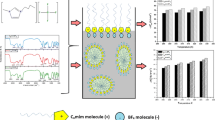
Abstract
Molecular interactions present between 1-pentadecyl-3-methylimidazolium bromide and the biologically important amino acid additives (glycine, l-alanine, and l-valine) have been examined in aqueous media by employing conductivity, UV–Visible, refractive index, and FT-IR spectroscopy at different compositions and temperatures. Furthermore, using the electrical conductivity data, critical micelle concentration, degree of counter-ion dissociation, and various thermodynamic parameters of micellization have been computed. To study the interactions in the micellar system, the effects of additives on these parameters have been carefully analyzed. UV–Visible and refractive index are carried out to outline the results stated above, along with FT-IR results to confirm the various interactions prevailing in the system.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Exploration of various kinds of interactions existing among the protein/amino acid-surfactant systems has attracted the interest of many academic researchers around the globe by virtue of their wide applications in foods and cosmetics, detergency, biosciences, drug delivery, and biotechnological processes [1,2,3,4,5,6,7]. The drug delivery application is the most appealing of them because different amino acid-surfactant combinations can be used to modulate drug distribution [8,9,10,11]. The application is intently associated with the surfactant’s micellization process since this is possible by understanding the interactions between amino acids and surfactants, which include both hydrophobic and electrostatic attraction forces. In the process of micellization, the surfactant monomers in an aqueous solution undergo self-association resulting in structures known as micelles. This self-association is found to take place at a threshold concentration termed as critical micelle concentration (CMC) of surfactant where micelles form. Usually, as the CMC is reached, the monomer surfactant concentration remains constant, and adding more surfactant results only in an increase in the concentration of micelles [12, 13]. The CMC value of a surfactant has been well recognized as an important parameter in several applications of medical, chemical, and biotechnology, for the evaluation and optimization of distinguished properties of micelles. Consequently, this leads to the increased investigation of these surfactants in aqueous as well as in the presence of numerous additives.
Ionic liquids (ILs) are now the subject of intense research because of their property of containing both hydrophobic and hydrophilic groups within the same molecule. As a result of this property, ILs have the potential to be utilised in a diverse array of contexts [14,15,16,17,18,19,20]. The ones consisting of long hydrophobic (alkyl chain) parts are found to possess surface-active properties analogous to those of traditional surfactants and as a consequence, ILs can form self-assembled structures in an aqueous solution such as micelles [21,22,23,24,25], gels [26, 27], vesicles [28,29,30,31], and lyotropic liquid crystals [32,33,34,35]. In these amphiphilic molecules, the aggregation phenomenon is governed by both attractive as well repulsive interactions. The repulsive interactions take place between polar head groups [36], while the hydrophobic interactions [37] among the non-polar tail constitute attractive interactions. Hence, it shows that any changes in the alkyl chain length (hydrophobic part) [38,39,40], the nature of the head group (hydrophilic part) [41, 42] as well as the nature of counter ions [43, 44] will influence the aggregation process, altering the CMC value, shape, size and also the aggregation number (Nagg) of aggregates. The aggregates formed provide important functional properties to the ionic liquids which in turn get modified in the presence of additives. In our previous researches, the micellization behavior of long-chain imidazolium ionic liquids, [C14mim][Br] [45] and [C15mim][Br] [46] in the presence of carbohydrates as additives has been explored with the main focus on the effect of hydrocarbon chain length on the CMC values as well as the IL-carbohydrates interactions. Therefore, we have expanded our previous published work as the literature survey and behavior of these ionic liquids have propelled us to carry out our investigation in this direction by studying the effect of different additives on their micellization behavior. So, in our present investigation, the aggregation study of the ionic liquid has been carried out in the presence of amino acids.
To explore the role of Ionic Liquid–amino acids mixtures in potential industrial applications, the study has been carried out involving the ionic liquid, 1-pentadecyl-3-methylimidazolium bromide [C15mim][Br], and three amino acids, glycine, l-alanine and l-valine (Scheme 1) at different concentrations and temperatures. Amino acids find use in a wide range of applications across many disciplines; nevertheless, the majority of their consumption occurs in the food industry. In addition to this, they are also useful as supplements in animal diets. The human nutrition industry makes use of technology that is analogous to that which is used for animal nutrition in order to treat the symptoms of mineral deficiencies, such as anemia [47, 48]. Combinations of amino acids and ILs may have a wide range of potential uses in the aforementioned fields. The techniques which are employed to study the present system are electrical conductivity, UV–Visible, Refractive index, and FT-IR spectroscopy. The ionic liquid [C15mim][Br] has been chosen based on its long hydrophobic alkyl chain along with its good water solubility. Since it is observed that with a decrease in the hydrophobic alkyl chain length, the CMC value of ionic liquid increases, due to which the amount of ionic liquid needed to form micelles also increases which ultimately poses a greater risk of toxicity. However, with increased alkyl chain length solubility issue arises. Therefore, a 15-carbon-atoms-long chain is used which forms micelles at lower micellar concentrations with good water solubility. Moreover, most of the micellization studies have been reported based on even-numbered alkyl chain ionic liquids and very less literature is available for odd-numbered alkyl chain ionic liquids. Further, the three amino acids have been selected keeping into consideration their commercial and biochemical importance and also their reproducible character of charged biomolecules. Also, these three amino acids differ in hydrophobicity, so chiefly the ionic liquid-amino acid hydrophobic interactions are investigated.
2 Experimental
2.1 Materials
All experiments have been accomplished with the use of double-distilled water having conductivity = ~ 1.0 × 10−6 S·cm−1, prepared from double distilling the water from alkaline KMnO4 to remove organic matter. The amino acids used in the experiment, i.e., glycine (mass fraction purity > 98%), l-alanine (mass fraction purity ≥ 99%), and l-valine (mass fraction purity > 99%) have been purchased from HIMEDIA Laboratories Pvt. Ltd, Mumbai, S D Fine Chem Ltd. India, and Merck, Germany, respectively and were used as received. 1-methylimidazole (mass fraction purity > 99%) and 1-bromo pentadecane (mass fraction purity > 97%) used in the synthesis of ionic liquid, 1-pentadecyl-3-methylimidazolium bromide have been procured from HIMEDIA Laboratories Pvt. Ltd, Mumbai and TCI Co. Ltd., Tokyo, Japan, respectively. The acetonitrile (mass fraction purity > 99.5%) solvent used in the experiment has been bought from LOBA Chemie Pvt. Ltd, Mumbai, and Hexane (mass fraction purity > 96%) from TCI Co. Ltd., Tokyo, Japan. A summary of the provenance and purity of chemicals used has also been provided in Table S1. All these chemicals have been vacuum dried in a vacuum desiccator for about 2 days to remove any moisture content prior to their use in an experiment.
2.2 Synthesis of [C15mim][Br]
The IL, 1-pentadecyl-3-methyl imidazolium bromide [C15mim][Br] has been synthesized according to the previously reported procedures [45]. Briefly, 1-methylimidazole and an excess molar amount of 1-bromopentadecane were mixed in acetonitrile solvent in a 500 ml round bottom flask, and then, the mixture was refluxed with continuous stirring at about 80 °C for at least 48 h. With the use of the thin-layer chromatography technique (TLC), the number of components present in the mixture was analyzed. After product formation, the resulting mixture was removed from refluxing and cooled down to room temperature (R.T.). Then, using the rota-evaporator, the acetonitrile solvent was removed. The end product was given washing with hexane 2 to 3 times using a separating funnel to eliminate any residual impurities and lastly, the purified product was vacuum dried in a vacuum desiccator for 18 h before use. The characterization of synthesized ionic liquid [C15mim][Br] was carried out with the help of 1H-NMR and FT-IR spectroscopy, which are given as Figs. S1–S2, respectively, in the supplementary data.
2.3 Instruments and Methods
Double distilled deionized water having a conductivity of ~ 1.0 × 10−6 S·cm−1, prepared from alkaline KMnO4 has been used in the preparation of stock solutions of ionic liquid as well as amino acids. The weighing balance Sartorius CPA 225 D with ± 0.00001 g precision has been utilized to accurately weigh the required amounts of chemicals used in the experiment and finally, the prepared solutions were homogenized using the magnetic stirrer. The pH of the amino acid solutions has been maintained at pH 7 using phosphate buffer, therefore they are expected to be present in their zwitter ionic forms. The change in pH may result in the ionization/protonation of their characteristic functional groups –COOH and –NH2 leading to different equilibrium forms of amino acids. Thus, the pH of amino acid solutions has been maintained equal to 7.
2.3.1 Conductivity Measurements
The conductivity measurements for the present system i.e., ionic liquid, 1-pentadecyl-3-methylimidazolium bromide [C15mim][Br] have been performed in the absence and the presence of different concentrations (0.05, 0.25, and 0.50) mol·dm−3 of three amino acids, glycine, l-alanine, and l-valine in a water-jacketed flow dilution cell in a range of temperatures (298.15 to 318.15) K with an interval of 10 K. For the measurements, a digital conductivity meter (Systronics 308) has been employed with a dip-type cell of unit cell constant. The calibration of the conductivity meter was done using 0.01 geq·dm−3 aqueous KCl solution in which the conductance value of 1413 µS·cm−1was noted at 298.15 K, afore the measurements. The various temperatures i.e., 298.15 K, 308.15 K, and 318.15 K at which conductivity measurements have been performed were kept constant by circulating the water around the solution under investigation through the jacket cell surrounding it. This is carried out with the aid of a refrigerated circulated water thermostat which is having an accuracy of ± 0.1 °C for a temperature range of 0–100 °C and is purchased from Macro Scientific Works Pvt. Ltd. Delhi. After the calibration has been performed and the constant temperature is maintained, the conductivity of the amino acid solution in the cell is measured first, and afterward, measuring the conductivity by stepwise adding 200 μL portions of the stock solution (concentration 15–20 times the CMC) of aqueous solution of IL [C15mim][Br] into the cell employing a micropipette (10–100 μL). At least three measurements were performed for each concentration and only the mean values have been taken into consideration with an uncertainty of ± 0.002 µS·cm−1.
2.3.2 UV–Vis Spectroscopic Studies
UV–Vis spectroscopic studies have been employed to determine the CMC of 1-pentadecyl-3-methylimidazolium bromide [C15mim][Br] in the absence and presence of aqueous solutions of amino acids, glycine, l-alanine, and l-valine at 298.15 K temperature. This is achieved with the help of the UV–Vis spectrophotometer of Agilent Technologies. The quartz cuvette of a 1 cm path length has been used to obtain the UV absorption plots and the measurements were performed at a fixed wavelength. The UV–Vis spectroscopic measurements are carried out without any external probe since the imidazolium ring is present in the ionic liquid. 1-pentadecyl-3-methylimidazolium bromide is found to absorb in the entire UV region of 200–800 nm [49, 50]. The obtained spectra corresponding to absorbance versus the concentration of IL [C15mim][Br] for the investigated system produced two straight lines with different slopes which intersect at a point and that point signifies the CMC value of the ionic liquid.
2.3.3 Refractive Index Measurements
An Abbemat 300 refractometer by Anton Paar has been employed for the determination of the refractive indices of the mixtures of ionic liquid and amino acids under investigation, which has a resolution of 0.00001 nD. The instrument has an inbuilt temperature regulator which maintains a particular temperature with an accuracy of ± 0.05 °C. The calibration of the instrument is carried out by recording the refractive index value of distilled water (1.3325 nD). Very little quantity of the sample is required for the measurements and the value of the refractive index is displayed within seconds. Before each measurement, the prism is cleaned with acetone and the reading is taken at least 3 times, and then the mean value is finally reported.
2.3.4 FT-IR Spectroscopic Studies
Fourier Transform-Infrared (FT-IR) spectra for the system composed of ionic liquid [C15mim][Br] in the absence as well as in the presence of aqueous solutions of different concentrations i.e., (0.05, 0.25, and 0.50) mol·dm−3 of three amino acids; glycine, l-alanine, and l-valine at 298.15 K have been obtained. These measurements are advantageous to study the various interactions prevailing among the ionic liquid and the three amino acids. Therefore, the FT-IR studies have been accomplished with the Shimadzu spectrometer 8400S operated in the accessible wavenumber region of 4000 to 400 cm−1.
3 Results and Discussion
The micellization properties of the aqueous solution of imidazolium-based ionic liquid [C15mim][Br] in the absence and the presence of amino acids have been studied employing conductivity, UV–Visible, refractive index, and FT-IR spectroscopic measurements. The details of the results and discussion acquired from each of the techniques are presented in the following subsections.
3.1 Conductivity Measurements
The experimental conductivity values for the aqueous solution of ionic liquid [C15mim][Br] and the ternary ([C15mim][Br] + water + amino acids) solutions at different concentrations and temperatures are measured and have been reported in Tables S2–S4. The critical micelle concentration (CMC) of [C15mim][Br] from the conductivity measurements has been evaluated by determining the change in the specific conductivity (κ) for the aqueous solutions of [C15mim][Br] in the absence as well as in the presence of different molar concentrations (i.e., 0.05, 0.25, and 0.50) mol·dm−3 of amino acids; glycine, l-alanine, and l-valine at different temperatures (i.e., 298.15 K, 308.15 K, and 318.15 K) as a function of the ionic liquid [C15mim][Br] concentration. The conductivity measurements have been performed by following the titration method which is carried out by adding fixed aliquots of concentrated stock solution of the ionic liquid into the fixed amount of water and the aqueous solution of different concentrations of the chosen three amino acids taken in a cell. Figures 1 and 2 plot the electrical conductivity versus concentration plot of ionic liquid [C15mim][Br] in the aqueous medium and in the presence of various concentrations of the amino acid glycine at different temperatures, respectively, while in the presence of l-alanine, and l-valine have been represented as Figs. S3–S4, respectively, in the supplementary data.
The experimentally obtained values of specific conductivity fit into two straight lines having dissimilar slopes and the location where the slope changes mark the value of CMC. Prior to reaching the CMC value, the measured conductivity primarily corresponds to the movement of free ions [C15mim]+ and [Br]− which is larger in value. As the CMC value is reached, this rate of increase of specific conductivity is lowered since the formed micelles possessing lesser mobility contribute less to the charge transport in comparison to the free ions, and also binding of some of the counter-ions to the micellar surface takes place which results in the effective loss of ionic charge [51,52,53]. The extent of counter-ions dissociating is specified by the degree of counter-ion dissociation (α) and is evaluated from the ratio of the slopes above and below the CMC from the specific conductivity vs ionic liquid concentration plot [54]. On the other hand, the degree of counter-ion binding (β) can be evaluated as β = 1 − α. The values of CMC and α for the investigated ionic liquid [C15mim][Br] in the aqueous and in the presence of three amino acids, glycine, l-alanine, and l-valine at various concentrations and temperatures that are obtained from conductivity measurements have been listed in Table 1. The obtained CMC value of the ionic liquid [C15mim][Br] in the aqueous media is 1.448 mmol·dm−3, 1.591 mmol·dm−3 and 1.710 mmol·dm−3 which is approximately parallel with the literature values [55] at 298.15 K, 308.15 K, and 318.15 K, respectively, determined by conductivity measurements.
3.1.1 Variation in CMC of IL with Change in Concentration and Nature of Amino Acid
The various interactions that are taking place between these amino acids and an aqueous solution of [C15mim][Br] can be categorized into four types; Type-1 interactions are the ion-ion interactions existing among ions of the amino acids (-NH3+ and –COO−), and the ionic liquid ([C15mim]+ and [Br]−). Type-2 interactions are the ion–hydrophobe interactions that involve the ions of amino acids and the hydrophobic chain of ionic liquid i.e., C15 chain and the methyl group. Type-3 interactions comprise hydrophobe–hydrophobe interactions involving the hydrophobic parts of the amino acids and the ionic liquid, while the Type-4 interactions are the hydrophile–hydrophobe interactions that are present among the hydrophilic and hydrophobic parts of amino acid and ionic liquid, respectively [56]. Some other interactions which are also prevailing in the system include ion–dipole and dipole–dipole interactions between the ionic parts of amino acids and ionic liquid, and hydrogen bonding interactions between the counter-ion of ionic liquid and the amino acid. It can be seen from Table 1, that at any given temperature the values of CMC of [C15mim][Br] in the presence of the three investigated amino acids are lesser than that in pure water, and also with an increase in the concentration of amino acid, the CMC values decrease which specifies that the amino acids enhance the micellization of the ionic liquid. This can be explained through reasons that (a) the electrostatic repulsions among the ionic liquid head groups are reduced due to attractive interactions between the cation [C15mim]+ of the IL and the amino acids which thereby enhances the micelle formation tendency of IL; (b) also the interactions among the Br− anion and the amino acids leads to increased solvation radius of the Br− anion, which is ultimately followed by an increase in polarizability [57]. This raised polarizability assists in increasing the binding of the anions at the micellar surface which also results in reducing the electrostatic repulsive interactions among the ionic liquid head groups. Therefore, these factors are responsible for the increased tendency of ionic liquid micellization [58], resulting in the reduction of the CMC of the IL [C15mim][Br] in the presence of amino acids. The hydrophobic interactions are known to favor the micellization process [3, 59, 60]. Some amino acids are more hydrophobic than others, and this property can be measured using “hydrophobicity scales”, [61,62,63,64,65,66] which rank the amino acids from “most hydrophilic” to “most hydrophobic”. For the studied amino acids, the hydrophobicity scale predicted by Bianco-Peled et al. [67] according to the Black and Mould scale was in the order: glycine (0.501) < l-alanine (0.616) < l-valine (0.825). Also, the number of methyl groups (or hydrophobic moiety) in the case of amino acids increases in the order: glycine < l-alanine < l-valine. Hence, the corresponding order of hydrophobic character of these amino acids is also identical. The outcome of this increased hydrophobicity of amino acids is that it reduces the requirement of ionic liquid molecules for the micelle formation as can be seen from the CMC values of ionic liquid [C15mim][Br] presented in Table 1 and the order of decreasing values of CMC of [C15mim][Br] in the presence of the investigated amino acids is the same i.e., glycine < l-alanine < l-valine as that of hydrophobic character of these amino acids. Also, from Table 1, we can observe that with the increase in the concentration of amino acids, the value of CMC of IL is decreasing. The zwitter ionic nature of these amino acids may result in their interactions with the surrounding water molecules which thereby leads to the dehydration of the hydrophilic head group of the ionic liquid micelles [59, 60]. Thus ultimately, favors the micelle formation of ionic liquid molecules at lower CMC values in the presence of amino acids.
3.1.2 Variation in CMC and α of IL upon the Change in Temperature
The value of CMC of ionic liquid [C15mim][Br] is found to increase with an increase in temperature in the aqueous as well as in the presence of all three investigated amino acids. The temperature variation has a complex influence on the CMC values of the ionic liquid surfactants [68]. The value of CMC may have two opposite trends upon temperature change: [38, 39, 59] (a) reduction in the value of CMC is observed on increasing the temperature which takes place as a result of increased dehydration of the hydrophilic layer around the ionic liquid monomer due to rise in temperature which results in inclined proximity among ionic liquid monomers and thereby, favoring the micellization process, (b) rise in the CMC value due to increased disruption of the structured layer of water around the hydrophobic group which thus leads to increase in value of CMC and accordingly, hindering the micellization process. From Table 1, it shows that the value of CMC of ionic liquid [C15mim][Br] is increasing upon an increase in temperature in aqueous as well as in the presence of different concentrations (0.05, 0.25, and 0.50) mol·dm−3 of the amino acids i.e., glycine, l-alanine and l-valine and hence, it is observed that in the temperature range studied for the present system, the second effect dominates. Also, the values of α of the IL, [C15mim][Br] presented in Table 1 are witnessed to increase with an upsurge in temperature in aqueous as well as in the presence of amino acids which can be explained by the fact that boost in thermal energy due to escalated temperature, leading to the dissociation of the counter ion Br− from the micellar surface and hence, this increase in the value of α is discernible. The variation of CMC of the IL, [C15mim][Br] with temperatures (298.15 K, 308.15 K, and 318.15 K) and concentrations (0.05, 0.25, and 0.50) mol·dm−3 of amino acids, glycine, l-alanine, and l-valine has been depicted by 2-D bar plots in Fig. 3.
3.1.3 Thermodynamic Parameters of Micellization
Additionally, thermodynamic parameters of micellization namely, Standard Gibb’s free energy (\(\Delta G_{{\text{m}}}^{0}\)), Standard enthalpy (\(\Delta H_{{\text{m}}}^{0}\)) and standard entropy (\(\Delta S_{{\text{m}}}^{0}\)) of micellization have been obtained from conductivity measurements. The values of critical micelle concentration determined at different concentrations and temperatures from conductivity data have been utilized in the calculation of thermodynamic parameters of micellization using equations (S1–S3) provided in supplementary information.
All the calculated thermodynamic parameters of micellization of the ionic liquid [C15mim][Br] in the aqueous and in the presence of different concentrations of the three amino acids at the studied temperatures are disclosed in Table 1. From Table 1, we can observe that the values of Standard Gibb’s free energy (\(\Delta G_{{\text{m}}}^{0}\)) and Standard enthalpy (\(\Delta H_{{\text{m}}}^{0}\)) of micellization for the investigated system are negative, which directs to the fact that the micelle formation is taking place spontaneously and is exothermic in nature. With an increase in temperature, the values get more negative, which can be explained by the fact that increasing temperature results in dehydration of the [C15mim][Br] monomers, hence, making the micellization process more favorable. Also, with the addition of amino acids, a comparatively more spontaneous micellization phenomenon was observed than that in aqueous. The obtained values of Standard entropy (\(\Delta S_{{\text{m}}}^{0}\)) of micellization are positive which indicates that the micellization process is entropy-driven. The micellization phenomenon is controlled by the entropy-enthalpy compensation effect which explains the trend obtained for the values of Standard entropy (\(\Delta S_{{\text{m}}}^{0}\)) of micellization. The values of \(\Delta S_{{\text{m}}}^{0}\) are found to decrease with an increase in temperature indicating that the process of micellization becomes enthalpy driven at higher temperatures and therefore, compensates for the contribution from entropy and enthalpy. The variation of these parameters with the change in temperature for the [C15mim][Br] in the aqueous and in the presence of different concentrations of amino acids has been presented in Figs. S5–S6. The values of CMC, alpha, and thermodynamic parameters obtained from the present study have also been compared with similar ILs and cationic surfactants (similar chain length like tetradecyl trimethyl ammonium bromide, TTAB and hexadecyl trimethyl ammonium bromide, HTAB) in presence of the studied amino acids in Table 2.
3.2 UV–Vis Spectroscopic Studies
Determination of the CMC value of the IL [C15mim][Br] in the presence of amino acids, namely glycine, l-alanine, and l-valine in the aqueous medium has been executed using the UV–Visible spectroscopy technique. The UV spectrum technique can be utilized to explore the self-organization mechanism of imidazolium-based IL in solutions since electronic transitions are sensitive to the imidazolium ring interaction. The π → π* type is the lowest-lying transition found in aromatic imidazolium rings. The absorbance measurements have been carried out for the system comprising of IL, [C15mim][Br] in the aqueous and in the presence of different concentrations (0.05, 0.25, and 0.50) mol·dm−3 of amino acids; glycine, l-alanine, and l-valine at 298.15 K temperature in the wavelength region of 200–800 nm. Therefore, the influence of variation in concentrations of amino acids on the CMC value of the ionic liquid has been examined. In the present investigation, the CMC determination using UV–visible spectroscopy does not require any external probe for the absorption of light because the ionic liquid employed contains the imidazolium ring which has the property to absorb in the entire UV region [50, 79,80,81]. The critical micelle concentration of the ionic liquid is obtained from the plots of absorbance versus the sample concentration [82], and the graphical plot for the ionic liquid [C15mim][Br] in the aqueous and the presence of (0.05, 0.25 and 0.50) mol·dm−3 glycine are presented in Fig. 4, while similarly obtained plots for different concentrations of l-alanine, and l-valine observed at wavelength λmax = 238 nm are presented in Figs. S7–S8, respectively. From these Figs., it is observed that the absorbance of the solution increases upon an increase in the concentration of the ionic liquid in the aqueous as well as in the presence of different concentrations of the three amino acids, and two straight lines are obtained with different slopes which intersect at some point, corresponding to the value of CMC of ionic liquid. The absorbance values of [C15mim][Br] solutions in the absence and in the presence of (0.05,0.25 and 0.50) mol·dm−3 glycine, l-alanine, and l-valine in the aqueous media have been reported in Table S5. The values of CMC obtained by UV–vis spectroscopy are compared with those obtained from conductivity measurements and are found to be in good agreement with each other as can be seen from the values listed in Table 3.
3.3 Refractive Index Measurements
In the determination of the critical micelle concentration (CMC) value of surfactants, the refractive index measurement is a simple and rapid method. The refractive index (η) of the solution is plotted against the concentration of the ionic liquid, where the breakpoint in the two straight lines obtained indicates the CMC value of the ionic liquid. The plots achieved from the refractive index measurements for the system of ionic liquid [C15mim][Br] in the absence and in the presence of 0.25 mol·dm−3 concentration of amino acid, glycine, have been presented in Fig. 5, while similarly obtained plots for different concentrations of glycine, l-alanine, and l-valine) have been presented in Figs. S9–S11, respectively. Also, CMC determination is not possible at a lower concentration of amino acid (i.e., 0.05 mol·dm−3) as no significant variation in the values of refractive index was observed (Table 3 where * denotes the non-significant variation in refractive index values). Molecular refraction and the refractive index are found to be based on the electronic polarization of constituent atoms and bonds [83, 84]. As a consequence, the variation of the refractive index observed for the investigated system shows that changes occur in electron distribution surrounding the ionic liquid which accompanies hydrogen bonding, ionic, van der Waals, and solvophobic interactions, and also restructuring of solvent molecules [85]. The values of critical micelle concentration obtained by refractive index measurements are in accordance with the results obtained by conductivity and UV–Vis measurements as can be noted from Table 3. The obtained values of the refractive index for all the investigated systems are reported in Tables S6–S9.
3.4 FT-IR Spectroscopic Studies
FT-IR spectroscopy technique has proven to be a remarkable tool for analyzing the various interactions occurring in the mixtures at a molecular scale. The mixtures composed of ionic liquid and amino acid additives are also found to show various structural alterations upon micelle formation. Thus, in the present work, to gain further insight into the interactions between the ionic liquid [C15mim][Br] and the three amino acids namely, glycine, l-alanine, l-valine in the aqueous media having different concentrations (0.05, 0.25, and 0.50) mol·dm−3, the system was investigated by FT-IR spectroscopy at 298.15 K in the wavenumber range 4000–250 cm−1. FT-IR spectra have been obtained for the before-mentioned system at below CMC, at CMC, and above CMC concentrations of the ionic liquid. The obtained spectra are found to display two major peaks, the one peak appearing in the wavenumber region of 1600–1690 cm−1, while the other major peak observed is the one appearing in the 3200–3300 cm−1. The peak appearing in the former wavenumber region may be due to N–H bending and C = C stretching, while in the latter wavenumber region, the peak may be due to N–H stretching. The various FT-IR spectra obtained for the system of [C15mim][Br] in the aqueous and the different concentrations (0.05, 0.25, and 0.50) mol·dm−3 of amino acid, glycine, l-alanine, l-valine are presented in Figs. S12–S15, respectively. From these figures it can be noticed that there is not much variation in the peak appearing in 1600–1690 cm−1, which can be explained by the greater bond strength of the C = C bond, while there is a significant shift observed for the peak appearing due to N–H stretching in 3200–3300 cm−1 region, which can be the result of breaking or making of the hydrogen bond, present between the hydrogen atom of imidazole ring and the anion. Therefore, we have enlisted the wavenumber values of the peaks appearing in the range 3200–3300 cm−1 in Table S10, where a significant shift in wavenumber is observed. Thus, FT-IR spectroscopy supports the fact that upon micellization, some interactions prevail in the mixtures of the studied ionic liquid and the amino acids.
4 Conclusion
In this work, micellization study carried out at different temperatures for different concentrations (0.05, 0.25, and 0.50) mol·dm−3 of amino acids (glycine, l-alanine, and l-valine) + ionic liquid ([C15mim][Br]) + water systems has been reported. The values of CMCs obtained using different techniques complement each other well. The critical micelle concentration of the ionic liquid [C15mim][Br] increases as temperature increases, and on the other side, the opposite trend for CMC is observed as the hydrophobic character of amino acid increases. Furthermore, the CMC of the IL decreases in the presence of amino acids additives in comparison to that in the absence and the ability of these amino acids to reduce the CMC of [C15mim][Br] in an aqueous solution follows the sequence: l-valine > l-alanine > glycine which is identical as the hydrophobic character of amino acids. The large negative values obtained for the standard enthalpy of micellization \(\Delta H_{{\text{m}}}^{0}\) indicate the exothermic nature of the micellization process. The standard entropy of micellization \(\Delta S_{{\text{m}}}^{0}\) values show that the standard state micellization process goes from entropy to enthalpy driven with increasing temperature and also, with increasing concentration of amino acid in the solution. This research was supposed to shed light on the interactions between ILs and amino acids, since the investigation of interactions between ILs and biomolecules is of great prominence in the study of the effects of ILs on animal and human health.
References
Horbett, T.A., Brash, J.L.: Proteins at interfaces: an overview. J. Dispers. Sci. Technol. 18, 557–557 (1997). https://doi.org/10.1080/01932699708943757
Chauhan, M.S., Kumari, N., Pathania, S., Sharma, K., Kumar, G.: A conductometric study of interactions between gelatin and sodium dodecyl sulfate (SDS) in aqueous-rich mixtures of dimethylsulfoxide. Colloids Surf. A Physicochem. Eng. Asp. 293, 157–161 (2007). https://doi.org/10.1016/j.colsurfa.2006.07.020
Sharma, K., Chauhan, S.: Effect of biologically active amino acids on the surface activity and micellar properties of industrially important ionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 453, 78–85 (2014). https://doi.org/10.1016/j.colsurfa.2014.04.003
Cid, A., Morales, J., Mejuto, J.C., Briz-Cid, N., Rial-Otero, R., Simal-Gándara, J.: Thermodynamics of sodium dodecyl sulphate-salicylic acid based micellar systems and their potential use in fruits postharvest. Food Chem. 151, 358–363 (2014). https://doi.org/10.1016/j.foodchem.2013.11.076
Cid, A., Mejuto, J.C., Orellana, P.G., López-Fernández, O., Rial-Otero, R., Simal-Gandara, J.: Effects of ascorbic acid on the microstructure and properties of SDS micellar aggregates for potential food applications. Food Res. Int. 50, 143–148 (2013). https://doi.org/10.1016/j.foodres.2012.10.009
Mittal, H., Morajkar, P.P., Al Alili, A., Alhassan, S.M.: In-situ synthesis of ZnO Nanoparticles using gum Arabic based hydrogels as a self-template for effective malachite green dye adsorption. J. Polym. Environ. 28, 1637–1653 (2020). https://doi.org/10.1007/s10924-020-01713-y
Mittal, H., Al Alili, A., Alhassan, S.M.: High efficiency removal of methylene blue dye using κ-carrageenan-poly(acrylamide-co-methacrylic acid)/AQSOA-Z05 zeolite hydrogel composites. Cellulose 27, 8269–8285 (2020). https://doi.org/10.1007/s10570-020-03365-6
Bhardwaj, T., Bhardwaj, V., Sharma, K., Gupta, A., Cameotra, S.S., Sharma, P.: Thermo-acoustical analysis of sodium dodecyl sulfate: Fluconazole (antifungal drug) based micellar system in hydro-ethanol solutions for potential drug topical application. J. Chem. Thermodyn. 78, 1–6 (2014). https://doi.org/10.1016/j.jct.2014.06.003
Madaeni, S.S., Rostami, E.: Spectroscopic investigation of the interaction of BSA with cationic surfactants. Chem. Eng. Technol. 31, 1265–1271 (2008). https://doi.org/10.1002/ceat.200700496
Bhardwaj, V., Bhardwaj, T., Sharma, K., Gupta, A., Chauhan, S., Cameotra, S.S., Sharma, S., Gupta, R., Sharma, P.: Drug-surfactant interaction: thermo-acoustic investigation of sodium dodecyl sulfate and antimicrobial drug (levofloxacin) for potential pharmaceutical application. RSC Adv. 4, 24935–24943 (2014). https://doi.org/10.1039/c4ra02177k
Mittal, H., Al Alili, A., Alhassan, S.M.: Solid polymer desiccants based on poly(acrylic acid-co-acrylamide) and Laponite RD: adsorption isotherm and kinetics studies. Colloids Surf. A Physicochem. Eng. Asp. 599, 124813 (2020). https://doi.org/10.1016/j.colsurfa.2020.124813
Gunnarsson, G., Jönsson, B., Wennerström, H.: Surfactant association into micelles. An Electrost. Approach. J. Phys. Chem. 84, 3114–3121 (1980). https://doi.org/10.1021/j100460a029
Kudryashov, E., Kapustina, T., Morrissey, S., Buckin, V., Dawson, K.: The compressibility of alkyltrimethylammonium bromide micelles. J. Colloid Interface Sci. 68, 59–68 (1998)
Abdelhamid, H.N.: Organic matrices, ionic liquids, and organic matrices@nanoparticles assisted laser desorption/ionization mass spectrometry. TrAC Trends Anal. Chem. 89, 68–98 (2017). https://doi.org/10.1016/j.trac.2017.01.012
Ventura, S.P.M., Gonçalves, A.M.M., Sintra, T., Pereira, J.L., Gonçalves, F., Coutinho, J.A.P.: Designing ionic liquids: The chemical structure role in the toxicity. Ecotoxicology 22, 1–12 (2013). https://doi.org/10.1007/s10646-012-0997-x
Abdelhamid, H.N.: Ionic liquids for mass spectrometry: matrices, separation and microextraction. TrAC Trends Anal. Chem. 77, 122–138 (2016). https://doi.org/10.1016/j.trac.2015.12.007
Bhaisare, M.L., Abdelhamid, H.N., Wu, B.S., Wu, H.F.: Rapid and direct MALDI-MS identification of pathogenic bacteria from blood using ionic liquid-modified magnetic nanoparticles (Fe3O4@SiO2). J. Mater. Chem. B. 2, 4671–4683 (2014). https://doi.org/10.1039/c4tb00528g
Marsh, K.N., Boxall, J.A., Lichtenthaler, R.: Room temperature ionic liquids and their mixtures—a review. Fluid Phase Equilibria 219, 93–98 (2004). https://doi.org/10.1016/j.fluid.2004.02.003
Sheldon, R.: Catalytic reactions in ionic liquids. Chem. Commun. 1, 2399–2407 (2001). https://doi.org/10.1039/b107270f
Abdelhamid, H.N.: Ionic liquids for nanomaterials recycling. Nanomater. Recycl. (2022). https://doi.org/10.1016/B978-0-323-90982-2.00024-X
Miskolczy, Z., Sebök-Nagy, K., Biczók, L., Göktürk, S.: Aggregation and micelle formation of ionic liquids in aqueous solution. Chem. Phys. Lett. 400, 296–300 (2004). https://doi.org/10.1016/j.cplett.2004.10.127
Geng, F., Liu, J., Zheng, L., Yu, L., Li, Z., Li, G., Tung, C.: Micelle formation of long-chain imidazolium ionic liquids in aqueous solution measured by isothermal titration microcalorimetry. J. Chem. Eng. Data. 55, 147–151 (2010). https://doi.org/10.1021/je900290w
Galgano, P.D., El Seoud, O.A.: Micellar properties of surface active ionic liquids: a comparison of 1-hexadecyl-3-methylimidazolium chloride with structurally related cationic surfactants. J. Colloid Interface Sci. 345, 1–11 (2010). https://doi.org/10.1016/j.jcis.2010.01.078
Mittal, H., Al Alili, A., Morajkar, P.P., Alhassan, S.M.: GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose —a versatile adsorbent for the treatment of dyes contaminated wastewater. Int. J. Biol. Macromol. 167, 1248–1261 (2021). https://doi.org/10.1016/j.ijbiomac.2020.11.079
Mittal, H., Al Alili, A., Alhassan, S.M.: Adsorption isotherm and kinetics of water vapors on novel superporous hydrogel composites. Microporous Mesoporous Mater. 299, 110106 (2020). https://doi.org/10.1016/j.micromeso.2020.110106
Zhao, Y., Chen, X., Jing, B., Wang, X., Ma, F.: Novel gel phase formed by mixing a cationic surfactive ionic liquid C 16mimCl and an anionic surfactant SDS in aqueous solution. J. Phys. Chem. B. 113, 983–988 (2009). https://doi.org/10.1021/jp809048u
Singh, W.P., Koch, U., Singh, R.S.: Gelation of ionic liquids by small-molecule gelators and their applications. Soft Mater. 18, 386–410 (2020). https://doi.org/10.1080/1539445X.2019.1697706
Zhang, J., Shen, X.: Temperature-induced reversible transition between vesicle and supramolecular hydrogel in the aqueous ionic liquid-β-cyclodextrin system. J. Phys. Chem. B. 117, 1451–1457 (2013). https://doi.org/10.1021/jp308877w
Yuan, J., Bai, X., Zhao, M., Zheng, L.: C12mim Br ionic liquid/SDS vesicle formation and use as template for the synthesis of hollow silica spheres. Langmuir 26, 11726–11731 (2010). https://doi.org/10.1021/la101221z
Gayet, F., Marty, J.D., Brûlet, A., Viguerie, N.L.D.: Vesicles in ionic liquids. Langmuir 27, 9706–9710 (2011). https://doi.org/10.1021/la2015989
Rao, K.S., Kumar, A.: Vesicles and reverse vesicles of an ionic liquid in ionic liquids. Chem. Commun. 49, 8111–8113 (2013). https://doi.org/10.1039/b000000x
Zhao, Y., Chen, X., Wang, X.: Liquid crystalline phases self-organized from a surfactant-like ionic liquid C16mimCl in ethylammonium nitrate. J. Phys. Chem. B. 113, 2024–2030 (2009). https://doi.org/10.1021/jp810613c
Zhang, G., Chen, X., Xie, Y., Zhao, Y., Qiu, H.: Lyotropic liquid crystalline phases in a ternary system of 1-hexadecyl-3-methylimidazolium chloride/1-decanol/water. J. Colloid Interface Sci. 315, 601–606 (2007). https://doi.org/10.1016/j.jcis.2007.07.012
Song, Z., Xin, X., Shen, J., Jiao, J., Xia, C., Wang, S., Yang, Y.: Manipulation of lyotropic liquid crystal behavior of ionic liquid-type imidazolium surfactant by amino acids. Colloids Surf. A Physicochem. Eng. Asp. 518, 7–14 (2017). https://doi.org/10.1016/j.colsurfa.2017.01.004
Mittal, H., Al Alili, A., Morajkar, P.P., Alhassan, S.M.: Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J. Mol. Liq. 323, 115034 (2021). https://doi.org/10.1016/j.molliq.2020.115034
Dixit, S.B., Bhasin, R., Rajasekaran, E., Jayaram, B.: Solvation thermodynamics of amino acids assessment of the electrostatic contribution and force-field dependence. J. Chem. Soc. Faraday Trans. 93, 1105–1113 (1997). https://doi.org/10.1039/a603913h
Nelson, D.L., Cox, M.C., Freeman, W.H.: Lehninger: Principles of Biochemistry. Worth Publishers, USA (2004)
Kumar, H., Kaur, G.: Effect of changing alkyl chain in imidazolium based ionic liquid on the micellization behavior of anionic surfactant sodium hexadecyl sulfate in aqueous media. J. Dispers. Sci. Technol. (2020). https://doi.org/10.1080/01932691.2020.1724796
Ebrahimi, M., Moosavi, F.: The effects of temperature, alkyl chain length, and anion type on thermophysical properties of the imidazolium based amino acid ionic liquids. J. Mol. Liq. 250, 121–130 (2018). https://doi.org/10.1016/j.molliq.2017.11.122
Hu, Y., Han, J., Guo, R.: Influence of the alkyl chain length of the imidazole ionic liquid-type surfactants on their aggregation behavior with sodium dodecyl sulfate. Langmuir 36, 10494–10503 (2020). https://doi.org/10.1021/acs.langmuir.0c01673
Mozrzymas, A.: On the head group effect on critical micelle concentration of cationic surfactants using molecular connectivity indices and atomic partial charges. J. Solution Chem. 48, 875–890 (2019). https://doi.org/10.1007/s10953-019-00887-x
Buckingham, S.A., Garvey, C.J., Warr, G.G.: Effect of head-group size on micellization and phase behavior in quaternary ammonium surfactant systems. J. Phys. Chem. 97, 10236–10244 (1993). https://doi.org/10.1021/j100141a054
Long, P., Chen, J., Wang, D., Hu, Z., Gao, X., Li, Z., Hao, J.: Influence of counterions on micellization of tetramethylammonium perfluorononanoic carboxylate in 1-butyl-3-methylimidazolium ionic liquid. J. Phys. Chem. B. 116, 7669–7675 (2012). https://doi.org/10.1021/jp300733x
Naskar, B., Dey, A., Moulik, S.P.: Counter-ion effect on micellization of ionic surfactants: a comprehensive understanding with two representatives, sodium dodecyl sulfate (SDS) and dodecyltrimethylammonium bromide (DTAB). J. Surfact. Deterg. 16, 785–794 (2013). https://doi.org/10.1007/s11743-013-1449-1
Kumar, H., Kaur, R.: Exploration of the soluting-out effect of carbohydrates on the micellization and surface activity of long-chain imidazolium ionic liquid in the aqueous medium. J. Mol. Liq. 319, 114209 (2020). https://doi.org/10.1016/j.molliq.2020.114209
Kumar, H., Kaur, R.: Studies on thermodynamics of micellization of imidazolium-based surface-active ionic liquid [C15mim][Br] in aqueous media: effect of D(+)-Xylose and D(+)-Glucose. J. Mol. Liq. 344, 117645 (2021). https://doi.org/10.1016/j.molliq.2021.117645
Arakawa, T., Tsumoto, K., Kita, Y., Chang, B., Ejima, D.: Biotechnology applications of amino acids in protein purification and formulations. Amino Acids 33, 587–605 (2007). https://doi.org/10.1007/S00726-007-0506-3
Chiang, C.-H., Yeh, M.-K.: Contribution of poly(amino acids) to advances in pharmaceutical biotechnology. Curr. Pharm. Biotechnol. 4, 323–330 (2005). https://doi.org/10.2174/1389201033489739
Rather, M.A., Rather, G.M., Pandit, S.A., Bhat, S.A., Bhat, M.A.: Determination of cmc of imidazolium based surface active ionic liquids through probe-less UV–vis spectrophotometry. Talanta 131, 55–58 (2015). https://doi.org/10.1016/j.talanta.2014.07.046
Bhat, M.A., Dutta, C.K., Rather, G.M.: Exploring physicochemical aspects of N-alkylimidazolium based ionic liquids. J. Mol. Liq. 181, 142–151 (2013). https://doi.org/10.1016/j.molliq.2013.02.021
Garcia, M.T., Ribosa, I., Perez, L., Manresa, A., Comelles, F.: Aggregation behavior and antimicrobial activity of ester-functionalized imidazolium- and pyridinium-based ionic liquids in aqueous solution. Langmuir 29, 2536–2545 (2013). https://doi.org/10.1021/la304752e
Aggarwal, R., Singh, S., Hundal, G.: Synthesis, characterization, and evaluation of surface properties of cyclohexanoxycarbonylmethylpyridinium and cyclohexanoxycarbonylmethylimidazolium ionic liquids. Ind. Eng. Chem. Res. 52, 1179–1189 (2013). https://doi.org/10.1021/ie3020473
Vanyúr, R., Biczók, L., Miskolczy, Z.: Micelle formation of 1-alkyl-3-methylimidazolium bromide ionic liquids in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 299, 256–261 (2007). https://doi.org/10.1016/j.colsurfa.2006.11.049
Shanks, P.C., Franses, E.I.: Estimation of micellization parameters of aqueous sodium dodecyl sulfate from conductivity data. J. Phys. Chem. 96, 1794–1805 (1992). https://doi.org/10.1021/j100183a055
Kumar, H., Sharma, P.: Investigations on the micellization behavior and thermodynamic characteristics of synthesized surface active ionic liquids [C14mim] [Br] and [C15mim] [Br] in the presence of oral antidiabetic drug metformin hydrochloride. J. Mol. Liq. 322, 114851 (2021). https://doi.org/10.1016/j.molliq.2020.114851
Ahmad Sajid, T., Jamal, M.A., Saeed, M., Atta-ul-Haq, M., Muneer, M.: Elucidation of molecular interactions between amino acid and imidazolium based ionic liquid in an aqueous system: Volumetric and acoustic studies. J. Mol. Liq. 335, 116513 (2021). https://doi.org/10.1016/j.molliq.2021.116513
Yan, Z., Geng, R., Gu, B., Pan, Q., Wang, J.: Densities, electrical conductances, and spectroscopic properties of glycyl dipeptide+ionic liquid ([C12mim]Br)+water mixtures at different temperatures. Fluid Phase Equilib. 367, 125–134 (2014). https://doi.org/10.1016/j.fluid.2014.01.038
Wang, H., Feng, Q., Wang, J., Zhang, H.: Salt effect on the aggregation behavior of 1-Decyl-3-methylimidazolium bromide in aqueous solutions. J. Phys. Chem. B. 114, 1380–1387 (2010). https://doi.org/10.1021/jp910903s
Ali, A., Ansari, N.H.: Studies on the effect of amino acids/peptide on micellization of SDS at different temperatures. J. Surfact. Deterg. 13, 441–449 (2010). https://doi.org/10.1007/s11743-010-1221-8
Rakshit, A.K., Sharma, B.: The effect of amino acids on the surface and the thermodynamic properties of poly[oxyethylene(10)] lauryl ether in aqueous solution. Colloid Polym. Sci. 281, 45–51 (2003). https://doi.org/10.1007/s00396-002-0743-7
Hopp, T.P., Woods, K.R.: Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 78, 3824–3828 (1981). https://doi.org/10.1073/PNAS.78.6.3824
Kyte, J., Doolittle, R.F.: A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 (1982). https://doi.org/10.1016/0022-2836(82)90515-0
Browne, C.A., Bennett, H.P.J., Solomon, S.: The isolation of peptides by high-performance liquid chromatography using predicted elution positions. Anal. Biochem. 124, 201–208 (1982). https://doi.org/10.1016/0003-2697(82)90238-X
Bull, H.B., Breese, K.: Surface tension of amino acid solutions: a hydrophobicity scale of the amino acid residues. Arch. Biochem. Biophys. 161, 665–670 (1974). https://doi.org/10.1016/0003-9861(74)90352-X
Berggren, K., Wolf, A., Asenjo, J.A., Andrews, B.A., Tjerneld, F.: The surface exposed amino acid residues of monomeric proteins determine the partitioning in aqueous two-phase systems. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1596, 253–268 (2002). https://doi.org/10.1016/S0167-4838(02)00222-4
Black, S.D., Mould, D.R.: Development of hydrophobicity parameters to analyze proteins which bear post- or cotranslational modifications. Anal. Biochem. 193, 72–82 (1991). https://doi.org/10.1016/0003-2697(91)90045-U
Bianco-Peled, H., Gryc, S.: Binding of amino acids to “smart” sorbents: where does hydrophobicity come into play? Langmuir 20, 169–174 (2004). https://doi.org/10.1021/la0357155
Zdziennicka, A., Szymczyk, K., Krawczyk, J., Jańczuk, B.: Critical micelle concentration of some surfactants and thermodynamic parameters of their micellization. Fluid Phase Equilib. (2012). https://doi.org/10.1016/j.fluid.2012.03.018
Ali, A., Malik, N.A., Uzair, S., Ali, M., Ahmad, M.F.: Hexadecyltrimethylammonium bromide micellization in glycine, diglycine, and triglycine aqueous solutions as a function of surfactant concentration and temperatures. Russ. J. Phys. Chem. A. 88, 1053–1061 (2014). https://doi.org/10.1134/S003602441406003X
Adane, D.F.: Surface and thermodynamic studies of micellization of surfactants in binary mixtures of 1,2-ethanediol and 1,2,3-propanetriol with water. Int. J. Phys. Sci. 10, 276–288 (2015). https://doi.org/10.5897/ijps2015.4288
Ali, A., Tasneem, S., Bidhuri, P., Bhushan, V., Malik, N.A.: Critical micelle concentration and self-aggregation of hexadecyltrimethylammonium bromide in aqueous glycine and glycylglycine solutions at different temperatures. Russ. J. Phys. Chem. A. 86, 1923–1929 (2012). https://doi.org/10.1134/S0036024412130031
Alam, M., Robel, M., Rana, S., Abdul, M., Azum, N., Hoque, A., Kabir, S.E.: Aggregation behavior of cetyltrimethylammonium bromide and tetradecyltrimethylammonium bromide in aqueous/urea solution at different temperatures: experimental and theoretical investigation. J. Mol. Liq. 285, 766–777 (2019)
Kumar, H., Kaur, J., Awasthi, P.: Scrutinizing the micellization behaviour of 14-2-14 gemini surfactant and tetradecyltrimethylammonium bromide in aqueous solutions of betaine hydrochloride drug. J. Mol. Liq. 338, 116642 (2021). https://doi.org/10.1016/j.molliq.2021.116642
Alam, M., Rana, S., Abdul, M., Hoque, A., Kabir, S.E., Asiri, A.M.: Influence of various electrolytes on the interaction of cetyltrimethylammonium bromide with tetradecyltrimethylammonium bromide at different temperatures and compositions: experimental and theoretical investigation. J. Mol. Liq. 278, 86–96 (2019). https://doi.org/10.1016/j.molliq.2018.12.112
Inoue, T., Ebina, H., Dong, B., Zheng, L.: Electrical conductivity study on micelle formation of long-chain imidazolium ionic liquids in aqueous solution. J. Colloid Interface Sci. 314, 236–241 (2007). https://doi.org/10.1016/j.jcis.2007.05.052
Dong, B., Zhao, X., Zheng, L., Zhang, J., Li, N., Inoue, T.: Aggregation behavior of long-chain imidazolium ionic liquids in aqueous solution: micellization and characterization of micelle microenvironment. Colloids Surf. A Physicochem. Eng. Asp. 317, 666–672 (2008). https://doi.org/10.1016/j.colsurfa.2007.12.001
Rojas, M., Miskolczy, Z., Biczók, L., Pavez, P.: Effect of amino acid addition on the micelle formation of the surface-active ionic liquid 1-tetradecyl-3-methylimidazolium bromide in aqueous solution. J. Phys. Org. Chem. 32, 1–9 (2019). https://doi.org/10.1002/poc.3814
Baltazar, Q.Q., Chandawalla, J., Sawyer, K., Anderson, J.L.: Interfacial and micellar properties of imidazolium-based monocationic and dicationic ionic liquids. Colloids Surf. A Physicochem. Eng. Asp. 302, 150–156 (2007). https://doi.org/10.1016/j.colsurfa.2007.02.012
Paul, A., Mandal, P.K., Samanta, A.: On the optical properties of the imidazolium ionic liquids. J. Phys. Chem. B. 109, 9148–9153 (2005). https://doi.org/10.1021/jp0503967
Ysambertt, F., Vejar, F., Paredes, J., Salager, J.L.: The absorbance deviation method: a spectrophotometric estimation of the critical micelle concentration (CMC) of ethoxylated alkylphenol surfactants. Colloids Surf. A Physicochem. Eng. Asp. 137, 189–196 (1998). https://doi.org/10.1016/S0927-7757(97)00203-3
Paul, A., Mandal, P.K., Samanta, A.: How transparent are the imidazolium ionic liquids? A case study with 1-methyl-3-butylimidazolium hexafluorophosphate, [bmim][PF6]. Chem. Phys. Lett. 402, 375–379 (2005). https://doi.org/10.1016/j.cplett.2004.12.060
Karimi, M.A., Mozaheb, M.A., Hatefi-mehrjardi, A., Tavallali, H.: A new simple method for determining the critical micelle concentration of surfactants using surface plasmon resonance of silver nanoparticles. J. Anal. Sci. Technol. 6, 1–8 (2015). https://doi.org/10.1186/s40543-015-0077-y
Partington, J.R.: An advanced treatise on physical chemistry. Longmans, London (1951)
Batsanov, S.S.: Refractometry and chemical structure. Van Nostrand, Princeton (1966)
Desando, M.A.: Refractive index in relation to solvent effects on the amphiphilic association of n-alkylammonium carboxylates. Colloid Polym. Sci. 294, 1789–1805 (2016). https://doi.org/10.1007/s00396-016-3924-5
Acknowledgements
Authors (H.K) are thankful to Science and Engineering Research Board (SERB), New Delhi for providing financial assistance to carry out research work vide sanction order number EMR/2015/002059. Ramanjeet Kaur is thankful to University Grants Commission (UGC), New Delhi for providing Junior Research Fellowship (JRF) (121213) vide letter UGC-Ref. no.: 131/(CSIR-UGC NET DEC. 2017). The authors are also thankful to DST, New Delhi for DST-FIST [CSI-228/2011] Program and The Director and Head, Department of Chemistry for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
Authors declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, R., Kumar, H. & Singla, M. Unraveling the Molecular Interactions of Imidazolium-Based Surface-Active Ionic Liquid [C15mim][Br] with Biologically Active Amino Acids Glycine, l-Alanine, l-Valine. J Solution Chem 52, 838–858 (2023). https://doi.org/10.1007/s10953-023-01273-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01273-4