Abstract
Herein, the synthesis of poly(4,4′-triphenylamine-co-9,9-dioctyl-2,7-fluorene) grafted with oligo N-(2-hydroxyethyl) carbazolyl methacrylate as side chains was performed in two steps. A macromonomer with dibromo-triphenylamine end was firstly synthesized by atom-transfer radical polymerization of N-(2-hydroxyethyl) carbazolyl methacrylate followed by Suzuki polycondensation of the macromonomer with 9,9-dioctylfluorene-2,7-diboronic acid. The graft copolymer was characterized by Fourier-transform infrared spectroscopy (FTIR), 1H and 13C-NMR spectroscopy while the optical properties were investigated by UV–vis and fluorescence methods. Cyclic votammetry studies evidenced that the redox processes were accompanied by the dimerization of carbazole pendant groups and polymer crosslinking with the formation of an insoluble network. The parent polymer was post-modified, in solution or bulk, by electrochemical oxidation leading to a crosslinked and insoluble network having electrochromic properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arylamine polymers have been explored due to their unique electro-physical properties [1] with potential applications in organic electronics, photonics and spintronics [2, 3]. Triphenylamine (TPA)-based polymers have good hole-transporting properties, high light-emitting efficiencies, photoconductivity and photorefractivity, large two-photon absorption cross-section and stabilization effect of high-spin polyradicals in organic magnets [4]. Due to the good electron-donating nature of TPA, its oligomers have been widely used as hole-transporting materials for a number of applications, such as in xerography, organic field-effect transistors, photorefractive systems, light emitting diodes, etc. [5, 6]. Soluble conjugated polytriphenylamines are usually obtained through introducing short alkyl side chains on the aromatic ring.
Among conjugated polymers, polyfluorene [7] and polythiophene [8, 9] have been intensively studied due to their interesting optoelectronic properties associated with good environmental and thermal stability. Unfortunately, owing to their intrinsic structure, theses conjugated polymers are non-moldable materials and have poor solubility. Moreover, polyfluorene is characterized by poor hole injection properties and synthesis of copolymers with arylamines (carbazole or triphenylamine) was carried out to balance hole and electron transport properties and improve electroluminescent performances of these materials. In this context great efforts have been made towards the synthesis of modified polyarylenes by copolymerization with arylamine derivatives and introduction of side alkyl and alcoxy substituents [10–12]. In order to increase solubility, processability and performances of these materials, in the last years a new approach based on the synthesis of graft polymers where the backbone is a conjugated chain while grafts are flexible chains was studied.
Polymer brushes are graft polymers that consist from a linear polymer backbone possessing densely grafted side chains [13]. Molecular brushes having stiff backbones based on conjugated main chains and flexible side chains have been synthesized mainly to improve the solubility and processability of conducting polymer and to modify the morphology with beneficial contribution on the photophysical properties. Three synthetic routes have been successfully applied for the synthesis of conducting polymer brushes and are based on: (a) the polymerization or polycondensation of macromonomers (grafting through” method) [14, 15], (b) the use of macroinitiators for growing the side chains by polymerization or polycondensation (“grafting from” method) [16–20], and (c) coupling of the main chain having functional groups with a monotelechelic oligomer (“grafting onto” method) [21, 22]. As main conjugated chain, poly(arylene-thienylene) with polystyrene grafts [14, 15] and poly(phenylene-fluorene) or polyfluorene with poly[2-(dimethylamino)ethyl methacrylate] [16], polyethyleneglycol [22] and polystyrene grafts [22] have been reported. Polythiophene decorated with polystyrene [19, 20, 23], polymethylacrylate [17], polyvinylquinoline [18], poly(N,N-dimethyl ethyl methacrylate), poly(N-isopropyl acrylamide) [24] or poly [N-(carbazolyl)propyl acrylate] [25] arms have been obtained using functionalized thiophene polymers for ATRP polymerization. Also, polypyrrole grafted with poly(epsilon-caprolactone) [26, 27], polytriphenylamine grafted with poly(epsilon-caprolactone) [28] or polyethylmethacrylate [29], polyphenylacetylene with poly(ethylene glycol) [30], polylactide [31], polydimethylsiloxane [32], polypeptide [33] and polyethyleneoxyde [34] as side chains were selected as electroactive backbones. In all cases polymer brushes showed good solubility in common organic solvents, new morphologies and improved photophysical properties. These polymers can to self-assemble into a wide array of morphologies that are very important for solar cells applications. The conservation of the morphology during the long operation time of electronic devices is a clear target because any morphological modification can change drastically their performances. Cross linking is recognized as a very promising approach to stabilize the nanoscale morphology of the thin photoactive layer [35] and presence of crosslinkable functional groups able to lead by UV, thermal and electrochemical post-reactions to conjugated networks is a necessary condition.
In this work we report the synthesis of a new crosslinkable graft copolymer, i.e., poly(4,4′-triphenylamine-co-9,9-dioctyl 2,7-fluorene) containing an oligo(2-hydroxyethyl) carbazolyl methacrylate as the grafted side chain in the para position of triphenylamine unit for improving the solubility and ability to form electrochemically cross linked thin films and to introduce new properties. The side chains are build by ATRP polymerization of N-(2-hydroxyethyl) carbazolyl methacrylate initiated by a low molecular weight ATRP initiator. Cyclic votammetric studies evidenced that the redox processes are accompanied by dimerization of carbazole pendant groups. The parent polymer was post-modified, in solution or bulk, by electrochemical oxidation to a crosslinked and insoluble network having electrochromic properties. The crosslinking reaction took place between carbazole pendants by reactive 3 and 6 positions.
Experimental
Materials
All the starting materials for synthesis of monomers and polymers were purchased from Aldrich and used as received, such as: 9,9-dioctylfluorene-2,7-diboronic acid, CuBr, 2-bromo-2-methyl-propionyl bromide, 2,2-bipyridine (bpy), tetrakis(triphenylphosphine)-palladium(0) [(PPh3)4Pd(0)], potassium carbonate, and triphenylamine (98 %). Solvents (Aldrich) were dried by the usual methods or used as received. The ATRP initiator, 4-[bis(4-bromophenyl)amino]benzyl 2-bromo-2-methylpropanoate (BBMP), was synthesized by a four-steps method. Starting from triphenylamine, 4-(diphenylamino) benzaldehyde was synthesized by formylation reaction using POCl3/DMF reagent, as was described in the literature [36], followed by bromination with bromine in CHCl3 at 0 °C [37] and reduction to 4-hydroxymethyl-N,N’-bis(4-bromophenyl)aniline with NaBH4 in tetrahydrofuran/methanol mixture [28]. The treatment of the alcohol with 2-bromo-2-methyl-propionyl bromide in toluene and presence of triethylamine led to ATRP initiator in good yield. Yield, 75 %. ESI-MS = 582.9 (M + H)+. 1H-NMR (CDCl3, ppm): 7.−6.8 (m, 12H Ar), 5.08 (s, 2H, −CH2O-) and 1.95 (s, 6H, −CH3). 13C-NMR (CDCl3, ppm): 171.52, 147.03, 146.28, 132.45, 129.30, 125.75, 124.75, 115.91, 77.01 (CDCl3), 67.19, 55.73, 30.78.
N-(2-Hydroxyethyl)carbazolyl methacrylate (HEKM) was synthesized according to the known method [38].
Synthesis of poly(N-(2-hydroxyethyl)carbazolyl methacrylate) macromonomer by ATRP method
HEKM (0.6 g; 2.14 mmol) and toluene (1.5 mL) were added to 10 mL Schlenk tube and subjected to three vacuum/nitrogen refill cycles. A solution of BBMP (16.0 mg; 0.028 mmol), bpy (8.9 mg; 0.057 mmol) and Cu(I)Br (4.1 mg; 0.028 mmol) in toluene (1.5 mL) was added to the Schlenk tube under nitrogen atmosphere, and then the whole mixture was degassed and filled with nitrogen. After stirring at room temperature for 1 h the Schlenk tube was placed in a temperature-controlled oil bath at 100 °C for 24 h. The mixture was cooled at room temperature, diluted with chloroform and passed through a short column of silicagel to remove copper salts. Finally, the macromonomer was precipitated in methanol, dried and purified by repeated precipitation. Yield = 74 % white powder with Mn = 12,460 and Mw/Mn = 1.13.
Synthesis of graft copolymer by “grafting through”
In a two-necked 25 mL flask equipped with a condenser and a magnetic stirrer 0.13 g of macromonomer, 0.0207 g (0.04 mmol) of 9,9-dioctylfluorene-2,7-diboronic acid and 5.5 mg (0.0047 mmol) tetrakis(triphenylphosphine) palladium (0) were introduced under inert atmosphere. The solvent mixture formed from 1 mL 2 M K2CO3 aqueous solution and 10 mL toluene (degassed by bubbling nitrogen) was introduced with a syringe through the septum. The mixture was kept at 85 °C for 3 days, maintaining vigorous stirring and the exclusion of oxygen and light. The copolymers were separated by precipitation in methanol, filtrated, washed several times with 1 N HCl (aqueous solution) and water for the removal of inorganic salts, and dried. Further purification of the product was achieved by dissolving the polymer in CHCl3 and precipitating in methanol. Yield = 72 %. Mn ~ 24,440 and polydispersity degree = 2.23.
Electrochemical crosslinking of graft copolymer
A CH2Cl2 solution of 10 mL 3 × 10−5 M of graft copolymer containing 1 × 10−3 M tetrabutylammonium perchlorate was introduced into a three-electrode electrochemical cell and polymerization carried out by scanning anodic potential between 0 and 1.8 V at 20 mV.sec−1. A very thin polymer film was deposited on ITO electrode due to the electrochemical crosslinking and solution was blue-greenish colored due to soluble low crosslinked product. The film was electrochemically dedoped in a monomer free solution containing only electrolyte and used for electrochemical and electrochromic studies.
Instruments
Fourier Transform Infrared (FTIR) spectra were recorded with a DIGILAB-FTS 2000 spectrometer. UV-visible and fluorescence measurements were carried out in CHCl3 solution with a Specord 200 spectrophotometer and Perkin Elmer LS 55 apparatus, respectively. 1H and 13C-NMR spectra were recorded at room temperature with a BrukerAvanceDRX-400 spectrometer (400 MHz) as solutions in CDCl3. The differential scanning calorimetry (DSC) measurements were performed with a Mettler DSC-12E apparatus, in nitrogen and a heating and cooling rate of 10 °C per minute. The glass transition temperatures (Tg) were determined from the second heating run. The relative molecular weights were determined by gel permeation chromatography (GPC) using a PL-EMD 950 Evaporative Mass Detector instrument and polystyrene standards for the calibration plot and chloroform (1 mL/min) as solvent. Electrochemical studies were carried out with a Bioanalytical System, Potentiostat–Galvanostat (BAS 100B/W). The all experiments were performed in a one-compartment cell using a standard three-electrode cell arrangement with a working electrode, an auxiliary electrode (platinum wire), and a reference electrode (consisted of a silver wire coated with AgCl). The working electrode potential was always measured against an Ag/AgCl reference electrode. Before each experiment the electrolyte solutions were degassed by bubbling argon for 5 min. All electrochemical experiments were carried out in stationary solutions and at room temperature (25 °C). Cyclic voltammograms of the investigated compounds were recorded in CH2Cl2, and tetrabutylammonium perchlorate (Bu4NClO4) as electrolyte. For spectroelectrochemical studies, a film of the cross-linked polymer was deposited upon ITO coated glass electrode by electrochemical polymerization using a solution of polymer in CH2Cl2 and Bu4NClO4 as electrolyte. The film is prepared by sweeping the potential between 0 and +1.8 V for 15 scan cycles and after deposition, the ITO/polymer film was dedoped and rinsed with distilled water and used as working electrode in a standard spectroelectrochemical cell (a quartz cuvette of 1cmx1cm dimensions) filled with supporting electrolyte (Bu4NClO4, solution 0.1 M in toluene/CH2Cl2, 1:1) and having a Pt wire as counter electrode. The electrodes were connected to a BAS Potentiostat–Galvanostat and spectroelectrochemical spectra were registered using an SEC-2000-UV/VIS-type spectrometer. The background correction was obtained by taking a UV–vis spectrum of a blank cell (an electrochemical cell with an ITO working electrode without the polymer film) with conditions and parameters identical to those of the polymer experiment.
Results and discussion
Synthesis and characterization
From the different synthetic methods, “grafting through” was followed to reach the graft copolymer structure, where the main backbone is a polyconjugated chain while the grafts are saturated short chains of oligo[N-(2-hydroxyethyl)carbazolyl methacrylate]. The “grafting through” approach has involved two steps: (a) dibromo triphenylamine-based initiator was used to obtain macromonomers with well-defined molecular weight, polydispersity and chain-end functionality by atom transfer radical polymerization (ATRP) of N-(2-hydroxyethyl)carbazolyl methacrylate. (b) Through a further step, macromonomer with dibromo-triphenylamine end is coupled with fluorene diboronic derivatives by Suzuki reactions to obtain graft copolymer (Scheme 1). This route has an important disadvantage, the equimolarity between coupling partners is very hard to be attained because the molecular weight of the macromonomer is not very precise known and this could explain the low molecular weight of the copolymer. To overpass this disadvantage, we have also tried the alternative route, the “grafting from” method, that suppose the obtaining of functional copolymer poly(4,4′-triphenylamine-co-9,9-dioctyl-2,7-fluorene) firstly, by Suzuki polycondensation of ATRP initiator with 9,9-dioctylfluorene-2,7-diboronic acid, followed by building of grafts by ATRP polymerization of N-(2-hydroxyethyl)carbazolyl methacrylate (Scheme 1). Thus, for synthesis of poly(triphenylamine-co-9,9-dioctyl-2,7-fluorene) chain the Suzuki polymerization reaction starting from the initiator 4-[bis(4-bromophenyl)amino] benzyl 2-bromo-2-methyl propanoate with 9,9-dioctylfluorene-2,7-diboronic acid using Pd(PPh3)4 as catalyst was carried out. The polyconjugated chain viewed as an ATRP polyinitiator, should contain the initiator group in every structural unit. Unfortunately, the expected structure was not obtained from the polycondensation, the product was obtained mostly as an insoluble material. The insolubility of the polymer could be assigned to the side reactions leading to crosslinking and damage of functional ATRP sites, because the side substituent (2-bromo 2-methyl propanoate group) contains reactive bromine atoms able to participate in side reactions.
Synthesis and characterization of macromonomer and graft copolymer
The triphenylamine-based initiator was used in ATRP polymerization of HEKM using the feed molar ratio between components: [HEKM]0: [initiator]0: [CuBr]0: [bipyridine]0 = 75:1:1:2 to obtain an oligo-HEKM with a well-defined molecular weight, narrow polydipersity and precise chain-end functionality (i.e., 4,4′-dibromo-triphenylamine end) (Scheme 1). This macromonomer, having a dibromo triphenylamine group at one end was used in Suzuki copolycondensation with 9,9-dioctylfluorene-2,7-diboronic acid to obtain graft copolymer. This step has a disadvantage, the equimolarity between coupling partners is very hard to be attained because the molecular weight of macromonomer is not very precise known so the synthesized graft copolymer has a short main conjugated chain. GPC traces of macromonomer is unimodal and symmetrical with average molecular weight, Mn ~ 12,460 and low polydispersity degree (1.13), a value of n = 42 was found for the polymerization degree of HEKM, but graft copolymer has a broad and bimodal GPC curve and shifted to higher molecular weights (Mn ~ 24,440 and polydispersity degree =2.23).
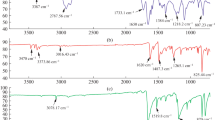
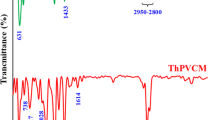
For confirmation of the macromonomer and copolymer structures, FTIR, 1H- and 13C-NMR spectra were registered. In Fig. 1 is shown the FT-IR spectrum of the macromonomer, characteristic bands are 3049 cm−1, 3020 cm−1 (aromatic CH stretching), 2961 cm−1 (aliphatic CH stretching), 1730 cm−1 (is attributed to the group C = O), 1627, 1597, 1485, 1460 cm−1 (in-plane stretching vibrations of aromatic rings), 1325–1261 cm−1 (corresponding to the vibration of the C-N bond from the carbazole group). The peaks at 802, 748 and 722 cm−1 are from CH deformation of trisubstituent benzene ring. Moreover, the characteristic band of the ester group (1730 cm−1) was clearly observed for macromonomer and graft copolymer. There are no obvious differences observed in the spectra because the chemical linkages and functional groups are quite similar. However, in the spectrum of copolymer, the broad bands between 2800 and 3000 cm−1 that derive from alkyl C-H stretching vibration have been strengthened prominently due to the increased content of alkyl C-H bonds from fluorene group.
Figures 2 and 3 show the representative 1H-NMR and 13C-NMR spectra of the macromonomer and graft copolymer. The results strongly suggest the formation of relative well-defined graft copolymer through Suzuki coupling reaction of dibromine triphenylamine end macromonomer with diboronic derivatives.
The 1H-NMR spectrum (CDCl3, δ, ppm) of macromonomer shows methyl and methylene protons from main chain at 0.18–0.5 ppm (Hh) and 1–1.5 ppm (Hg), respectively, between 3.97 and 4.11 ppm are protons assigned to the two methylene group (−CH2-CH2-, Hi and Hj), while in the region 6.8 and 8.1 ppm appear aromatic protons of the carbazolyl group (Hk, Hm and Hm). The signals attributed to the initiator are 7.52 ppm (Ha) and 6.79 ppm (for the protons Hb and Hc), whereas the signal in the region of 4.7 to 5.0 ppm correspond methylene protons He. The copolymer shows peaks in the same region as macromonomer but shows two signals in the region 7.51 and 7.65 ppm assigned to the aromatic proton of the fluorene ring. In addition, it is observed in the aliphatic region changes that are assigned to the alkyl substituents of fluorene.
The 13C-NMR spectral analysis of macromonomer (Fig. 3), recorded in CDCl3 shows the following signals: 17.18 to 18.29 ppm (Ch), 40.83 (Cg), 44.31 (Cj), 53.22 (Ct), 62.38 (Ci) ppm characteristic peaks of methyl carbons, methylene carbon and quaternary carbons respectively. Aromatic carbon signals are assigned as follows: 108.68 (Ck), 119.28 (Cm), 120.48 (Cw), 122.96 (Cl), 125.75 (Cy), 140.20 (Cv) [39, 40], but the initiator is assigned to a single signal at 132.24 ppm (Ca) other signals overlap with the signals of the monomeric unit. Carbonyl carbons belonging monomeric unit can be observed in the region 175–179 ppm [39, 40]. Moreover, 13C-NMR spectrum of copolymer G1 shows not only peaks of the main polymer chain from macromonomer, but also due to the newly introduced moiety. The peaks are as follows: 14.41, 24.21, 28.12, 29.56, 29.90, 30.40, and 44.73 ppm (for aliphatic carbon) and small peak at 152 ppm corresponding unit the fluorene.
The glass transition temperature measured by differential scanning calorimetry (DSC) is 142.8 °C for the macromonomer and 146.2 °C for graft copolymer. For the graft copolymer only one Tg was observed assigned to the methacrylate side chains. The increasing of Tg from macromonomer to copolymer can be explained by increase of molecular weight and the influence of the conjugated sequence on the mobility of polymethacrylate chains.
Optical properties
The optical properties of macromonomer and copolymer were investigated by UV–vis and fluorescence spectroscopy, in diluted THF solution and in Fig. 4 are presented typical absorption spectra.
ATRP initiator shows an UV–vis absorption spectrum with a maximum at 310 nm while the fluorescence spectrum shows two emission maxima at 388 nm and 477 nm, characteristic for triphenylamine group. By ATPR polymerization of HEKM, UV–vis spectrum of macromonomer shows absorption peaks characteristic, at 262, 294, 328, and 342 nm, as is observed in Fig. 4, assignable to the π-π* transition from carbazole rings. The absorption and emission of triphenylamine end are very low and covered by absorption and emission of carbazole rings. When compare the absorption spectra of macromonomer and graft copolymer, one can readily deduce that the broad absorption band at around 380 nm is originated from main conjugated chain. Furthermore, the strong fluorescence in solution can be observed, for macromonomer (excited with a wavelength of 328 nm) with maxima at 348, 363 and 400 nm. For graft copolymer (λexc = 328 nm) the maxima are situated at 349m, 364 and 426 nm (Fig. 5).
Electrochemical characterization
Electrochemical modification of the graft copolymer
The electrochemical properties of the graft copolymer were investigated using cyclic voltammetry by recording the current intensity - potential curves of the solution (~3.5 × 10−3 M copolymer unit concentration), in CH2Cl2 as solvent and containing 0.1 M tetrabutylammonium perchlorate as electrolyte. The ITO working electrode potential was swept on the 0.0 to 1.8 V window with a scan rate of 20 mVs−1 for 5 cycles. The multiple cyclic voltammograms of polymer are showed in Fig. 6. As it can be seen, in the first scan only an oxidation peak at 1.44 V with the oxidation onset at 1.25 V was observed assigned to the oxidation of the carbazole groups from grafts to cation-radicals, followed by their rapid and irreversible coupling and forming of 3,3′-dicarbazolyl dications [41–43] (Scheme 2).
The reverse scanning the voltammogram reveals two cathodic peaks at 1.12 and 0.90 V, assigned to the reduction of the oxidized species (cation-radical monomer and dimer species, respectively) that are formed at the solution-electrode interface. As the CV scans continued, the subsequent cycles showed two oxidation and two reduction peaks, with the same oxidation onset at 0.85 V. The oxidation and reduction of the dimer units occurs at lower potential then monomer units due to extended conjugation path. In the same time, on the working electrode a thin polymer film was deposited and its thickness gradually increased upon CV scanning and intensification of oxidation waves indicate the formation of polymer film. Therefore, the electrodimerization of carbazole groups by 3 and 6-reactive positions convert the soluble graft copolymer into a crosslinked and insoluble network, deposited on the working electrode. The oxidation peaks are shifted at higher potential, probably the electrical resistance of the film increased and higher potential was needed to overcome the higher resistance [44]. Starting with the fourth cycle the oxidation and reduction peaks are hardly observed, because the bulky dopant ions cannot easy free move in and out of the dense crosslinked copolymer film. The insertion and expulsion of anions that assure neutrality is hindered by the compactness of film.
For comparison, carbazole monomer (HEKM), has presented a different electrochemical behavior (Fig. 7). In the first oxidation scan, the oxidation of carbazole group to cation-radicals was observed at 1.49 V while the reduction of monomer and dimer forms was observed at 1.19 and 0.93 V, respectively. The subsequent scans show two oxidation and two reduction peaks and process has a quasi-reversible response. Starting with the fourth cycle the deposition of a thin film polymer on electrode surface was observed and the oxidation and reduction peaks can also be evidenced.
After coating of graft copolymer onto the electrode, the ITO-glass surface was dedoped electrochemically in a monomer free solution containing only electrolyte and used for electrochemical and electrochromic studies. The cyclic voltammetric response of this film, cycled in the copolymer-free 0.1 M solution of Bu4NClO4, is shown in Fig. 8.
The crosslinked copolymer becomes less electroactive with cycling that is probable linked to the bulkiness and mobility of the dopant anions and the compactness of film. Diffusion of anions between the film and the electrolyte during the oxidation and reduction processes is hindered.
Electrochromic properties
It was observed that the film of crosslinked copolymer upon transparent ITO electrode surface shows different colors depending on the applied potential. For a more detailed study, in-situ UV–vis spectroelectrochemistry was further employed, using the crosslinked copolymer film deposited on a transparent ITO/glass working electrode and cyclic voltammetry technique coupled with UV–vis absorbance measurements. This procedure allowed simultaneous recording of the electronic absorption spectra in the range of 300 – 800 nm and the current intensity versus applied potential. The changes in the absorption spectra were recorded with a spectrophotometer while the working electrode potential was swept from 0.0 to 1.8 V, with 50 mVs−1 scan rate. The typical absorption spectra of the polymers films are presented in Fig. 9, as a series of UV–vis absorbance curves correlated to electrode potentials during the oxidation (up) and reduction (down) processes.
In the neutral state (at 0.0 V) the film is transparent in the visible region and exhibits absorption spectrum with maximum at 336 nm assignable to the π-π* transition from carbazole rings and conjugated main chain. Upon oxidation by increasing the applied potential, new absorption band at 421 nm is observed. As the applied potential reaches 1.25 V a new absorption band arises at 697 nm and grows. Upon oxidation by increasing the applied potential from 0.0 to 1.8 V, the intensity of 336 nm absorption gradually decreases in intensity, while the 421 and 697 nm new absorption bands were formed. Absorption band located at 421 nm can be associated with the formation of radical cation species (polaron state) while the band at 697 nm to dicationic species (bipolaron state) formed by oxidation of 3,3′-dicarbazolyl segments (Scheme 2). During the reduction process, the absorption bands decrease in intensity along with potential value. A more pronounced decrease in intensity has absorption bands located at 421 and 697 nm, while the band at 336 nm increases slightly in intensity to potential 0.5 V and later decrease in intensity, as a consequence of the reduction process.
Electrochromic switching studies were carried out to monitor absorbance change with time during repeated potential steps between reduced and oxidized states. The ITO/poly-G1 film oxidation state was switched by stepping the potential between 0.0 and 1.5 V, with time intervals of 5 s, and the current and absorbance at 421 and 697 nm were monitored as a function of time. The switching time was defined as the time required for 90 % of the full change in transmittance after the switching of the potential. The percentage transmittance change (ΔT %) of crosslinked copolymer between neutral (0 V) and oxidized states (at 1.5 V) was found to be 16 % for 421 nm and 21 % for 697 nm. Moreover, the response time of the polymer film was found to be 1.8 s from color switching and 0.6 s for bleaching (Fig. 10). Coloration efficiency (η) of the crosslinked copolymer at 697 nm was calculated to be 450 cm2/C, using the equation using the equation η = ΔOD/Qd = log [Tneut/Toxi]/Qd (cm2/C), where Qd is the injected charge per unit electrode area during a redox step, and Tneut and Toxi are the bleached and colored transmittance values, respectively [45]. The crosslinked copolymer loses 29 % of its original optical contrast after several runs.
Conclusions
A new graft copolymer having a conjugated main chain of poly(4,4′-triphenylamine-co-9,9-dioctyl 2,7-fluorene) type and grafts containing carbazole groups was synthesized by two-step method combining controlled radical polymerization method and Suzuki coupling. The copolymer structure was proved by spectral methods (FTIR, 1H- and 13C-NMR). The molecular weight and polydispersity was slightly higher than the one corresponding to the macromonomer precursor. The fluorescence spectrum of the graft copolymer shifted to higher wavelength values as compared to the macromonomer. Cyclic voltammetry measurements revealed the electrochemical dimerization of carbazole pendant groups and formation of a crosslinked structure. Furthermore, the deposition of a polymer film onto the electrode surface was observed. Electrochemical and electrochromic properties were not reversible due to the presence of a dense crosslinked network.
References
Iwan A, Sek D (2011) Prog Polym Sci 36:1277–1325
Friend RH, Gymer RW, Holmes AB, Burroughes JH, Markes RN, Taliani C (1999) Nature 397:121–128
Mitchke U, Bauerle P (2000) J Mater Chem 10:1471–1479
Thelakkat M (2002) Macromol Mater Eng 287:442–461
Walzer K, Maennig B, Pfeiffer M, Leo K (2007) Chem Rev 107:1233–1271
Law KY (1993) Chem Rev 93:449–486
List EJW, Scherf U (2007) In Skotheim TA, Reynolds JR (eds) Handbook of conducting polymers, 3rd edn. CRC Press, New York
Jeffries-El M, McCullough RD (2007) In: Skotheim TA, Reynolds JR (eds) Handbook of conducting polymers, 3rd edn. CRC Press, New York
McCullough RD (1998) Adv Mater 10:93–116
Giovanella U, Pasini M, Destri S, Porzio W, Botta C (2008) Synth Met 158:113–119
Kruzinauskiene A, Matoliukstyte A, Michaleviciute A, Grazulevicius JV, Musnickas J, Gaidelis V, Jankauskas V (2007) Synth Met 157:401–406
Tomkeviciene A, Grazulevicius JV, Volyniuk D, Jankauskas V, Sini G (2014) Phys Chem Chem Phys 16:13932–13942
Sheiko SS, Sumerlin BS, Matyjaszewski K (2008) Prog Polym Sci 33:759–785, and references therein
Cianga I, Mercore VM, Grigoras M, Yagci Y (2007) J Polym Sci A Polym Chem 45:848–865
Cianga I, Mercore VM, Grigoras M, Yagci Y (2007) Polymer 48:6501–6509
Zhang Z, Lu X, Fan Q, Hu W, Huang W, Polym Chem 2: 2369–2377
Costanzo PJ, Stokes KK (2002) Macromolecules 35:6804–6810
Economopoulos SP, Chochos CL, Gregoriou VG, Kallitsis JK, Barrau S, Hadziioannou G (2007) Macromolecules 40:921–927
Shen J, Tsuchiya T, Ogino K (2008) J Polym Sci A Polym Chem 46:1003–1013
Strover L, Roux C, Malmstrom J, Pei Y, Williams DE, Travas-Sejdic J (2012) Synth Met 162:381–390
Bolognesi A, Galeotti F, Mroz W, Gancheva V, Terlemezyan L (2010) Macromol Chem Phys 211:1488–1495
Pu KI, Li K, Liu B (2010) Adv Funct Mater 20:2770–2777
Wang MF, Zou S, Guerin G, Shen L, Deng KQ, Jones M, Walker GC, Scholes GD, Winnik MA (2008) Macromolecules 41:6993–7002
Balamurugan SS, Bantchev GB, Yang YM, McCarley RL (2005) Angew Chem Int Ed 44:4872–4876
Shen J, Masaoka H, Tuchiya K, Ogino K (2008) Polym J 40:421–427
Mecerreyes D, Stevens R, Nguyen C, Pomposo JA, Bengoetxea M, Grande H (2002) Synth Met 126:173–178
Strover LT, Malmstrom J, Laita O, Reynisson J, Aydemir N, Nieuwoudt MK, Williams DE, Dunbar PR, Brimble MA, Travas-Sejdic J (2013) Polymer 54:1305–1317
Bendrea AD, Vacareanu L, Grigoras M (2010) Polym Int 59:624–629
Cao Z, Abe Y, Nagahama T, Tsuchiya K, Ogino K (2013) Polymer 1:269–276
Qin Z, Chen Y, Zhou W, He X, Bai F, Wan M (2008) Eur Polym J 44:3732–3740
Zhang C, Wang H, Su G, Li R, Shen X, Zhang S, Geng Q, Liu F, Otsuka I, Satoh T, Kakuchi T (2012) Polym Int 61:1158–1162
Zhang W, Shiotsuki M, Masuda T (2007) Polymer 48:2448–2553
Maeda K, Kamiya N, Yashima E (2004) Chem Eur J 10:4000–4010
Hiraoka S, Hirata K, Shionoya M (2004) Angew Chem Int Ed 43:3814–3818
Qian D, Xu Q, Hou X, Wang F, Hou J, Tan Z (2013) J Polym Sci A Polym Chem Ed 51:3123–3131
Grigoras M, Vacareanu (Stafie) L (2009) Des Monom Polym 2:177–196
Vacareanu L, Grigoras M (2011) High Perf Polym 23:112–124
Simionescu CI, Percec V, Natansohn A (1980) Polymer 21:417–422
Karali A, Froudakis GE, Dais P, Heatley F (2000) 33, 3180–3183
Mailhot-Jensen B, Robu S, Rivaton A, Pilichowski JF, Chirita A, Chilat E, Dragalina G (2010) Int J Photoen. doi:10.1155/2010/945242
Fulghum TM, Taranekar P, Advincula RC (2008) Macromolecules 41:5681–5687
Frau AF, Estillore NC, Fulghum TM, Advincula RC (2010) Appl Mater Interfaces 2:3726–3737
Tria MC, Liao KS, Alley N, Curran S, Advincula RC (2011) J Mater Chem 21:10261–10264
Andrikaityte E, Cekaviciute M, Simokaitiene J, Buka G, Grazulevicius JV, Rubeziene V (2012) React Funct Polym 72:11–16
Monk PMS, Mortimer RJ, Rosseinsky DR (1995) Electrochromism: Fundamentals and Applications. Weinheim, VCH
Acknowledgments
The authors thank to the Romanian National Authority for Scientific Research (UEFISCDI) for financial support (Grant PN-II-ID-PCE-2011-3-0274, Contract 148/2011).
Conflict of interest
Authors confirm that this article content has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Negru, O.I., Grigoras, M. Electrochemically generated networks from poly(4,4′-triphenylamine-co-9,9-dioctyl-2,7-fluorene) with grafts containing carbazole groups. J Polym Res 22, 637 (2015). https://doi.org/10.1007/s10965-014-0637-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0637-7