Abstract
Novel Ni-Al layered double hydroxide (LDH) intercalated with bio-active amino acid containing dicarboxylate was synthesized via co-precipitation reaction of Ni(NO3)2. 6H2O, Al(NO3)3. 9H2O and N,N′-(pyromellitoyl)-bis-L-phenylalanine under ultrasonic irradiation. The X-ray diffraction (XRD) results of the organo-modified LDH show that the dianion are intercalated in the interlayer region of LDH and expanded the interlayer distance. An optically active and organo-soluble poly(amide-imide) was prepared by the direct step-growth polymerization of L-phenylalanine based diacid and 3,5-diamino-N-(4-hydroxyphenyl)benzamide in the presence of molten tetra-butylammonium bromide as a green solvent. Then nanocomposite materials built from the assembly of L-phenylalanine amino acid containing polymer and two-dimensional organo-modified LDH were synthesized by solution intercalation method. The structures and morphology of the synthesized materials were investigated by XRD, Fourier transform infrared spectra, field emission scanning electron microscope (FE-SEM) and transmission electron microscopy (TEM) techniques. The thermal properties of the modified LDH and the resulting nanocomposites were studied by thermogravimetry analysis techniques and the results showed improved in the thermal properties of the nanocomposites in comparison with the neat polymer. The FE-SEM, TEM and XRD results revealed a coexistence of exfoliated and intercalated modified LDH in polymer matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Layered double hydroxide (LDH) constitutes a family of inorganic layered materials that possess unique nanostructures and can be considered as promising nanoscale building blocks for novel materials [1, 2]. They are a class of natural and synthetic mixed metal hydroxides, historically known for having extensive anion-exchanging capabilities [3]. The structure of LDHs is closely related to the layered structure of brucite (Mg(OH)2), but replacement of some of the divalent metal ions with trivalent metal ions results in a net positive charge in each of the layers. This net positive charge is balanced by any of a wide assortment of anions, which, along with water, hold the individual layers together. By description, it is the charge-balancing behavior that has defined LDHs as anion-exchanging, clay-like materials for several decades. The general formula for LDH is: [M(II)1-xM(III)x(OH)2] [Ax/y]y-1 nH2O, although some materials have been prepared using monovalent cations. The values for x vary, but the most common are x = 0.33 and x = 0.25, which correspond to a 2:1 and 3:1 M(II):M(III) ratio, respectively. The most familiar naturally occurring LDH is known as hydrotalcite and is a 3:1 Mg: Al LDH with carbonate as the interlayer anion. Because of the characteristics of tunable compositions and exchangeable anions, LDHs have been widely used in various fields like catalysts, drug delivery materials, electrode materials, flame retardant polymer additives, and chemically tailored functional materials [4–10].
In recent time significant attention has been paid to Ni-containing LDHs due to the high catalytic activity of the deriving homogeneous Ni-Al oxides, with high thermal and chemical stability, for a variety of reactions such as steam reforming of methanol, oxydehydrogenation of ethylbenzene, hydrogenation of acetonitrile, and aldol condensation of acetone [11–16].
The synthetic routes to layered hydroxides include co-precipitation, hydrolysis of urea, sol–gel method, and so on [17–19]. The most widely used method for the preparation of LDHs is the co-precipitation method, in which the mixed alkali soda solution is added to a mixed salt solution and the resultant slurry is aged at a desired temperature [20].
The LDH nanosheets are anticipated to be used as building blocks for the production of different functional nanocomposites (NCs). However, for a successful development of high quality NCs containing lamellar materials, it is an essential requirement to develop methods of preparing stable uniform dispersions of the inorganic phase in the nanometer length scale. One approach that can yield miscible clay-polymer dispersions is the direct intercalation of macromolecules into the lamellar structure of the inorganic host. On the other hand, a more effective but much more demanding approach would be the exfoliation of the layers into individual nanoplatelets. In this method, inorganic layers with a very high aspect ratio may become thoroughly separated and dispersed in the polymer. NCs synthesized in this form will show greater thermal and mechanical properties as a result of the molecular-level interactions between the clay particles and the polymers [21–26].
Poly(amide-imide)s (PAI) as a high performance condensation polymer, combine the superior mechanical properties associated with the amide group and the high thermal stability of the imide ring in the same polymer [27, 28]. Aromatic PAIs with chiral structures are biologically very important. In polycondensation, we use amino acids as chiral inducting agents. So polymers based on amino acids are expected to be biodegradable and biocompatible [29, 30].
This work aims at reporting the synthesis of the organically-modified LDH by the co-precipitation reaction of the Al(NO3)3. 9H2O, Ni(NO3)2. 6H2O and bio-active N,N′-(pyromellitoyl)-bis-L-phenylalanine under ultrasonic irradiation and its influence on the properties of polymer-based NCs. The model polymer used is a PAI containing amino acid and phenol moiety. This polymer is so interesting material and there are several reasons for choosing it as a functional polymer. First of all, it is good processable polymer. Furthermore, the introduction of several functional groups as well as phenol moiety and amino acid bulky substituents will result in increasing chain packing distances and decrease intermolecular interactions, leading to the better interaction with modified LDH. NCs of the above polymer and modified NiAl LDH were prepared by ultrasonic techniques. The properties of the hybrid materials were studied by Fourier transform infrared (FT-IR), X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM) and thermogravimetric analysis (TGA) techniques.
Experimental
Materials
Nikel (II) nitrate hexahydrate [Ni(NO3)2.6H2O], aluminum (III) nitrate nonahydrate [Al(NO3)3.9H2O], pyromellitic dianhydride (PMDA), sodium hydroxide (NaOH), tetra-butylammonium bromide (TBAB), 3,5-dinitrobenzoylchloride, 4-aminophenol, 3,5-dinitrobenzoylchloride, glacial acetic acid, propylene oxide, hydrazine monohydrate, 10 % palladium on activated carbon and L-phenylalanine were purchased from Merck Chemical Co. (Darmstadt, Germany) and used without further purification. N,N-Dimethylformamide (DMF) was distilled over barium oxide under reduced pressure before use.
Equipment
X-ray diffraction analysis (XRD) over 2θ = 1.8 to 40°, in steps of 0.02° was carried out using a X-ray diffractometer (Bruker, D8 Advance, Germany) with Cu-Kα radiation (λ = 0.154 nm, monochromatization by primary graphite crystal) generated at 100 mA and 45 kV. The diffraction patterns were collected at a scanning rate of 0.05°/min. Basal spacing were determined from the position of the d(003) reflection. The d-spacing of the hybrid materials was analyzed by using Bragg’s equation (nλ = 2d sinθ). Where n is an integer, λ is the wavelength, θ is the glancing angle of incidence, and d is the interplanar spacing of the crystal. FT-IR spectra of the samples were recorded with a Jasco-680 (Japan) spectrometer at a resolution of 4 cm−1 from 400 to 4000 cm−1. Vibration bands were reported as wavenumber (cm−1). The powdered samples were mixed with dry KBr and pressed in the form of pellets for the measurement. Band intensities are assigned as weak (w), medium (m), shoulder (sh), strong (s), and broad (br). Inherent viscositie was measured by a standard procedure using a Cannon-Fenske routine viscometer (Germany) at the concentration of 0.5 g/dL at 25 °C. Specific rotation was measured by a Jasco Polarimeter (Japan). TGA was performed on a STA503 TA instrument (Hullhorst, Germany) in a nitrogen atmosphere by heating rate of 10 °C/min from ambient temperature to 800 °C at nitrogen atmosphere. The morphology of the nanostructure materials was examined by FE-SEM [HITACHI; S-4160, (Tokyo, Japan)]. The powdered sample was dispersed in H2O, and then the sediment was dried at room temperature before gold coating. The nanostructure morphology of the novel materials was also examined by TEM. The TEM images were obtained from a Philips CM120 (Eindhoven, Netherland) using an accelerator voltage of 100 kV. The reaction was carried out on a MISONIX ultrasonic liquid processors, XL-2000 SERIES (Raleigh, North Carolina, USA). Ultrasound was a wave of frequency 2.25 × 104 Hz and power of 100 W.
Preparation of chiral diacid
N,N′-(Pyromellitoyl)-bis-L-phenylalanine as diacid compound was prepared by procedure reported elsewhere [30].
Preparation of organo-modified Ni2Al LDH under ultrasonic irradiation
Intercalation of N,N′-(pyromellitoyl)-bis-L-phenylalanine in the NiAl–LDH was carried out in one step under ultrasonic irradiation. 0.02 mol of Al(NO3)3.9H2O and 0.04 mol of Ni(NO3)2.6H2O were dissolved in deionized water to obtain solution 1. An aqueous solution containing NaOH (0.02 mol) and N,N′-(pyromellitoyl)-bis-L-phenylalanine (0.02 mol) was prepared and stirred at R.T. Solution 2 was added to solution 1 and the obtained suspension was sonicated for 1 h to furnished modified NiAl-LDH. Finally, the resulting precipitate was filtered and washed by deionized water and then dried at 60 °C for 24 h. For comparative study, NiAl LDH-CO3 2− was prepared under identical reaction conditions without using diacid compound.
Synthesis of diamine with phenol side chain
3,5-Diamino-N-(4-hydroxyphenyl)benzamide as a diamine monomer was prepared according to the reported procedure [31].
Polymer synthesis
The one-step polyamidation reaction of equimolecular amounts of diamine 4 and diacid 3, using molten TBAB as a reaction medium and TPP as a homogenizer, gave the optically active PAI. A typical reaction was carried out as follows: 0.10 g (3.43 × 10−4 mol) of diacid monomer 3, 0.083 g (3.43 × 10−4 mol) of diamine 4, and 0.44 g of TBAB (1.37 × 10−3 mol) were placed in a round-bottom flask and ground completely for 5 min; then, 0.36 mL (13.72 × 10−4 mol) of TPP was added and the mixture was ground for 3 min. It was heated until a homogeneous solution was formed and then, 0.52 mL (1.98 × 10−3 mol) of TPP was added. After that, the solution was stirred for 12 h at 120 °C to obtain a viscous solution. The resulting viscous solution was poured into 30 mL of methanol, filtered and dried at 80 °C for 6 h under vacuum to give 0.16 g (90 %) of yellow powder PAI. The optical specific rotation was measured ([α] 25 Na,589 = − 61.20) at a concentration of 0.5 g/dL in DMF at 25 °C. The inherent viscosity was also measured (η inh = 0.36 dL/g) under the same conditions.
FT-IR peaks (KBr, cm−1): 3311 (m, br, NH and OH stretching), 3100 (w, C-H aromatic), 2970 (w, C-H aliphatic), 2922 (w, C-H aliphatic), 1776 (m, C=O imide, asymmetric stretching), 1717 (s, C=O imide, symmetric stretching), 1665 (s, C=O amide, stretching), 1600 (s), 1544 (s), 1512 (s), 1446 (s), 1377 (s, CNC axial stretching), 1210 (m, CNC transverse stretching), 1071 (m), 865 (m), 835 (s), 757 (m), 727 (s, CNC out-of-plane bending), 687 (w), 526 (w) [31].
Synthesis of PAI/modified NiAl-LDH NCs
For preparations of polymer based NCs, 0.1 g of the PAI was dispersed in 20 mL of absolute ethanol and a uniform colloidal dispersion was obtained after sonication for 30 min at room temperature. Then the suspension was mixed with the different amounts of diacid modified NiAl-LDH (2, 4 and 8 %wt) to produced polymer based NCs followed by sonication for 1 h at room temperature. The solvent was removed and the obtained solid was dried in vacuum at 80 °C for 2 h.
Result and discussion
Synthesis of novel modified NiAl-LDH
Owing to its layered structure, LDH is an outstanding choice as nanofiller considered for preparation of multifunctional polymer/layered crystal NCs. But, its use as nanofiller is limited by its layers high-charge density and high content of anionic species and water molecules. To facilitate the intercalation of polymer in the layers of LDH, the interlayer space should be modified with appropriate organic anions with intention of increasing both the interlayer distance and the hydrophobicity of LDH layers. In this study, at first, chiral biodegradable dicarboxylic acid based on pyromellitic dianhydride (1) and L-phenylalanine amino acid (2) was prepared in reflux acetic acid solution (Scheme 1a). Then this bio-safe diacid was used for efficient synthesis of novel modified NiAl LDH in one-step under ultrasonic irradiation as shown in Fig. 1. This Figure only serves to emphasize the process of adsorption and does not provide information on the order in which the adsorbent molecules are present along with carboxylate ions ionically bound on the surface.
Synthesis of amino acid containing PAI
In the recent time for the more efficient and at the same time more green polymerization process, attracted attention has been done to the new class of potential solvents a namely ionic liquid. In the present investigation, molten TBAB was used as solvent and catalyst for the formation of optically active and organo-solube PAI by the direct polymerization reaction of chiral diacid three and diamine four as shown in Scheme 1b. The incorporation of a chiral unit into the polymer backbone was obtained by measuring the specific rotations of resulted polymer. Chemical structure and purity of this polymer was confirmed in our previous study [31]. Several polymer based NCs were prepared from the reaction of the L-phenylalanine containing PAI and different amount of the diacid modified-NiAl LDH under ultrasonic irradiation. The presence of amide, imide and phenol groups in the backbone of the polymer matrix causes hydrogen interactions with functional groups of the modified-LDH.
Characterized techniques
FT-IR analysis
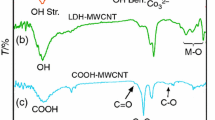
The FT-IR spectra of the NiAl LDH structure with interlayer carbonate anions and modified LDH with chiral dicarboxylate are shown in Fig. 2. The broad band at 3500–3400 cm−1 is due to the O–H stretching modes of layer hydroxyl groups and water molecules. The broadness arises from the very different strengths of hydrogen bonding between these species. The bands are asymmetric and abroad shoulder around 3000 cm−1 is due to the O–H stretching mode of interlayer water molecules hydrogen-bonded to interlayer carbonate anions (Fig. 2a). Water molecules are also responsible for the medium-weak band at 1640 cm−1, owing to the irregular deformation mode [32–34]. The band at 1370 cm−1 is due to the anti symmetric υ3 mode of carbonate anions in the interlayer. The bands at 600, 570 and 425 cm−1 are due to Ni–O–Al deformation mode and Ni–O and Al–O stretching modes [35].
In the FT-IR spectrum of modified NiAl LDH, the broad band in the range of 3200–3600 cm−1 mainly from O-H groups of the hydroxide layers. The absorption bands at 2930–3100 cm−1 correspond to the ν asym and ν sym (C-H) modes of CH2 group in the chiral dicarboxylate molecules (Fig. 2b). The other dicarboxylate bands originated from various functional groups are also found at 1770–1700 cm−1 (C=O), and 1300–1000 cm−1 (C-O). This collective information indicated that diacid molecules were combined within LDH layers through electrostatic interactions.
The FT-IR of the neat PAI and NCs of PAI and different amount of modified NiAl MLDH (2, 4 and 8 %) are shown in Fig. 3a-d. The FT-IR spectrum of chiral PAI showed absorptions of amide (N-H) and phenol (OH) bonds appeared around 3300–3600 cm−1. The aliphatic C-H stretching peak was also appeared at around 2965 cm−1. Strong absorption bands were observed at 1778 and 1726 cm−1, which they can be attributed to the asymmetric and symmetric stretching vibrations of the imide carbonyl groups. The bands of C-N stretching and ring deformation appeared at 1377 and 727 cm−1, respectively. In the spectrum of NCs in compression with the neat PAI, the formation of new absorption bands in the range of 450–700 cm−1 could be attributed to the M-O stretching. Thus from the FT-IR spectroscopy, the interaction between PAI and modified LDH and their complex formation have been confirmed (Fig. 3b-d).
XRD analysis
Figure 4a and b show the powder XRD patterns of NiAl-LDH and modified NiAl-LDH. The XRD patterns of both samples display the characteristic reflections corresponding to two-dimensional hydrotalcite-like materials i.e. (003), (006) and (110) in each case, indicative of R3m symmetry and a hexagonal lattice [35]. The basal spacing value (d 003 ) of LDH phase in pristine NiAl–LDH is approximately 0.76 nm, consistent with that of carbonate-intercalated LDH materials [36]. The position of the basal reflections in diacid modified LDH was shifted towards higher d value indicating the expansion in the interlayer distance (Fig. 4b). In the XRD pattern of modified LDH, the d003 has shifted to 4.20°, corresponding to an interlayer distance of 2.16 nm. It is noteworthy that basal spacing d003 is expanded by 1.40 nm after intercalation of dicarboxylated anions. Hence the XRD results reveal that the diacid anions are successfully intercalated into the LDH galleries (Fig. 4b).
The XRD patterns of the neat PAI, PAI/modified NiAl-LDH NC2 %, PAI/modified NiAl-LDH NC4 % and PAI/modified NiAl-LDH NC8 % are shown in Fig. 4c-f. For neat PAI, no crystalline peak was observed, indicating that this polymer is amorphous. The XRD patterns of the PAI/modified NiAl-LDH NCs (2, 4 and 8 %) are characterized by the disappearance of the diffraction peaks corresponding to the LDH irrespective of the variation in LDH content (Fig. 4c-e). This complete disappearance of LDH peaks may be due to the partial exfoliated structure, in which the gallery height of intercalated layers is large enough and the layer correlation is not detected by XRD (Fig. 4c-f). Although XRD provides a partial picture about distribution of nanofiller and disappearance of peak corresponding to d-spacing does not always confirm the exfoliated NCs, a complete characterization of NC morphology requires microscopic investigation.
Thermal properties
The thermal behavior of the pristine NiAl–LDH and diacid modified NiAl-LDH was examined by TGA techniques (Fig. 5). As can be seen in Fig. 5, the weight loss for pristine NiAl–LDH occurs essentially in two steps: the first one at low temperature (ca. 40–210 °C) corresponds to removal of water physisorbed on the external surface of the crystallites as well as water intercalated in the interlayer galleries; the second one in a wide temperature range of ca. 230–680 °C involves dehydroxylation of the layers as well as removal of volatile species (CO2) arising from the interlayer carbonate anions [15, 37]. The organic modification of the LDH changes it’s the thermal decomposition behavior in comparison to the unmodified sample. According to Fig. 5b, the presence of a larger weight loss step within 400–600 °C for modified-LDH compared to the LDH confirmed the presence of interlayer surfactant anions in LDH. The residual weight percent values at 800 °C of the NiAl-LDH and diacid modified LDH which was obtained from the TGA thermogram are 63, and 49 wt %, respectively. In comparison to LDH-CO3 2−, residual weight percent of the diacid modified LDH were decreased. These results confirmed the presence of interlayer surfactant anions in LDH (Fig. 5).
The thermal stability of the neat PAI, PAI/modified NiAl-LDH NC2 %, PAI/modified NiAl-LDH NC4 % and PAI/modified NiAl-LDH NC8 % were study by TGA technique and the temperatures at which 5 % (T5), 10 % (T10) degradation occur and the residue at 800 °C are shown in Fig. 6 and Table 1. The onset of decomposition temperature of the NCs was higher than that of pure PAI, shifting toward higher temperatures as the amount of modified-LDH was increased. The increase in thermal stability of PAI matrix upon incorporation of modified-LDH could be attributed to the higher heat transfer capacity of modified-LDH that facilitated heat dissipation within the composites, thus preventing the accumulation of heat at certain points for degradation. The residues at 800 °C of the NCs with different modified LDH content are higher than that of pure PAI (Fig. 6). According to the TGA data, a small amount of NiAl-LDH acted as effective thermal degradation resistant reinforcement in the PAI matrix, increasing the thermal stability of the resulting hybrid materials.
The limiting oxygen index (LOI) is a measure of the percentage of oxygen to be present to support the combustion of the materials and it can be used to evaluate the flame-retardancy of them. Theoretically, char residue (CR) can also be used as a criterion for evaluating LOI of materials.
According to Van Krevelen [38], there is a linear relationship between LOI and CR only for halogen-free polymers (Eq. 1):
From this equation, a higher char yield will improve flame retardance. PAI and NCs, containing 2, 4, and 8 wt. % of NiAl-LDH had LOI values 34, 36, 38, and 40, respectively, were calculated from their CR (Table 1). On the basis of the LOI values, such materials can be classified as self-extinguishing materials.
TEM study
TEM images of diacid modified NiAl LDH and NC of PAI with 4 % of diacid modified LDH with two different magnifications are shown in Fig. 7. For modified LDH the nanoparticles show plate-like morphology. The average diameter of dispersed particles is around 20–50 nm (Fig. 7a and b). In NC4 %, TEM observations reveal a coexistence of organo-nanosilicate layers in the intercalated and partially the exfoliated states. TEM micrograph shows two-dimensional objects which are oriented largely parallel to the grid surface and thin sheet like object with similar lateral dimensions. Both modified LDH (Fig. 7a and b) and PAI/modified LDH NC4 % (Fig. 7c and d) present disc-like images, which are actually the LDH platelets lying flat on the substrate. In addition, the picture also shows that some platelets are overlapping on the edge.
FE-SEM study
Morphological information of diacid modified NiAl LDH and NC of PAI with 2 and 8 % of the modified LDH characterized by FE-SEM is shown in Fig. 8. The crystal morphology of modified LDH displays platelet-like structure with lateral dimensions ranging over few micrometer and thickness over few hundred nanometers (Fig. 8a and b). Modified LDH is composed of densely-packed plate-like particles with the visible edges and the thickness of about 30–50 nm. The FE-SEM images of PAI/modified LDH NC2 % (Fig. 8c and d) and PAI/modified LDH NC8 % (Fig. 8e and f) are shown in Fig. 8. According to these photograph in the NCs, the micrograph exhibits the good dispersion of LDH into polymer matrix. It seems that the particles are distributed uniformly in the polymer matrix.
Conclusions
Novel organo-modified NiAl LDH was prepared in one-step by co-precipitation reaction under green conditions by using ultrasonic irradiation. The XRD results of the modified LDH show that the diacid is intercalated in the interlayer region of NiAl-LDH and enlarge the interlayer distance. An optically active and thermally stable PAI with phenol moiety was prepared in high purity and good yields by the direct polymerization of diamine with phenol side chain and diacid monomer based on L-phenylalanine amino acid in TBAB as a green solvent. Three NCs of synthesized PAL and modified LDH were prepared by loading different amounts of LDH (2, 4 and 8 %) into PAI matrix. The hydroxyl groups on the side chains of the PAI could provide better compatibility and bonding, which cause to suitable homogenous dispersion of modified LDH in the obtained NCs. XRD and FT-IR results pointed to the incorporation of the modified LDH within the PAI matrix. The morphology of the modified LDH and NCs was examined by FE-SEM and TEM and the results indicated that the LDH platelets are distributed within the PAI matrix. Thermal stability from TGA measurements was shown to be enhanced compared with those of pure polymer. The presence of amino acid as a biodegradable segment in both novel modified LDH and optically active PAI, make the obtained NCs materials more susceptible for better biodegradation process.
References
Rives V (2001) Layered double hydroxides: present and future. Nova, Huntington
Braterman PS, Xu ZP, Yarberry F (2004) Layered double hydroxides. In: Auer-bach SM, Carrado KA, Dutta PK (eds) Handbook of layered materials. Marcel Dekker, Inc., New York, pp 373–474
Evans DG, Slade RCT (2006) Structural aspects of layered double hydroxides. Struct Bond Springer Berlin Heidelb 119:1–87
Choy JH, Kwak SY, Jeong YJ, Park JS (2000) Angew Chem Int Ed 39:4041–4045
Zhao Y, Li F, Zhang R, Evans DG, Duan X (2002) Chem Mater 14:4286–4291
Zimmermann A, Silvia J, Sonia FZ, Wypych F (2013) J Polym Res 20:224
Tonelli D, Scavetta E, Giorgetti M (2013) Anal Bioanal Chem 405:603–614
Wang Q, Tang SVY, Lester E, O’Hare D (2013) Nanoscale 5:114–117
Wei Z, Chen G, Shi Y, Song P, Zhan M, Zhang W (2012) J Polym Res 19:9930
Zhao C, Peng G, Liu B, Jiang Z (2011) J Polym Res 18:1971
Segal SR, Anderson KB, Carrado KA, Marshall CL (2002) Appl Catal A 231:215–226
Qi C, Amphlett JC, Peppley BA (2006) Appl Catal A 302:237–243
Qi C, Amphlett JC, Peppley BA (2007) J Power Sources 171:842–849
Wang L, Lu Z, Li F, Duan X (2008) Ind Eng Chem Res 47:7211–7218
Coq B, Tichit D, Ribet S (2000) J Catal 189:117–128
Hua M, Ji X, Lei L, Lu X (2013) J Alloys Compd 578:17–25
Wang H, Xiang X, Li F (2010) J Mater Chem 20:3944–3952
Zhang X, Li SP (2013) Appl Surf Sci 274:158–164
Qi FL, Zhang XQ, Li SP (2013) J Phy Chem Solids 74:1101–1108
Mallakpour S, Dinari M, Behranvand V (2013) RSC Adv 3:23303–23308
Martínez AB, Realinho V, Antunes M, Maspoch ML, Velasco JI (2011) Ind Eng Chem Res 50:5239–5247
Matusinovic Z, Wilkie CA (2012) J Mater Chem 22:18701–18704
Wang DY, Das A, Leuteritz A, Mahaling RN, Jehnichen D, Wagenknecht U, Heinrich G (2012) RSC Adv 2:3927–3933
Wang Q, Zhang X, Wang CJ, Zhu J, Guo Z, O’Hare D (2012) J Mater Chem 22:19113–19121
Lonkar SP, Leuteritz A, Heinrich G (2013) RSC Adv 3:1495–1501
Mallakpour S, Dinari M (2013) Polymer 54:2907–2916
Liaw DJ, Chen WH (2006) Polym Degrad Stab 91:1731–739
Menahem T, Mastai Y (2006) J Appl Polym Sci 44:3009–017
Mallakpour S, Asadi P, Sabzalian MR (2011) Amino Acids 41:1215–1222
Mallakpour S, Dinari M (2011) J Macromol Sci A Pure Appl Chem 48:644–679
Mallakpour S, Hatami M (2012) Prog Org Coat 74:564–571
Leroux F, Adachi-Pagano M, Intissar M, Chauviere S, Foranoand C, Besse J (2001) J Mater Chem 11:105–112
Jitianu M, Gunness DC, Aboagye DE, Zaharescu M, Jitianu A (2013) Mater Res Bull 48:1864–1873
Hausslerand L, Heinrich G (2008) Appl Clay Sci 38:153–164
Dinari M, Mallakpour S (2014) J Polym Res 21:350–357
Millange F, Walton RI, O’Hare D (2000) J Mater Chem 10:1713–1720
Wu G, Wang L, Evans DG, Duan X (2006) Eur J Inorg Chem 3185–3196
Van Krevelen DW, Hoftyzer PJ (1976) Properties of polymers, 3rd edn. Elsevier scientific publishing, New York
Acknowledgments
We gratefully acknowledge the partial financial support from the Research Affairs Division at Isfahan University of Technology (IUT), Isfahan. The partial support of Iran Nanotechnology Initiative Council (INIC), and National Elite Foundation (NEF) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallakpour, S., Dinari, M. & Nabiyan, A. Production of NiAl-layered double hydroxide intercalated with bio-safe amino acid containing organic dianion and its utilization in formation of LDH/poly(amide-imide) nanocomposites. J Polym Res 22, 11 (2015). https://doi.org/10.1007/s10965-015-0663-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0663-0