electrochim Acta 49:3671–3686
Lukachova LV, Shkerin EA, Puganova EA, Karyakina EE, Kiseleva SG, Orlov AV, Karpacheva GP, Karyakin AA (2003) Electroactivity of chemically synthesized polyaniline in neutral and alkaline aqueous solutions. J Electroanal Chem 544:59–63
Karyakin AA, Strakhova AK, Yatsimirsky AK (1994) Self-doped polyanilines electrochemically active in neutral and basic aqueous solutions.: electropolymerization of substituted anilinesactive in neutral and basic of substituted anilines. J Electroanal Chem 371:259–265
Mažeikiene R, Malinauskas A (2004) Electrochemical preparation and study of novel self-doped polyanilines. Mater Chem Phys 83:184–192
Rahmanifar M, Mousavi M, Shamsipur M (2002) Effect of self-doped polyaniline on performance of secondary Zn–polyaniline battery. J Power Sources 110:229–232
Thiemann C, Brett CM (2001) Electrosynthesis and properties of conducting polymers derived from aminobenzoic acids and from aminobenzoic acids and aniline. Synth Met 123:1–9
Mazeikiene R, Statino A, Kuodis Z, Niaura G, Malinauskas A (2006) In situ Raman spectroelectrochemical study of self-doped polyaniline degradation kinetics. Electrochem Commun 8:1082–1086
Ghenaatian HR, Mousavi MF, Rahmanifar MS (2011) High performance battery-supercapacitor hybrid energy storage system based on self-doped polyaniline nanofibers. Synth Met 161:2017–2023
Bian J, Li Z, Chen Z, Zhang X, Li Q, Jiang S, He J, Han G (2012) Double-potentiostatic electrodeposition of Ag nanoflowers on ITO glass for reproducible surface-enhanced (resonance) Raman scattering application. Electrochim Acta 67:12–17
Gospodinova N, Terlemezyan L (1998) Conducting polymers prepared by oxidative polymerization: polyaniline. Prog Polym Sci 23:1443–1484
Zotti G, Cattarin S, Comisso N (1988) Cyclic potential sweep electropolymerization of aniline: the role of anions in the polymerization mechanism. J Electroanal Chem Interfacial Electrochem 239:387–396
Zotti G, Cattarin S, Comisso N (1987) Electrodeposition of polythiophene, polypyrrole and polyaniline by the cyclic potential sweep method. J Electroanal Chem Interfacial Electrochem 235:259–273
Wallace GG, Spinks Geoffrey M, Kane-Meguire LAP, Teasdale PR (2009) Conductive electroactive polymers, 3rd. Teyor& Francis Group, Boca Raton,
Albuquerque J, Mattoso LH, Balogh D, Faria R, Masters J, MacDiarmid A (2000) A simple method to estimate the oxidation state of polyanilines. Synth Met 113:19–22
De Albuquerque JE, Mattoso LHC, Faria RM, Masters JG, MacDiarmid AG (2004) Study of the interconversion of polyaniline oxidation states by optical absorption spectroscopy. Synth Met 146:1–10
Chen S, Lee H (1993) Polyaniline plasticized with 1-methyl-2-pyrrolidone: structure and doping behavior. Macromol Biosci 26:3254–3261
Yang D, Lu W, Goering R, Mattes BR (2009) Investigation of polyaniline processibility using GPC/UV–vis analysis. Synth Met 159:666–674
Salavagione HJ, Acevedo DF, Miras MC, Barbero C (2003) Redox coupled ion exchange in copolymers of aniline with aminobenzoic acids. Port Electrochim Acta 21:245–254
Acknowledgments
This work was financially supported by The Ministry of Education and Science, Republic of Serbia, under Contract No. OI 172046 and III 45019.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jokić, B.M., Džunuzović, E.S., Grgur, B.N. et al. The influence of m-aminobenzoic acid on electrochemical synthesis and behavior of poly(aniline-co-(m-aminobenzoic acid). J Polym Res 24, 146 (2017). https://doi.org/10.1007/s10965-017-1313-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1313-5