Abstract
Solid dispersions can play a significant role in the enhancement of drug dissolution and stability. Still, the polymeric effect can vary according to the possibility of intermolecular forces with the drug. The objective of this study was to evaluate the effect of several polymers on enhancement in-vitro dissolution behavior of celecoxib; in addition to comparing prepared dispersions with selected commercial products. Solid dispersions of celecoxib were prepared with different ratios between the drug and selected polymer (Soluplus®, polyvinyl pyrrolidine, Chitosan, polyethylene glycol). Physicochemical characterizations were performed using Powder X-ray diffraction, Differential Scanning Calorimetry, Fourier Transform Infra-Red analysis and Scanning Electron Microscopy. Dispersions were subjected to in-vitro drug release studies. Results revealed enhancement in dissolution rate for all dispersions prepared except for Chitosan-based dispersions that showed clear retardation in the drug release. Prepared dispersions from other polymers succeeded to match with release profile of two commercially marketed products (Celebrex® and Flamex®). Further Characterization of Chitosan dispersions revealed presence celecoxib in its crystalline form entrapped inside Chitosan carrier with the presence of two hydrogen bonding between Chitosan and celecoxib. Although both Polyvinylpyrrolidone, and polyethylene glycol dispersions showed a great enhancement in drug release; both failed to maintain stability. Sticky paste formation occurred to dispersions, and recrystallization took place in polyethylene glycol dispersions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The challenge of enhancement of solubility of class II drugs remains one of the critical areas in the pharmaceutical industry. Low solubility leads to inadequate absorption and variable bioavailability [1]. One of the class II drugs that exhibit low solubility is celecoxib. It was introduced as the first selective COX-2 inhibitor with fewer side effects compared to conventional nonsteroidal anti-inflammatory drugs (NSAIDs) which acts as COX-1 inhibitors [2]. It has been used successfully for the treatment of rheumatoid arthritis, osteoarthritis, primary dysmenorrhea and acute pain [3]. It is available commercially as capsules [4]. Celecoxib (CXB) (Fig. 1) is classified as Class II drug, and it is practically insoluble at physiological pH, which causes variability in oral bioavailability [5].
Several techniques were reported in the literature to enhance solubility and dissolution rate of class II drugs [6]. Solid dispersion is considered a fruitful technique to disperse drugs in hydrophilic polymers or stabilize the amorphous form of the drug. Several conventional techniques were used in the preparation of solid dispersions [7]. These include melting method [8], solvent evaporation [9], hot-melt extrusion [10], lyophilization [11], spray drying [12], and supercritical fluid technique [13].
Polymers can play a crucial role in solubilization of drugs and stabilization of its amorphous form. Soluplus® is a polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer [14]. It is a new polymer with amphiphilic properties. It has bifunctional characters which enable it to act as a matrix polymer in solid solution and as an active solubilizer for poorly soluble drugs in aqueous media attributed to micelle formation [15–17]. Polyvinylpyrrolidone (PVP) is a synthetic polymer made from a monomer N- vinylpyrrolidone. It is known as Povidone in the USP [18]. PVP has many properties make it suitable for many pharmaceutical applications; in addition to its solubility in many solvents, with thickened ability, amphiphilic properties, ability to form complexes, and it is physiologically inert [19]. PVP-K30 was also used in solubility enhancement of many drugs [20, 21]. Chitosan is a linear polysaccharide composed of randomly distributed β-(1–4)-linked D-Glucosamine (deacetylated unit) and N-acetyl-DGlucosamine (acetylated unit) [22]. Chitosan possesses many intrinsic properties in the pharmaceutical field. These include hydrophilic character, low toxicity, biocompatibility, biodegradability, low immunogenicity and mucoadhesion. Previous studies found that Chitosan enhances the solubility of poorly soluble drugs, such as Griseofulvin or Prednisolone enhanced their dissolution rate [23, 24]. Polyethylene glycol (PEG) is a linear or branched, neutral polyether, available in different molecular weight [25]. It has excellent solubility property and can enhance wettability. PEG 4000, 6000 have improved its efficiency in the enhancement of dissolution of many poorly soluble drugs [26].
What we aimed for in this work was to evaluate the effect of several polymers on the improvement of in-vitro dissolution and stability of celecoxib in prepared dispersions. The comparison will be performed for selected dispersions with commercial products (Celebrex® and Flamex®).
Materials and methods
Materials
Celecoxib was kindly donated by JOSWE (Amman, Jordan), High molecular weight Chitosan polymer (600 kDa) was supplied by Shanghai Hanshare Industry Co., Ltd., China. Hydrochloric acid (37% w/w) was provided by Biosolve, France. Soluplus® (13% PEG 6000 / 57% vinyl caprolactam / 33% vinyl acetate) was kindly supplied by the BASF company (Ludwigshafen, Germany). Absolute ethanol was supplied by Solvochem, Holland. Sodium tripolyphosphate (STPP) was supplied by Sigma-Aldrich, USA. Polyvinylpyrrolidone K30 (PVP K30) by Aldrich Chemistry, USA. Polyethylene glycol 6000 (PEG 6000) was provided by Fluke Biochemika, Switzerland. Sodium Lauryl Sulfate was supplied by Lonover, England. Potassium bromide (IR spectroscopy grade), sodium hydroxide (granulated, synthesis grade), and Sodium dihydrogen phosphate (extra pure) were supplied by Scharlau Chemie, Spain. The nylon filter membrane was provided by Bonna-Agela Technologies. Acetonitrile and methanol were provided by Fisher chemical, UK. Distilled water. Chemicals were used as supplied without further modification except for Chitosan. 11 KDa Chitosan oligomer was prepared according to R. Obaidat et al. [27]. Prepared Chitosan oligomer was stored in glass vials at room temperature. Also, 11 kDa Chitosan carrier was prepared according to R. Obaidat et al. [13, 28].
Methods
Preparation of celecoxib-polymeric dispersions (SDs) of Soluplus®, chitosan and PVP-K30
The SDs were prepared according to the following method in three polymers to celecoxib weight ratios 9:1, 8:2, and 7:3 by weighing 0.1, 0.2, and 0.3 g of the drug in 0.9, 0.8, and 0.7 g of the polymer, respectively. The weighed polymer was dissolved in 10 ml ethanol in the sonicator until a clear solution was obtained. Accurately weighed Celecoxib was dissolved in a mixture of 4 ml methanol and 6 ml ethanol. The drug solution was then added to the polymeric solution. The clear solution was transferred into a petri-dish and dried using the vacuum oven at 110 °C for 15 min for Soluplus® and Chitosan dispersions, while the rotary evaporator was used for drying of PVP-K30 at 90 RPM and 95 °C for 1 h.
After preparation, samples were collected, grounded in a mortar and pestle and sieved through mesh screen 300–180 μm, and stored in glass desiccators for further use.
Preparation of celecoxib-polymeric SDs of PEGs
The melting method was used for the preparation of PEGs dispersions in three polymers to celecoxib weight ratios 9:1, 8:2, and 7:3. The weighed PEG was melted in a water bath at 70 °C, then accurately weighed Celecoxib was added to the molten polymer and the mixture was stirred manually using glass rod repeatedly for 15 min were mixed using magnetic stirrer until homogeneity was attained. The amount of celecoxib was chosen based on the selected drug to polymer ratio. The mixture was allowed to be cooled in ice bath. After solidification, the sample was scraped gently with a spatula, grounded in a mortar and pestle and sieved through mesh screen 300–180 μm, and stored in glass desiccators for further use.
Preparation of physical mixtures
Physical mixtures (PMs) of Celecoxib with different polymers were prepared at various polymer-to-drug weight ratios 9:1, 8:2, and 7:3 by weighing 0.1, 0.2, and 0.3 g of the drug in 0.9, 0.8, and 0.7 g of the polymer, respectively. Accurately weighed amounts of Celecoxib and the polymer was homogeneously mixed in a mortar and pestle without titration, and sieved through 300–180 μm sieve and then stored in the desiccator until further analysis. The ratio of (7:3) was used in DSC, PXRD, FTIR characterization and in-vitro drug release test for Chitosan based PM. While (9:1) ratio was employed in SEM analysis and in-vitro drug release test for remaining polymers. All physicochemical characterization was performed to exclude the dilution effect.
Characterization procedures
Drug content percent and yield percent determination
The average of three different SDs for the same drug ratio were used to determine drug content percent and yield percent. To determine drug content percent, 100 mg of each SDs was dissolved in 25 ml methanol then further diluted to get UV absorbance concentration that ranges from 5 mg/L to 20 mg/L. The drug concentration was measured at (λmax =253 nm) using UV double beam spectroscopy (UV-1800 Shimadzu) according to F. Primo et al. [29], and the drug content percent was calculated by using the following equation:
To determine yield percent, the amount of product obtained from each SDs preparation was weighed, and the yield percent was calculated by using the following equation:
Differential scanning Calorimetry analysis
Differential Scanning Calorimetry (DSC) analysis were carried out for raw materials, PMs, and SDs using DSC 204 F1 Phoenix (Netzsch, Germany) equipped with a liquid nitrogen subambient accessory, the enthalpic response was calibrated with indium. Approximately 5 mg of each sample was heated in a sealed aluminum pan (P/N 201–52,943) and heated in a temperature range from 30 to 200 °C at a heating rate 10 °C/min under a nitrogen flow of 20 ml/min. An empty sealed pan was used as a reference. Sealing of the pans was performed using a sample crimper.
Fourier transform infrared spectroscopy
Fourier Transform Infrared (FTIR) of raw materials, PMs, and SDs were recorded using an IRAffinity-1 spectrophotometer, (Shimadzu, Japan). FTIR spectra were obtained by blending the samples with a small amount of potassium bromide with mortar and pestle to get a homogenous sample.
Powder X-ray diffraction analysis
Powder X-ray diffractions (PXRD) of raw materials, PMs and SDs, were recorded using a powder X-ray diffractometer (Ultima IV X-ray diffractometer, Rigaku, Japan) using cobalt radiation at a voltage of 4 kV and a current of 30 mA. The samples were analyzed in the 2θ angle range 0–80°, and the step scans mode was used with a step size of 0.02°.
Scanning electron microscopy (SEM) analysis
The surface morphology of raw materials, PMs, and SDs were obtained at different magnifications by using a Jeol- JSM-5300 scanning electron microscope, Tokyo, Japan. Operating at 2.00 kV after placing the sample on an aluminum stub, then they were coated with platinum by using Emitech K550X sputter coater under vacuum to form a conductive film. Different magnifications were recorded to study the morphology of the solid dispersions.
In-vitro drug release studies
For the selection of dissolution media, sodium phosphate buffer (pH 6.8) with different concentrations of Sodium Lauryl Sulfate (SLS) (0.05%, 0.075%, 0.1%, 0.5% w/v) were examined on their effect on the drug release rate and only one concentration was used to investigate the in-vitro release study for all prepared SDs. SLS can solubilize Celecoxib by micellization and is used to improve the wettability of Celecoxib by dissolution media [30].
The result showed that upon increasing concentration of surfactant added (SLS) in the dissolution media, the dissolution rate increased. Among different SLS concentrations, 0.075% (w/v) in 900 ml of phosphate buffer (pH 6.8) were selected as a dissolution medium to investigate the possible differences in the in-vitro release study for the prepared SDs.
An amount of SD equivalent to 50 mg Celecoxib was accurately weighed and filled manually into hard gelatin capsule size (0). The dissolution tests are performed using USP apparatus II Paddle (Erweka, Germany), with 100 RPM and 37 °C using 900 ml of dissolution media. 10 ml samples were withdrawn at (10, 20, 30, 45, 60, 90, 120 and 150 min), filtered through 0.45 μm membrane filter (Sartorius, Gottingen, Germany), analyzed by UV at 255.2 nm (λmax was selected based on scanning of samples in phosphate buffer with SLS which matches with K. Chowdary et. Al. [31] and M. Nasr et. Al [32]. Replacement of the sample was performed directly with new dissolution media to maintain sink condition.
Selected formulations which provide best release profile from Soluplus®, PVP-K30, PEG 4000 and PEG 6000 were compared separately with each of commercially marketed Celecoxib Capsules (Celebrex® and Flamex®).
Celebrex® which is considered the internationally marketed Celecoxib by Pfizer, USA and Flamex® which represents the locally marketed Celecoxib by JOSWE, Jordan.
All resulted data were expressed as mean value ± S.D. The statistical analysis was performed using the one-tailed t-test to investigate if each selected formulation provided significantly higher release profile than each marketed Celecoxib (Celebrex® and Flamex®), p < 0.05 was considered to be statistically significant. A tailed t-test was used because each selected formulation was compared separately with each selected marketed Celecoxib. The kinetics of drug release data were determined with respect to Higuchi model and Korsmeyer and Peppas models according to Obaidat et al. [28, 33, 34].
Stability studies
The prepared SDs were subjected to accelerated stability studies for three months at 30 °C and 40 °C under 75% RH. Samples were collected after three months and analyzed by PXRD to check out any crystal type changes and tested for drug content to evaluate the physical stability. While chemical stability was evaluated by using RP-HPLC system (ELSD-LІІ Shmadzu Chromatografe, Japan) as published by K. Jadhavo et. Al. on a C18 column (4.6 mm × 250 mm). The mobile phase used was Methanol: Acetonitrile (7:3 v/v) [35]. The flow rate was 1 ml/min, the injection volume was 20 μl, and the detection wavelength was 253 nm. The HPLC analysis method was validated for inter and intra-day variability.
Results
Drug content percent and yield percent determination
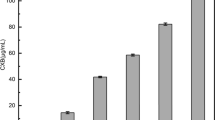
All prepared SDs showed good drug content (Table 1) with values higher than 97%, except in PEG 4000 based preparations which showed yield values that ranged from (86.02 to 86.98%). On the other hand, drug content was excellent in Chitosan reaching values that are higher than 100% in Chitosan SDs. This can be related to polymeric loss during preparation. The SDs prepared with PEG showed the highest yield among all other polymers with drug content values ranging from 81.61% to 99.22%. Variability between PEGs was seen in the results as PEG 6000 containing preparations showed better drug content than PEG 4000.
Characterization procedures
DSC
A single, sharp endothermic peak (Fig. 2) at 165 ± 0.56 °C was observed for Celecoxib. While broad endothermic peaks were observed for Soluplus®, PVP-K30, and chitosan at 71.7 °C, 59.4–120.8 °C, 90.6–146.7 °C; respectively. The thermal behavior of PEG 4000 and PEG 6000 showed endothermic peaks at 64.4 °C and 65.1 °C; respectively. All PM showed the presence of endothermic peaks related to the drug and the polymer, except for PEGs which showed only one endothermic peak related to PEG, while all SDs showed single endothermic peak associated with the used polymer, except for Chitosan where polymer peak appeared along with drug peak.
a DSC thermogram of Celecoxib, Soluplus®, Soluplus®:Celecoxib 7:3 PM, Soluplus®:Celecoxib 9:1 SD1, Soluplus®:Celecoxib 8:2 SD2 and Soluplus®:Celecoxib 7:3 SD3. From top to the bottom respectively. b DSC thermogram of Celecoxib PVP-K30, PVP-K30:Celecoxib 7:3 PM, PVP-K30:Celecoxib 9:1 SD4, PVP-K30:Celecoxib 8:2 SD5 and PVP-K30:Celecoxib 7:3 SD6. From top to the bottom respectively. c DSC thermogram of Celecoxib, 11 KDa chitosan, Chitosan:Celecoxib 7:3 PM, Chitosan:Celecoxib 9:1 SD7, Chitosan:Celecoxib 8:2 SD8 and Chitosan:Celecoxib 7:3 SD9. From top to the bottom respectively. d DSC thermogram of Celecoxib, PEG 4000, PEG 4000:Celecoxib 7:3 PM, PEG 4000:Celecoxib 9:1 SD10, PEG 4000:Celecoxib 8:2 SD11 and PEG 4000:Celecoxib 7:3 SD12. From top to the bottom respectively. e DSC thermogram of Celecoxib, PEG 6000, PEG 6000:Celecoxib 7:3 PM, PEG 6000:Celecoxib 9:1 SD13, PEG 6000:Celecoxib 8:2 SD14 and PEG 6000:Celecoxib 7:3 SD15. From top to the bottom respectively
PXRD
PXRD pattern (Fig. 3) of Celecoxib showed intense peaks between 10° and 25° at 2θ (5.36°, 10.71°, 14.81°, 16.11°, 19.67°, 21.53°, 22.19°). The PXRD pattern of Soluplus® showed no diffraction peaks; while PXRD of PVP-K30 showed a couple of broad bands of 2θ equal to 11° and 22°. The PXRD pattern of Chitosan carrier exhibited characteristic peaks at 2θ equals to 15° and 25°. Although PXRD of PEGs showed similarities in patterns, a slight difference in the value 2θ of was observed. PXRD spectrum for PEG 4000 showed a distinct pattern with two peaks having the highest intensity at 2θ equal 19.35° along with other peaks at 2θ equal 23.5°, while PEG 6000 exhibited diffraction peaks at 2θ equal to 19.12° and 23.28°.
a PXRD of Celecoxib, Soluplus®, Soluplus®:Celecoxib 7:3 PM, Soluplus®:Celecoxib 9:1 SD1, Soluplus®:Celecoxib 8:2 SD2, and Soluplus®:Celecoxib 7:3 SD3. From bottom to the top respectively. b PXRD of Celecoxib, PVP-K30, PVP-K30:Celecoxib 7:3 PM, PVP-K30:Celecoxib 9:1 SD4, PVP-K30:Celecoxib 8:2 SD5, and PVP-K30:Celecoxib 7:3 SD6. From bottom to the top respectively. c PXRD of Celecoxib, 11-KDa Chitosan, Chitosan:Celecoxib 7:3 PM, Chitosan:Celecoxib 9:1 SD7, Chitosan:Celecoxib 8:2 SD8, and Chitosan:Celecoxib 7:3 SD9. From bottom to the top respectively. d PXRD of Celecoxib, PEG 4000, PEG 4000:Celecoxib 7:3 PM, PEG 4000:Celecoxib 9:1 SD10, PEG 4000:Celecoxib 8:2 SD11, and PEG 4000:Celecoxib 7:3 SD12. From bottom to the top respectively. e PXRD of Celecoxib, PEG 6000, PEG 6000:Celecoxib 7:3 PM, PEG 6000:Celecoxib 9:1 SD13, PEG 6000:Celecoxib 8:2 SD14, and PEG 6000:Celecoxib 7:3 SD15. From bottom to the top respectively
The PXRD pattern of PMs showed the existence of all diffraction peaks related to the individual materials. In contrast, the PXRD patterns of SDs prepared by Soluplus® and PVP-K30 at different ratios showed a complete absence of Celecoxib diffraction peaks. However, the PXRD pattern of Chitosan SDs showed some diffraction peak for Celecoxib in SD9, while SDs containing higher carrier to drag ratios (SD7 and SD8) showed a diffraction pattern similar to Chitosan alone.
In the case of PEGs SDs, decrease in the intensity of the diffractogram appeared in SD11, SD12, SD14 and SD15. On contrast, the characteristic peaks of Celecoxib disappeared completely in SD10 and SD13.
FTIR
Celecoxib spectra (Fig. 4) showed a characteristic S = O symmetric stretching at 1165 cm-1. Medium intensity bands were observed at 3336.85 cm-1, 3234.62 cm-1 which were attributed to the N-H stretching vibration of -SO2-NH2 group. Another two characteristic peak was observed at 1274.95 and 1228.66 cm-1 related -CF3 stretching.
a FTIR spectra of Soluplus®, Celecoxib, Soluplus®:Celecoxib 7:3 PM, Soluplus®:Celecoxib 9:1 SD1, Soluplus®:Celecoxib 8:2 SD2, and Soluplus®:Celecoxib 7:3 SD3, respectively from bottom to the top. b FTIR spectra of PVP-K30, Celecoxib, PVP-K30:Celecoxib 7:3 PM, PVP-K30:Celecoxib 9:1 SD4, PVPK30:Celecoxib 8:2 SD5, and PVP-K30:Celecoxib 7:3 SD6, respectively from bottom to the top. c FTIR spectra of 11 KDa chitosan, Celecoxib, Chitosan:Celecoxib 7:3 PM, Chitosan:Celecoxib 9:1 SD7, Chitosan:Celecoxib 8:2 SD8, and Chitosan:Celecoxib 7:3 SD9, respectively from bottom to the top. d FTIR spectra of PEG 4000, Celecoxib, PEG 4000:Celecoxib 7:3 PM, PEG 4000:Celecoxib 9:1 SD10, PEG 4000:Celecoxib 8:2 SD12, and PEG 4000:Celecoxib 7:3 SD13, respectively from bottom to the top. e FTIR spectra of PEG 6000, Celecoxib, PEG 6000:Celecoxib 7:3 PM, PEG 6000:Celecoxib 9:1 SD13, PEG 6000:Celecoxib 8:2 SD14, and PEG 6000:Celecoxib 7:3 SD15, respectively from bottom to the top
Soluplus® showed main characteristic absorption bands at 3653.18 cm-1, 2962.66 cm-1, 1693.50 cm-1, and 1469.76 cm-1 attributed to O-H stretching, C-H stretching, C = O stretching, and C-O-C stretching, respectively. Significant vibrations were detected in the spectra of PVP-K30 were the (C = O) at 1658.78 cm-1, C-H stretch at 2949.16 cm-1 and O-H stretching at 3446.79 cm-1. The FTIR spectra of Chitosan showed a broad absorption band range from 3701.40 to 2366.66 cm-1 due to stretching vibrations of OH groups. A peak at 2923 cm-1 was related to symmetric -CH2 stretching, vibration attributed to pyranose ring. The absorption bands at 1155.36 cm-1 were assigned to C-O-C bridge anti-symmetric stretching. The broad peak at 1041.56 and 1068.56 cm-1 indicated the C-O stretching, vibration in Chitosan and area between 1627.92 and 1541.12 cm-1 were due NH2 scissoring vibrations. Same characteristic peaks appeared for both PEGs. The spectra revealed major peaks for O-H stretch at 3425.58 cm-1, C-O-C stretch at 1107.14 cm-1 and C-H stretch at 2887.44 cm-1.
It is clear that the characteristic bands of both drug and the polymers were maintained in all PMs. Whilst in the FTIR spectra of all SDs, the two Celecoxib bands at 3336 cm-1 and 3234 cm-1 for N-H stretching were lost except in PEG 6000 where those two peaks were broadened and shifted to lower wavelength indicating that Celecoxib was engaged in hydrogen bond with the polymers in all SDs prepared. On the other hand, Chitosan SDs also exhibited absence of Celecoxib S = O stretching peak at 1165 cm-1. Whereas, a negligible shift in the position of C = O stretching, vibration band related to Soluplus® and PVP-K30 could be observed in SD samples.
SEM
SEM images of Celecoxib (Fig. 5) showed the appearance of long needle shaped particles of different irregular sizes with smooth surfaces. Soluplus® showed an irregular spherical shape. While PVP-K30 appeared as irregular smooth rounded spheroids with concave depression. Chitosan carrier appeared cross-linked with relatively strong wall. PEGs displayed as bulky smooth surface particles with irregular shape and few smaller particles.
All PMs revealed the presence of drug crystals and unchanged polymers. On the contrary, SDs prepared with Soluplus®, PVP-K30, PEGs showed that the drug crystals were totally absent demonstrating that the drug was entrapped inside Chitosan, while Chitosan SDs appeared that the drug was entrapped in the pores of cross-linked Chitosan in the crystalline form and evidence of strong network interaction was established between Celecoxib and Chitosan.
In-vitro release study
Celecoxib release profile (Fig. 6) exhibited low dissolution rate with 11.8% release in 30 min and only 52.72% release after 150 min. It is evident that PMs prepared with PVP-K30, PEGs improved the dissolution rate, Whereas PM prepared with Soluplus® and Chitosan showed a decrease in the dissolution rate of Celecoxib.
a In-vitro release profile of Celecoxib, Soluplus®:Celecoxib 9:1 PM, Soluplus®:Celecoxib 9:1 SD1, Soluplus®:Celecoxib 8:2 SD2, and Soluplus®:Celecoxib 7:3 SD3. b In-vitro release profile of Celecoxib, PVPK30:Celecoxib 9:1 PM, PVP-K30:Celecoxib 9:1 SD4, PVP-K30:Celecoxib 8:2 SD5, and PVP-K30:Celecoxib 7:3 SD6. c In-vitro release profile of Celecoxib, Chitosan:Celecoxib 7:3 PM, Chitosan:Celecoxib 9:1 SD7, Chitosan:Celecoxib 8:2 SD8, and Chitosan:Celecoxib 7:3 SD9. d In-vitro release profile of Celecoxib, PEG 4000:Celecoxib 9:1 PM, PEG 4000:Celecoxib 9:1 SD10, PEG 4000:Celecoxib 8:2 SD11, and PEG 4000:Celecoxib 7:3 SD12. e In-vitro release profile of Celecoxib, PEG 6000:Celecoxib 9:1 PM, PEG 6000:Celecoxib 9:1 SD13, PEG 6000:Celecoxib 8:2 SD14, and PEG 6000:Celecoxib 7:3 SD15. f In-vitro release profile of Celecoxib, Celebrex®, Flamex®, Soluplus®:Celecoxib 9:1 SD1, PVP-K30:Celecoxib 9:1 SD4, PEG 4000:Celecoxib 9:1 SD 10, and PEG 6000:Celecoxib 9:1 SD13
SDs showed different effects of dissolution behavior depending on the polymer used, its molecular weight, the polymer to drug ratio and method of preparation. In SDs prepared with Soluplus® and PVP-K30 showed a higher dissolution rate compared to Celecoxib and PM. Surprisingly, the Chitosan SDs with different ratios did not enhance the dissolution rate, conversely, the retardation effect was observed. On contrast with other polymers used, the dissolution enhancing the power of PEGs SDs depends on the molecular weight of the used PEGs and polymer to drag ratio. Release behavior from SDs of PVP-K30 and Chitosan matched with PMs, while changes in release behavior were observed for Soluplus® and PEGs.
SD1 has lower dissolution rate in the first 60 min compared to the SD2; then the release rate increased rapidly up to (102.75%) after 150 min. So, the highest release of all Soluplus® SDs ratios was obtained from highest Soluplus® concentration compared to only 92.54% for SD2 followed by SD3 showing 87.9% drug release.
SD4 showed a higher dissolution rate with 108.93% release after 150 min, while at lower PVP-K30 to Celecoxib weight ratio (SD5) release rate was about 100% in 150 min. On the other hand, SD6 enhanced the release to 92.85%, which is the lowest upon PVP-K30 SDs ratios. It is clear that the increasing PVP-K30 concentration in the prepared SDs resulted in an increasing dissolution rate.
The effect of the carrier to drug ratio on Chitosan release profile showed that the increasing Chitosan ratio resulted in a slower release, with SD7 showing lowest dissolution rate with only 17.81% after 150 min, compared to SD9 that achieved a higher release rate 25.69%.
Regarding PEGs, results demonstrated higher drug dissolution of the binary system containing PEG 4000 than that of containing PEG 6000 at low drug concentration. Effect of polymeric ratio was also observed for PEGs. The remarkable enhancement was seen in 9:1 ratio (SD10 and SD13); in which Celecoxib release from PEG 4000 to PEG 6000 was 85.18% to 75.3% in the first 10 min, and 104.84% to 96.84% at the end of 150 min;whereas SDs with the other two ratios showed a lower dissolution rate. The highest cumulative release was achieved by PVP-K30 SDs, followed by PEG 4000, then Soluplus® and finally PEG 6000 of 9:1 ratio, while Chitosan SDs showed retardation release effect.
Noteworthy, PEGs SDs reached plateau faster than other polymers; where more than 75% of the drug were released in the first 10 min. On the other hand, PVP-K30 and Soluplus® SD required more than 30 min and 45 min, respectively, to reach same percent release.
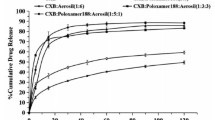
The best release obtained from each polymer was in formulations SD1, SD4, SD10, and SD13. Those were selected to be compared with commercial products (Celebrex® and Flamex®). Figure 6G demonstrates that Celebrex® and Flamex® showed the almost similar dissolution rate and both exhibit higher release rates than Celecoxib. Results proved (Table 2) significant (P-value lower than 0.5) dissolution enhancement effect for Soluplus® and PEGs SDs compared to Celebrex® or Flamex®. On the other hand, only PVP-K30 SDs did not show significant differences from commercial products [36].
Stability studies
PVP-K30 SDs were excluded from stability study cause it formed a sticky paste after three months compared to other SDs prepared with other polymers which remained dry and powdery [37].
Drug content analysis showed that there is no significant difference in the drug content. PXRD patterns of SDs were similar before and after the stability study period for Soluplus® SDs. Whereas PXRD of Chitosan SDs showed diffraction peaks of Celecoxib crystals upon storage, which were not observed with the freshly prepared SDs, while PXRD of PEGs SDs revealed that Celecoxib diffraction peaks started to reappear upon storage. The chemical stability study showed that SDs containing Soluplus®, Chitosan, PEGs were chemically stable.
Discussion
Physicochemical characterization of raw materials, PMs, and SDs
Celecoxib was proved to be pure and crystalline, regarding the presence of single, sharp endothermic melting peak in DSC analysis [38], and high-intensity peaks in PXRD. SEM analysis proved needle shape with FTIR analysis showed that Celecoxib could engage in hydrogen bond by N-H stretching [39–45]. The broad endothermic peak in DSC analysis for Soluplus®, PVP-K30 and Chitosan relates to water evaporation. Other researchers related the Soluplus® peak to its glass transition temperature, while the sharp endothermic peak in PEGs was related to its melting point. Also, complete disappearance of Celecoxib peak form PEGs dispersions indicated complete miscibility of Celecoxib in the melted carrier which melted at 65 °C. On the other hand, the absence of diffraction peaks in Soluplus® and PVP-K30 PXRD proved its amorphous nature, with purity confirmed by FTIR.
Physicochemical characterization of PMs indicates that Celecoxib is still in its crystalline form because of the presence of drug peak. While Celecoxib precipitated as an amorphous form in SDs prepared by.
Soluplus® and PVP-K30. This is indicated by the absence of the drug peaks in DSC and PXRD analysis.
On the other hand, the two Celecoxib bands at 3336 cm-1 and 3234 cm-1 in the FTIR spectra were lost, suggesting that the N-H group in Celecoxib engaged in hydrogen bonding with C = O bond in Soluplus® and PVP-K30. However, the negligible shift of C = O bond in Soluplus® and PVP-K30 could be due to the high content of the polymer in SDs. SEM analysis also showed the absence of drug crystals, suggesting that the drug was dispersed in the polymer at the molecular level in an amorphous form [46, 47].
In contrast, PEGs SDs showed behavior related to the polymer to drag ratio. Semicrystalline state appeared in certain ratios, and complete amorphous precipitation occurred in the proportion 9:1. In DSC analysis, this was evident by the absence of endothermic drug peak, which can be related to solubilization effects of the melted polymer, while the PXRD diffractogram showed decreases in the intensity of Celecoxib peaks in semicrystalline ratios as reported previously by Guleria R. et al. (2012) [48]. On contrast, the Celecoxib peaks disappeared completely in 9:1 ratio, confirming that Celecoxib converted to an amorphous state.
Sambhakar S. et al. (2013) and Hu L. et al. (2014) confirmed similar results for PEG 4000 and PEG 6000, respectively [49, 50]. On the other hand, the FTIR spectra of PEG 4000 SD exhibited absence of the two Celecoxib characteristic bands, while the spectra of PEG 6000 SDs showed that those bands were broadened and shifted to lower wavelength, suggesting the possibility of hydrogen bond formation between N-H of Celecoxib and either O-H of PEGs or electron pair of carrier oxygen atoms, that could not be demonstrated. Reported results showed the similar type of interaction between N-H of Gliclazide and PEG 4000 [51], and between Celecoxib and PEG 6000 in SD prepared by spray drying method [52].
Celecoxib precipitated in Chitosan SDs as crystalline form. Physicochemical characterization showed that the drug maintained its crystallinity as shown by the presence of Celecoxib sharp melting peaks in DSC analysis for all samples. Dilution effect on the PXRD pattern of SDs containing higher carrier to drug ratios (SD7 and 8SD8) was observed upon comparison with PM patterns. The drug was entrapped inside the Chitosan in the crystalline form as confirmed by SEM analysis. FTIR spectra of Chitosan SDs showed absence of Celecoxib characteristic peaks in addition to the absence of Celecoxib S = O stretching peak at 1165 cm-1, indicating that those two functional groups interacted with functional group of Chitosan carrier and two hydrogen bonds formed, which could give evidence of stronger interaction between Celecoxib and Chitosan compared to other SD that was prepared by the other polymers. While SEM showed an interesting results where Chitosan and Celecoxib formed a unique structure that explained many results. Celecoxib was entrapped in the pores of cross-linked Chitosan in the crystalline form and evidence of strong network interaction was formed between Celecoxib and Chitosan, caused by the high number of hydrogen bonds generated compared to the other polymers as indicated by FTIR analysis.
In-vitro release study
Reduction in dissolution rate was clearly observed in PMs of Soluplus® and Chitosan. For Soluplus®, this can be related to gel formation and sticky properties of Soluplus® when it came in contact with water that hindered the release of Celecoxib [46]. Naelapaa K. et al. also showed that physical mixing of Soluplus® and Guaifenesin decreased the dissolution rate of Guaifenesin [53]. However, for Chitosan this can be justified by formation of high viscous solution of Chitosan that resulted in an increase in resistance diffusion pathway, thus, retarded drug release.
In contrast, the increase in dissolution rate was observed in PVP-K30 and PEGs PMs. For PVPK30, it can be explained by the surface tension lowering effect of the PVP-K30 to the dissolution media, which provided better wettability for the hydrophobic surface of the crystalline Celecoxib [54]. While for PEGs, this can be explained by the formation of a high concentration region of the dissolved polymer at the surface of crystalline drug in which the drug can be solubilized and subsequently diffused to the bulk dissolution media [55].
Different release behavior was obtained for SDs. The enhancement of dissolution rate of all Soluplus® SDs could be related to the conversion of the drug from crystalline to amorphous state [56]. Also, it can be seen that better release of Celecoxib achieved as the Soluplus® ratio increase. This agrees with what was reported previously by Shamma R. et al. (2013) when they found the same effect of Soluplus® concentration with carvedilol [40]. This could be attributed to the amphiphilic nature of Soluplus® which above certain concentration will form micelles. Soluplus® has a low critical micelle concentration (CMC) which was determined to be 0.0007% (w/v) at 37 °C.
On the other hand, the dissolution rate enhancement of PVP-K30 SDs was attributed to conversion of crystal form of Celecoxib to amorphous form, improve wettability and the role of PVP in suppression the recrytallization of Celecoxib [57, 58]. PVP is known as an inhibitor of drug crystallization [59].
Also, decreasing the dissolution rate of Chitosan SDs was related to the presence of Celecoxib in the crystalline state as confirmed by DSC, PXRD and SEM data, and also the entrapment of Celecoxib in Chitosan which hindered the release of Celecoxib due to the higher number of hydrogen bond formed between Chitosan and the drug compared to other polymers which predict stronger interaction that might hold the Celecoxib in the entangled, cross-linked Chitosan. FTIR and SEM analysis were consistent with this result.
In contrast, results showed that PEGs SDs have a different dissolution behavior with respect to molecular weight of PEG used and polymer to drug weight ratio. The higher dissolution rate of SDs prepared by low molecular weight PEG compared to higher molecular weight was explained by the shorter chain length of PEG 4000 compared to PEG 6000 [60]. Furthermore, the polymer to drug ratio demonstrated the critical role in controlling the mechanism of drug release form SD. The remarkable enhancement of dissolution of PEGs SDs at only 9:1 ratio was related to carrier controlled release mechanism where the dissolution of drug particles into the polymer concentrated layer occurs at a sufficiently rapid rate where the drug becomes molecularly dispersed within that layer because there is insufficient time for the particles to be released intact into the medium [61]. Surprisingly, the retardation in the release rate that was observed in the other two ratios of PEGs SDs compared to Celecoxib may be due to drug-controlled dissolution mechanism. This mechanism demonstrates that drug particles dissolve in the polymer concentrated layer at comparatively slow rate and the drug is released as solid particles. Consequently the dissolution will not be influenced with the polymer but will instead be dominated by the properties (physical form, size, etc.) of the drug itself [50]. Another proposed mechanism to explain the effect of polymeric ratio is the ability of the polymer to inhibit crystallization at high ratio [62].
PVP-K30 SDs presented better drug-release profiles over corresponding PEGs SD. This phenomenon may be due to the inherent differences between the two polymers in terms of intrinsic dissolution rate and hydration, and the possibility of complexation of the drug with PVP-30 which leads to decrease the crystallinity of the coprecipitated drug and inhibit the crystallization of Celecoxib amorphous state [63].
Korsmeyer-Peppas models (Table 3) showed fitting for the various ratios of Soluplus® SDs, having n values ≤0.43 as well as for commercial products (Flamex® and Celebrex®), suggesting that the drug release mechanism from these SDs was based on Fickian diffusion [64]. On the contrary, SDs containing PVP-K30 with various ratios exhibited Higuchi model, indicating that the release mechanism was mainly based on diffusion mechanism [65]. Model fitting for PEG 4000 SDs showed the best fit for Korsmeyer-Peppas equation, where the release was controlled by Fickian diffusion [66]. According to PEG 6000 SDs, only SD15 was proved to best fit Higuchi model, a similar result was observed for Stiripentol SDs containing PEG 6000 [67]. While SD13 and SD14 showed the best fit for Korsmeyer-Peppas equation, where n values were ≤0.43, suggesting that release mechanism was Fickian diffusion [68]. Chitosan SDs followed Korsmeyer-Peppas, where n values were ≤0,43, suggesting Fickian diffusion [69], except for SD9 which followed the Higuchi model [70].
Stability studies
All SDs appeared chemically stable; except for PVP-K30 SDs which formed a sticky paste during storage. On the other hand, only Soluplus® was capable of maintaining physical stability (amorphous form).
Compared to other polymers; in contrast to PEGs SD at which re-crystallization occurred upon storage. Also, the reappearance of diffraction peaks in PXRD of Chitosan SDs indicated that Celecoxib, which was presented with Chitosan as crystals, was released out from Chitosan carrier upon storage which was confirmed by SEM analysis.
Conclusions
In conclusion, Soluplus®, PVP-k30 and PEGs proved to enhance the dissolution rate of the drug matching two commercially marketed products (Celebrex® and Flamex®). Unlike Chitosan which is caused retardation of the drug release due to the presence of hydrogen bonding with Celecoxib. Maximum enhancement was achieved for PVP-K30, followed by PEG 4000, Soluplus®, and PEG 6000 exhibiting the minimum effect. Although both PVP and PEG dispersions showed a great enhancement in drug release; both failed to maintain stability. Sticky paste formation occurred to PVP dispersions, and re-crystallization happened in PEG dispersions.
References
Rajesh K, Rajalakshmi R, Umamaheswari J, Ashok Kumar CK (2011) Liquisolid technique a novel approach to enhance solubility and bioavailability. International Journal of Biopharmaceutics 2(1):8–13
Ventura CA, Giannone I, Paolino D, Pistara V, Corsaro A, Puglisi G (2005) Preparation of celecoxib-dimethyl-beta-cyclodextrin inclusion complex: characterization and in vitro permeation study. Eur J Med Chem 40(7):624–631
Chow HHS, Anavy N, Salazar D, Frank DH, Alberts DS (2004) Determination of celecoxib in human plasma using solid-phase extraction and high-performance liquid chromatography. J Pharm Biomed Anal 34(1):167–174
Dolenc A, Kristl J, Baumgartner S, Planinsek O (2009) Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm 376(1–2):204–212
Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP (2004) Formulation Design of Self-Microemulsifying Drug Delivery Systems for improved oral bioavailability of celecoxib. Biol Pharm Bull 27(12):1993–1999
Savjani KT, Gajjar AK, Savjani JK (2012) Drug solubility: importance and enhancement techniques. International Scholarly Research Network Pharmaceutics 2012:1–10
Chokshi RJ, Zia H, Sandhu HK, Shah NH, Malick WA (2007) Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution: pros and cons. Drug Deliv 14(1):33–45
Sekiguchi K, Obi N, Ueda Y (1964) Studies on absorption of eutectic mixture. II. Absorption of fused conglomerates of chloramphenicol and urea in rabbits. Chem Pharm Bull (Tokyo) 12:134–144
Arunachalam A, Karthikeyan M, Konam K, Prasad P, Sethuraman S, Ashutoshkumar S (2010) Solid dispersions: A review. Current Pharmaceutical Research 1(1):82–90
Breitenbach J (2002) Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm 54:107–117
Kumar BP, Rao S, Murthy KVR, Sahu RK, Ramu B (2011) Solid dispersion technique: a tool for enhancing bioavailability of poorly soluble drugs. Journal of Chemical and Pharmaceutical sciences 4(4):170–179
Vasconcelos T, Sarmento B, Costa P (2007) Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today 12(23–24):1068–1075
Obaidat RM, Tashtoush BM, Awad AA, Al Bustami RT (2016) Using supercritical fluid technology (SFT) in preparation of tacrolimus solid dispersions. AAPS PharmSciTech
Vo CL, Park C, Lee BJ (2013) Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm 85(3 Pt B):799–813
group B (2010). Soluplus Technical Information, pp 1–8
Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K (2013) Preparation of carbamazepine-Soluplus® solid dispersions by hot-melt extrusion, and prediction of drug-polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm 84(1):228–237
Thakral NK, Ray AR, Bar-Shalom D, Eriksson AH, Majumdar DK (2012) Soluplus--solubilized citrated camptothecin--a potential drug delivery strategy in colon cancer. American Association of Pharmaceutical Scientists 13(1):59–66
Fischer F, Bauer S (2009) An all rounder in the chemistry polyvinylpyrrolidone. Chem unserer Zeit 43:376–383
Folttmann H, Quadir A (2008) Polyvinylpyrrolidone (PVP) – one of the most widely used excipients in pharmaceuticals: an overview. Drug Delivery Technology 8:7–22
Shah J, Vasanti S, Anroop B, Vyas H (2008) Enhancement of dissolution rate of valdecoxib by solid dispersions technique with PVP K 30 & PEG 4000: preparation and in vitro evaluation. J Incl Phenom Macrocycl Chem 63(1–2):69–75
Singh A, Sharma PK, Meher JG, Malviya R (2011) Evaluation of enhancement of solubility of paracetamol by solid dispersion technique using different polymers concentration. Asian Journal of Pharmaceutical and Clinical Research 4(1):117–119
Badawy MEI, Rabea EI (2011) A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. International Journal of Carbohydrate Chemistry 2011:1–29
de Alvarenga ES (2011) Characterization and properties of chitosan. In: Biotechnology of biopolymers, pp 91–108
Rodrigues S, Dionisio M, Lopez CR, Grenha A (2012) Biocompatibility of chitosan carriers with application in drug delivery. Journal of Functional Biomaterials 3(3):615–641
Caliceti P (2003) Pharmacokinetic and biodistribution properties of poly(ethylene glycol)–protein conjugates. Adv Drug Deliv Rev 55(10):1261–1277
Kumar SGV, Mishra DM (2006) Preparation, characterization and in vitro dissolution studies of solid dispersion of meloxicam with PEG 6000. The Pharmaceutical Society of Japan 126(8):567–664
Obaidat R, Al-Jbour N, Al-Sou’d K, Sweidan K, Al-Remawi M, Badwan A (2010) (2010). Some Physico-chemical properties of low molecular weight Chitosans and their relationship to conformation in aqueous solution. J Solut Chem 39(4):575–588
Obaidat RM, Tashtoush BM, Bayan MF, Al Bustami RT, Mohammad A (2015) Drying using supercritical fluid technology as a potential method for preparation of chitosan aerogel microparticles. American Association of Pharmaceutical Scientists. 16(6):1235–1244
Primo FT, Froehlich PE (2005) Celecoxib identification methods. Acta Farm Bonaer 24(3):421–425
Schott H, Kwan LC, Feldman S (1982) The role of surfactants in the release of very slightly soluble drugs from tablets. J Pharm Sci 71(9):1038–1045
Chawdary KPR, Srinivas SV (2006) Influence of hydrophilic polymers on CelecoxibComplexation with Hydroxypropyl β-cyclodextrin. American Association of Pharmaceutical Scientists. 7(3):E1–E6
Nasr M (2010) In vitro and in vivo evaluation of proniosomes containing celecoxib for oral administration. American Association of Pharmaceutical Scientists 11(1):85–89
Shoaib MH, Tazeen J, Merchant HA, Yousuf RI (2006) Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm Sci 19(2):119–124
Parize AL, Stulzer HK (2012) Evaluation of chitosan microparticles containing curcumin and crosslinked with sodium tripolyphosphate produced by spray dryin. Quim Nov. 35(6):1127–1132
Jadhav K, Gowekar N, Gowekar S (2012) A validated RP-HPLC method for the determination of celecoxib in bulk and pharmaceutical dosage form. Int J Res Pharmaceut Biomed Sci 3(3):1312–1316
Lee JH, Kim MJ, Yoon H, Shim CR, Ko HA, Cho SA, et al (2013) Enhanced dissolution rate of celecoxib using PVP and/or HPMC-based solid dispersions prepared by spray drying method. Journal of Pharmaceutical Investigation 43(3):205–213
Caron V, Hu Y, Tajber L, Erxleben A, Corrigan OI, McArdle P, et al (2013) Amorphous solid dispersions of sulfonamide/Soluplus(R) and sulfonamide/PVP prepared by ball milling. American Association of Pharmaceutical Scientists 14(1):464–474
Chawla G, Gupta P, Thilagavathi R, Chakraborti AK, Bansal AK (2003) Characterization of solid-state forms of celecoxib. Eur J Pharm Sci 20(3):305–317
Gupta VR, Mutalik S, Patel MM, Jani GK (2007) Spherical crystals of celecoxib to improve solubility, dissolution rate and micromeritic properties. Acta Pharma 57:173–184
Shamma RN, Basha M (2013) Soluplus®: a novel polymeric solubilizer for optimization of carvedilol solid dispersions: formulation design and effect of method of preparation. Powder Technol 237:406–414
Sethia S, Squillante E (2004) Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm 272(1–2):1–10
Ujang Z, Diah M, Abdul Rashid AH, Halim AS (2011) The development, characterization and application of water soluble chitosan. Biotechnol Biopolymers 109–31
El-Badry M, Fetih G, Fathy M (2009) Improvement of solubility and dissolution rate of indomethacin by solid dispersions in Gelucire 50/13 and PEG4000. Saudi Pharmaceutical Journal 17(3):217–225
Patil M, Gaikwad N (2011) Characterization of gliclazide-polyethylene glycol solid dispersion and its effect on dissolution. Brazilian Journal of Pharmaceutical Sciences 47:161–166
Homayouni A, Sadeghi F, Nokhodchi A, Varshosaz J, Garekani HA, Vyas H (2014) Preparation and characterization of celecoxib solid dispersions; comparison of poloxamer-188 and PVP-K30 as carriers. Iranian Journal of Basic Medical Sciences 17:322–331
Homayouni A, Sadeghi F, Nokhodchi A, Varshosaz J, Garekani HF (2015) Preparation and characterization of celecoxib dispersions in Soluplus®: comparison of spray drying and conventional methods. Iranian Journal of Pharmaceutical Research 14(1):35–50
Zhang X, Sun N, Wu B, Lu Y, Guan T, Wu W (2008) Physical characterization of lansoprazole/PVP solid dispersion prepared by fluid-bed coating technique. Powder Technol 182(3):480–485
Guleria R, Kaith NS, Singh R (2012) PEG based solid dispersions of gliclazide a comparative study. Int J Pharm Pharm Sci 4(1):507–511
Sambhakar S, Singh B, Madan K, Singh M, Kashyap N, Mayle S (2013) Solid dispersions: a tool for improving the solubility and dissolution of metronidazole. International Journal of Drug Delivery 5:94–98
Hu L, Gu D, Hu Q, Shi Y, Gao N (2014) Investigation of solid dispersion of atorvastatin calcium in polyethylene glycol 6000 and polyvinylpyrrolidone. Trop J Pharm Res 13(6):835
Liu C, Desai KG, Liu C (2005) Enhancement of dissolution rate of valdecoxib using solid dispersions with polyethylene glycol 4000. Drug Dev Ind Pharm 31(1):1–10
Fouad EA, El-Badry M, Mahrous GM, Alanazi FK, Neau SH, Alsarra IA (2011) The use of spray-drying to enhance celecoxib solubility. Drug Dev Ind Pharm 37(12):1463–1472
Naelapää K, Boetker J, Müllertz A, Rades T, Rantanen J, Bar-Shalom D. Soluplus® for modifying the release of highly water soluble APIs
Tantishaiyakul V, Kaewnopparat N, Ingkatawornwong S (1996) Properties of solid dispersions of piroxicam in polyvinylpyrrolidone K-30. Int J Pharm 143:59–66
Verheyen S, Blaton N, Kinget R, Van den Mooter G (2002) Mechanism of increased dissolution of diazepam and temazepam from polyethylene glycol 6000 solid dispersions. Int J Pharm 249:45–58
Dangprasirt P, Ritthidej G (1995) Development of diclofenac sodium controlled release solid dispersions by spray drying using optimization strategy I. Powder formulation. Drug Dev Ind Pharm 21(20):2323–2337
Soliman M, Abdel Malak NS, El Gazayerly ON, Abdel Rehim AA (2011) Preparation of celecoxib solid dispersions for dermal application: in vitro characterization and skin irritation test. J Drug Del Sci Tech 21(6):509–516
Kim EJ, Chun MK, Jang JS, Lee IH, Lee KR, Choi HK (2006) Preparation of a solid dispersion of felodipine using a solvent wetting method. Eur J Pharm Biopharm 64(2):200–205
Sekikawa H, Fujiwara JE, Naganuma T, Nakano M, Arita T (1987) Inhibitory effect of polyvinylpyrrolidone on the crystallization of drugs. Chem Pharm Bull (Tokyo) 26:118–126
Leuner C, Dressman J (2000) Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 50:47–60
Craig DQM (2002) The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 231:131–144
Katharya AK, Chaudhary R, Sharma R, Singh Y, Teotia UVS (2013) Development and optimization of solid dispersion of olanzapine in poly ethylene glycol by D-optimal response surface factorial design. International Journal of PharmTech Research 5(2):700–710
Narang AS, Srivastava AK (2002) Evaluation of solid dispersions of Clofazimine. Drug Dev Ind Pharm 28(8):1001–1013
Ullah M, Ullah H, Murtaza G, Mahmood Q, Hussain I (2015) Evaluation of influence of various polymers on dissolution and phase behavior of carbamazepine-succinic acid Cocrystal in matrix tablets. Biomed Res Int 2015:1–10
Abd Alaziz DM, Sammour OA, Elshamy AEAA, Neseem DI (2014) Formulation and evaluation of binary and ternary solid dispersions of domperidone by solvent evaporation method. Afr J Pharm Pharmacol 8(3):66–80
Parmar KR, Shah SR, Sheth NR (2011) Studies in dissolution enhancement of Ezetimibe by solid dispersions in combination with a surface adsorbent. Dissolution Technologies 18(3):55–61
Afifia S (2015) Solid dispersion approach improving dissolution rate of Stiripentol: a novel antiepileptic drug. Iranian Journal of Pharmaceutical Research 14(4):1001–1014
Ahuja N, Katare OP, Singh B (2007) Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur J Pharm Biopharm 65(1):26–38
Murtaza G, Ahmad M, Khan S, Hussain I (2012) Evaluation of Cefixime-loaded chitosan microspheres: analysis of dissolution data using DDSolver. Dissolution Technologies 19(2):13–19
Raghavendra Rao NG, Kulkarni U, Deshmukh A, Suresh DK (2010) Preparation and characterization of Ionotropic cross-linked chitosan microparticles for controlled release of Aceclofenac. International Journal of Pharmaceutical Sciences and Drug Research 2(2):107–111
Acknowledgements
This work is funded by Deanship of Research at Jordan University Science and Technology (JUST) with Fund number (302/2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obaidat, R.M., AlTaani, B. & Ailabouni, A. Effect of different polymeric dispersions on In-vitro dissolution rate and stability of celecoxib class II drug. J Polym Res 24, 58 (2017). https://doi.org/10.1007/s10965-017-1215-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1215-6