Abstract
The phase equilibrium of the quaternary system Na+, K+, Mg2+//Cl−–H2O and the quinary system Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O were investigated at 258 K using the isothermal dissolution equilibrium method. The phase diagrams were obtained based on the measured data. Double salt KCl·MgCl2·6H2O was found in the quaternary and the quinary systems at 258 K. In the quaternary system Na+, K+, Mg2+//Cl−–H2O, there are two invariant points, five univariant curves, and four crystallization fields. The quinary system saturated with NaCl·2H2O contains four invariant points, nine univariant curves, and six crystallization fields corresponding to KCl, NaNO3, KNO3, Mg(NO3)2·6H2O, KCl·MgCl2·6H2O and MgCl2·8H2O. Mg(NO3)2·6H2O and MgCl2·8H2O that have higher concentrations and stronger salting-out effect on other salts. Therefore, low-temperature pretreatment can offer an alternative treatment of brines in accordance with the necessity for current brine treatment processes to reduce the presence of double salts and be crucial for purer products to be separated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrate brines are found in the area close to Lop Nur in Xinjiang Province of China, containing Na+, K+, Mg2+, Cl−, \({\text{NO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\), which can be used to produce potassium nitrate or sodium nitrate, and play important roles in many industrial fields [1, 2]. However, the brine treatment process is difficult because the separation processes are extremely complex at normal temperature and cannot even produce pure products, due to the characteristics of multicomponent system and large numbers of complex salts, such as NaNO3·Na2SO4·H2O, Na2SO4·3K2SO4, KCl·MgCl2·6H2O, etc. [3,4,5,6,7,8,9].
The solid–liquid phase equilibrium of a salt-water system is closely affected by temperature, especially for normal temperatures, and the phase diagrams are complex because of the presence of various double salts. For example, at 298 K, six, two and four types of double salts have been found in the Na+, K+, Mg2+//Cl−, \({\text{SO}}_{4}^{2 - }\)–H2O system [6, 10], the Na+, K+//Cl−, \({\text{NO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\)–H2O system [6, 11], and the Na+, K+, Li+//Cl−, \({\text{SO}}_{4}^{2 - }\)–H2O system [12], respectively. However, at a higher operating temperature, the crystallization regions of the double salts involving nitrate or magnesium chloride, such as KCl·MgCl2·6H2O and NaNO3·Na2SO4·H2O, decrease or even disappear; at a lower temperature, a similar tendency could also be observed that crystallization zones of sulfate-type double salts, such as Na2SO4·3K2SO4 and K2SO4·MgSO4·H2O, decrease or even disappear. All of the crystallization zones of sulfate-type double salts in these systems disappear when the temperature is below 298 K [13,14,15]. This indicates that the temperature has a strong influence on the crystallization behavior and the solubility of salts. The low-temperature treatments have some advantages in terms of fewer species of complex salts, high separation efficiency and purer final quality of salts products. Thus, the phase equilibria at low temperature are suitable for applications associated with crystallization and purification processes.
Currently, there are few reports in the aspect of the brine associated with Na+, K+, Mg2+, Cl−, \({\text{NO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\), H2O at low temperature. However, it can be speculated that the types of double salts involved in this multicomponent system diminish at low temperature, which could simplify the phase diagram relationship. Thus, an alternative route in terms of dealing with the brine, that is, a relatively simple low-temperature processing method, can be promoted. The cooling crystallization processes have proved to play an efficient role in some fields [16,17,18,19,20,21,22]. Accurate knowledge of the phase diagrams is thus essential at low temperatures.
To exploit the valuable brine resources economically, it is important to adopt the local natural conditions including low temperatures. The winter in salt lake areas is both long and cold, with an average temperature of 258 K, providing large cold capacity. So, the study of phase equilibrium at this temperature would have more practical significance to better guide salt lake production in winter.
The Na+, K+, Mg2+//Cl+, \({\text{NO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\)–H2O system saturated with NaCl·2H2O has four quinary subsystems at 258 K, which are necessary for studying the solid–liquid equilibrium of the complex six-component system. Among them, the quinary system Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O is an important system of natural brine type associated with nitrate deposits, also involved in other chemical processes or some industrial wastewater treatments, but the phase equilibrium of this system has not been reported so far. This quinary system saturated with NaCl contains three quaternary subsystems and eight ternary subsystems. Except for the solubilities of the quaternary subsystems Na+, K+, Mg2+//Cl−–H2O, Na+ and Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O, those of other subsystems can be found in some compilations. The results show that the subsystems Na+//Cl−, \({\text{NO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\)–H2O [13] and Na+, K+//Cl−, \({\text{NO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\)–H2O [14] are both of simple types without double salts, whereas the quinary subsystem Na+, K+, Mg2+//Cl−, \({\text{SO}}_{4}^{2 - }\)–H2O [15] is of a complex type with the double salt KCl·MgCl2·6H2O formed at 258 K.
In this paper, we will supply the solubility data of the Na+, K+, Mg2+//Cl−–H2O and Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O systems; these data can be used as a theoretical reference for the design and optimize the process and is the basis for the utilization of brine resource.
2 Experimental Section
2.1 Reagents and Apparatus
The experimental materials were of analytical grade with purities not less than 98.0% mass fraction and all of them were used without any further purification. As tabulated in Table 1. NaCl, KCl, NaNO3 and KNO3 were purchased from the Chemical Reagent Factory of Tianjin Kermel, China, and were dried for 5–8 h at 105 °C prior to their use, the MgCl2·6H2O and Mg(NO3)2·6H2O supplied by Tianjin First Reagent Corporation. Doubly distilled water (DDW) with an electrical conductivity less than 1.0 × 10−4 s·m−1 and pH of about 6.6 at room temperature was used.
A magnetically stirred thermostatic bath (DHJF-4010A, Zhengzhou Changcheng Instrument Co., Ltd., China), maintaining the temperature range from –40 to 99 °C with an accuracy of ± 0.1 °C, was employed for the equilibrium experiments, and its temperature control accuracy was calibrated using a precise thermometer. As presented in Fig. 1, the apparatus mainly included four parts: a stainless steel tank, the heating tube, the cooling coils and a stirring motor. An X-ray diffraction analyzer (D8 Advance, Bruker, Germany) was employed for solid phase characterization. Raman spectra(LabRAM HR Evolution, HORIBA Scientific, France) was recorded from 1000 to 4000 cm−1 using the 532 nm line from an argon ion laser at a power of 100 mW and am integration time of 30 s at the sample.
2.2 Experimental Procedure
The solubilities data of the systems were determined by means of the isothermal solution method in this study. The other third or fourth salt was added gradually to the initial samples until the quaternary or quinary system’s invariant point was seen at 258 K. The appropriate salts and distilled water were mixed together and sealed in a 250 cm3 glass bottle. Then the bottle was placed in the magnetic stirring thermostatic bath, which was set to the desired temperature. The magnetic stirrer in the bottom of flask worked at a fixed speed. Samples of the liquid phase were taken periodically for chemical analysis. It was assumed that equilibrium was achieved when the concentration of the solution remained constant. The experimental results showed that the equilibrium time for the three systems was at least 16 h. After equilibration, stirring was stopped, and the equilibrated system was allowed to rest for 3 h to ensure that the solid settled and the liquid cleared.
The liquid phases were taken out with a pre-cooled pipette at 258 K and transferred to a 250 mL volumetric flask and diluted for the measurement of the compositions by chemical or instrument analysis. The solid phases were identified by powder X-ray diffraction. By changing the amount of the salts and deionized water added, other points in the liquid and their equilibrium solids can be determined, thus allowing the phase diagram to be plotted. All of these were applied to the above systems.
2.3 Analytical Methods
The concentration of (Cl−) was analyzed by 0.1 mol·L−1 AgNO3 volumetric method with a precision of 0.3%; The (Mg2+) ion concentration was determined by titration with an EDTA at pH = 9.0–10.0 (ammonia buffer) with the indicator Eriochrome Black-T with a standard uncertainty of less than 0.5%; The (K+) ion concentration was measured by a gravimetric method (uncertainty of 0.2%), The (\({\text{NO}}_{3}^{ - }\)) ion concentration was analyzed by potassium dichromate oxidization (uncertainty of 0.6%). The (Na+) ion concentration was evaluated from an ion balance.
3 Results and Discussion
3.1 Quaternary Homo-Ion System Na+, K+, Mg2+//Cl−–H2O
The experimental data on the solubilities of the equilibrated solution and equilibrated solid phases in the quaternary system determined experimentally are given in Table 2.
As a quaternary conjugate salt system, the phase diagram of the quaternary system with a common anion can be represented by a trigonal prism as shown in Fig. 2. In this diagram, its three coordinates are J(2Na+), J(2 K+) and J(H2O), respectively, which are respective Jänecke index values, and can be calculated according to the following correlations. The Jänecke index values were listed in Table 2.
Stereodiagram of the quaternary system Na+, K+, Mg2+//Cl−–H2O at 258 K (the region near point A3, where the KCl and ice are crystallized simultaneously at 258 K because of the low solubility and high eutectic temperature of the solution, was not investigated in this paper, therefore not plotted in proportion)
The Jänecke indices were calculated using the following equations. Letting
where w(ion) or w(H2O) is the mass of the ion or water in g per 100 g of solution, respectively. J(ion) or J(H2O) are the Jänecke index values of the ion or water, respectively.
On the basis of the Jänecke index values in Table 2, the stereodiagram of the quaternary system Na+, K+, Mg2+//Cl−–H2O at 258 K was plotted as shown in Fig. 2 and the planar projection of the phase diagram and the diagram of water contents are plotted in Figs. 3, 4 and 5.
Partial enlargement diagram of Fig. 3
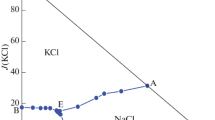
The phase diagram is composed of two invariant points, five univariant curves, and six crystallized regions of single salts. Three vertices A1, A2, A3 of the triangle represent salts of NaCl, MgCl2 and KCl. B1 and B2 are the invariant points of ternary systems Na+, K+//Cl−–H2O, Na+, Mg2+//Cl−–H2O, at 258 K, respectively. The K+, Mg2+//Cl−–H2O ternary system at 258 K is a complex type with two invariant points of point B3 and B4. The invariant points of the quinary system are labeled as C1 and C2. The point C1 is co-saturated with three salts NaCl·2H2O + KCl + KCl·MgCl2·6H2O and an equilibrium solution with w(Cl−) = 20.29 wt%, w(Mg2+) = 6.28 wt%, and w(K+) = 0.85 wt%. The point C2 is co-saturated with three salts NaCl·2H2O + MgCl2·8H2O + KCl·MgCl2·6H2O and an equilibrium solution with w(Cl−) = 24.29 wt%, w(Mg2+) = 8.26 wt%, and w(K+) = 0.04 wt%.
As shown in Fig. 6, the solids at two invariant points C1 and C2 were analyzed by X-ray diffraction, allowing the determination of the natures of the solid phases in equilibrium. The existence of the double salt KCl·MgCl2·6H2O can be determined. The salts can form a hydrated phase, which is only stable in a particular temperature range, and melt with changes of the conditions. Magnesium chloride can be formed from various hydrated salts, including MgCl2·12H2O, MgCl2·8H2O and MgCl2·6H2O. The literature demonstrated the existence of the hydrate MgCl2·12H2O in the temperature range 239–256 K, MgCl2·8H2O at 256–269 K and MgCl2·6H2O at 273–389 K. The research on this system shows that MgCl2·8H2O is the crystallization form of magnesium chloride [5, 6, 23,24,25,26]. In addition, the literature [5, 6, 25,26,27] shows that at 258 K, sodium chloride can form hydrated (NaCl·2H2O) in binary or ternary systems. Besides, Raman microscopy makes it possible to determine precisely the salt hydrates that crystallize upon cooling [28, 29]. We measured the Raman spectra of various aqueous solutions at different temperatures and showed that NaCl·2H2O exists in the systems at 258 K, consistent with the literature.
It is worth mentioning that, compared to the phase studies at higher temperature, studies at 258 K are more difficult, the salts can form various hydrated salts and the solutions have greater viscosity, which increases the complexity of the study on the phase equilibrium. Besides, because hydrate phases are only stable in special temperature and pressure ranges, they melt with the change of conditions. The limitation of experimental and equipment conditions, MgCl2·8H2O and NaCl·2H2O were difficult to detect by X-ray diffraction. So, in Figs. 7 and 12, the X-ray diffraction patterns of the invariant points consist of MgCl2·6H2O and NaCl, instead of MgCl2·8H2O and NaCl·2H2O.
The salts corresponding to the five-univariant curves B1C1, B4C1, C1C2, B3C2 and B2C2 are NaCl·2H2O + KCl, KCl·MgCl2·6H2O + KCl, KCl·MgCl2·6H2O + NaCl·2H2O, KCl·MgCl2·6H2O + MgCl2·8H2O and MgCl2·8H2O + NaCl·2H2O, respectively.
The four crystallization fields correspond to three single salts NaCl·2H2O, KCl, MgCl2·8H2O, and one double salts KCl·MgCl2·6H2O. The crystallization area of MgCl2·8H2O occupies the smallest part because of its high solubility. Whereas the crystallization areas of KCl and NaCl·2H2O are larger, which indicates that they are more easily saturated and crystallize out from the solution in this system as a result of the strong salting-out effect of other salts.
The water-content diagram is essential for the design and calculation of crystallization processes. As shown in Fig. 5, with J(2Na+) as the abscissa, and J(H2O) as the ordinate, we can see that at the univariant curves B1C1, B2C1 and C1C2, the water content changes obviously, while at the other univariant curves, the water content changes slightly, which can be explained by the increase of the mass fraction of MgCl2. On the univariant curve B1C1, J(H2O) increases to a maximum value with the decreasing of J(2Na+) and then decreases with further reduction of J(2Na+).
4 Quinary System Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O
Findings obtained from our previous research show that, NaCl should crystallize first from the solution during the cooling process because of the high concentration of NaCl in actual nitrate brine. So, the phase diagrams on the condition saturated with NaCl·2H2O at low temperatures have more practical value. Besides, the equilibrium of the quinary system is beneficial to the construction of the phase diagrams of the complex six-component system.
The measured solubilities data of the quinary system Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O saturated with NaCl·2H2O are presented in Tables 3 and 4. The concentration of each ion is expressed in mass fraction.
Na+, K+, Mg2+//Cl −, \({\text{NO}}_{3}^{ - }\)–H2O is a complicated quinary system, the isothermal phase behavior of which has four dimensions. The solvent-less projection (the dry salt phase diagram) obtained by elimination of the solvent coordinate decreases the dimensionality to three and can be represented in form of a trigonal prism as shown in Fig. 8, the coordinates of which are the Jänecke index J(2 K+), J(Mg2+) and J(2Cl−) respectively. These Jänecke index can be calculated according to the following equations and listed in Table 3.
Letting
According to the Jänecke index in Table 3, the crystallization space of NaCl·2H2O can be plotted in Fig. 7, which has four invariant points saturated with NaCl·2H2O and other three salts, nine univariant curves saturated with NaCl·2H2O and other two salts, and six crystallization fields saturated with NaCl·2H2O and the other salts.
Figure 7 is a stereodiagram and not easy to apply. The Cl-less projection (planar projection diagram) obtained by deducting the variable of Cl can reduce the dimension to two, and be plotted in a 2-D figure as shown in Figs. 8 and 9, the two coordinates of which are J(Mg2+) and J(2 \({\text{NO}}_{3}^{ - }\)); The water and contents of Cl can be indicated by the water-content diagram and chlorine-content diagrams respectively as shown in Figs. 10 and 11, the two coordinates of which are J(Mg2+) and J(H2O), J(Mg2+) and J(Cl−). These Jänecke indices are listed in Table 4.
Partial enlargement diagram of Fig. 8
As shown in Table 4 and Figs. 8, 9, 10 and 11, the quinary system Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O is of a complex type, with the double salt KCl·MgCl2·6H2O formed at 258 K, the phase diagram of which contains four invariant points, nine univariant curves, and six crystallization fields.
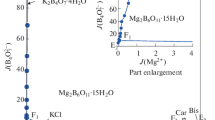
Each vertex B1, B2 and B5 represents the invariant points of NaCl–KCl, MgCl2–NaCl, and NaNO3–NaCl salts in water at 258 K, respectively. The points C1, C2, C3, C4, C5, and C6 on the sides correspond to the invariant points of quarternary subsystems Na+, K+, Mg2+//Cl−–H2O, Na+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O, and Na+, K+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O respectively.
The points D1, D2, D3 and D4 are invariant points of the quinary system Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O. The mass fraction compositions of the corresponding equilibrated solution and equilibrated solids of D1, D2, D3 and D4 are listed as follows.
Point D1 is analyzed as 9.36% mass (\({\text{NO}}_{3}^{ - }\)), 16.64% mass (Cl−), 0.71% mass (K+), 6.63% mass (Mg2+), and co-saturated with salts NaCl·2H2O, NaNO3, KNO3 and Mg(NO3)2·6H2O.
Point D2 is analyzed as 2.82% mass (\({\text{NO}}_{3}^{ - }\)), 18.78% mass (Cl−), 1.37% mass (K+), 5.91% mass (Mg2+), and co-saturated with salts NaCl·2H2O, KCl, KNO3 and KCl·MgCl2·6H2O.
Point D3 is analyzed as 9.67% mass (\({\text{NO}}_{3}^{ - }\)), 18.03% mass (Cl−), 0.42% mass (K+), 7.72% mass (Mg2+), and co-saturated with salts NaCl·2H2O, KNO3, KCl·MgCl2·6H2O and Mg(NO3)2·6H2O.
Point D4 is analyzed as 8.52% mass (\({\text{NO}}_{3}^{ - }\)), 20.34% mass (Cl−), 0.15% mass (K+), 8.36% mass (Mg2+), and co-saturated with salts NaCl·2H2O, KCl·MgCl2·6H2O, MgCl2·8H2O, and Mg(NO3)2·6H2O.
Figure 12 shows the X-ray diffraction patterns corresponding to the equilibrium solid phases of the invariant points D1, D2, D3 and D4, respectively. The XRD spectra in Fig. 12 can only determine that MgCl2·6H2O and NaCl, in the solid phases, as a result of the limitations of sample and testing conditions. It is very difficult to find MgCl2·8H2O and NaCl·2H2O.
The nine univariant curves, C5D1, C3D1, D1D3, C4D4, D3D4, D2D3, C6D2, C1D2 and C2D4, are co-saturated with NaCl·2H2O and two other salts, respectively.
The quinary phase diagram has six crystallization fields saturated with NaCl·2H2O and another salt, the second salts being NaNO3 (B5C5D1C3 field), KNO3 (C5C6D2D3D1 field), KCl (C6B1C1D2 field), Mg(NO3)2·6H2O (C3D1D3D4C4 field), KCl·MgCl2·6H2O (C1D2D3D4C2 field), and MgCl2·8H2O (C2 D4C4 B2 field).
It can be seen that the areas of the crystallization zones saturated with NaCl·2H2O decrease in the order of KCl, KNO3, NaNO3, KCl·MgCl2·6H2O, MgCl2·8H2O, Mg (NO3)2·6H2O. Among them, KNO3 and KCl have larger crystallization areas and lower solubility than the other salts. This means that separation processes from the mixture solutions for the two salts are feasible due to their relatively large areas of crystallization. The fields of Mg(NO3)2·6H2O and MgCl2·8H2O are extremely small, which are the result of their high solubility.
Figure 10 shows that the water-contents for the invariant points decrease apparently from D2–D1–D3 to D4, reaching the J(H2O) values of 832.19 mol·(100 mol)−1 (2 K+ + 2\({\text{NO}}_{3}^{ - }\) + Mg2+) at the invariant point D4. It can be concluded that the point D4 has the highest concentration in the quinary system. The water content gradually decreases at the univariant curves C6D2 and it is almost unchanged at the univariant curves C1D2, C2D4 with increasing of the Jänecke index values of J(Mg2+). The high concentration of Mg2+ ion in the quinary system implies that Mg2+ has a strong salting-out effect on other salts, which can decrease the solubilities of the other salts.
Figure 11 indicates that the chlorine-content of the points C3, C4, D1, D3 and D4 are similar, about 65–70 mol·(100 mol)−1 (2 K+ + NO3− + Mg2+); The lowest contents of Cl is at point D3; The concentration of magnesium and nitrate ion significantly influence the chlorine content, especially on univariant curves C6D2, C1D2D3 and C2D4. It clearly shows that the regularity in the variation of the chlorine content related to the J(Mg2+) is a feature similar to that of the water content. The point D4 possess the lowest water content, which is because of the higher solubility of MgCl2 and Mg(NO3)2 compared to other salts at 258 K.
5 Conclusions
The phase equilibria of the quinary system Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O and the quaternary system Na+, K+, Mg2+//Cl−–H2O have been investigated by the method of isothermal solution saturation. The results demonstrated that the quinary system Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O saturated with NaCl·2H2O at 258 K belongs to a complex type, which contains four invariant points, nine univariant curves, and six crystallization fields. The salts Mg(NO3)2·6H2O and MgCl2·8H2O have smaller crystallization areas and higher solubility than the other salts, so they are more difficult to separate from the solution in this system at the research temperature. The concentrations of Mg2+ and \({\text{NO}}_{3}^{ - }\) in the solution are the main factors affecting the water content and solution density. The findings of this study can be applicable in various industrial salts recovering units.
References
Zheng, M.P.: On saline lakes of China. Miner. Dep. 20, 181–189 (2001)
Zhang, B.Q.: Present status of development and utilization of nitrate resources and its development prospect in China. Ind. Miner. Proces. 36, 1–5 (2007)
Steiger, M.: The geochemistry of nitrate deposits : I. Thermodynamics of Mg (NO3)2–H2O and solubilities in the Na+–Mg2+–\({\text{NO}}_{3}^{ - }\)–\({\text{SO}}_{4}^{2 - }\)–H2O system. Chem. Geol. 36, 84–97 (2016)
Chan, C.K., Liang, Z., Zheng, J., Clegg, S.L., Brimblecombe, P.: Thermodynamic properties of aqueous aerosols to high supersaturation: I–Measurements of water activity of the system Na+–Cl−–\({\text{NO}}_{3}^{ - }\)–\({\text{SO}}_{4}^{2 - }\)–H2O at 298.15 K. Aerosol. Sci. Technol. 27, 324–344 (1997)
Weast, R.C., Astle, M.J., Beyer, W.H.: CRC handbook of Chemistry and Physics. CRC Press, Boca Raton (1983)
Howard, S., Silcock, H.L.: Solubililies of Inorganic and Organic Compounds, 3rd edn. Pergamon Press, New York (1979)
Huang, X.L., Song, P.S., Chen, L.J., Lu, B.L.: Liquid–solid equilibria in quinary system Na+, Mg2+//Cl−,\({\text{SO}}_{4}^{2 - }\)–\({\text{NO}}_{3}^{ - }\)–H2O at 298.15 K. Calphad 32, 188–194 (2008)
Huang, X.L., Zhu, L.J., Liang, T., Song, P.S.: Study on the phase equilibrium for the quinary system Na+, K+, Mg2+//Cl−,\({\text{NO}}_{3}^{ - }\)–H2O at 298.16 K. Acta Chim. Sinica 65, 798–802 (2007)
Yang, J., Wang, Y.F., Shu, M., Yang, L.B., Zhu, L., Zhao, X.Y., Shan, Z.L.: Solid–liquid equilibrium of quaternary system Na+//Cl−,–\({\text{NO}}_{3}^{ - }\)–\({\text{SO}}_{4}^{2 - }\)–H2O at 373.15 K. Fluid Phase Equilib. 445, 7–13 (2017)
Jin, Z.M., Xiao, X.Z., Liang, S.M.: Study of the metastable equilibrium for pentanary system of (Na+, K+, Mg2+), (Cl−, \({\text{SO}}_{4}^{2 - }\)), H2O. Acta Chim. Sin. 38, 313–321 (1980)
Zhou, T., Wang, X.F., Huang, X.L., Li, H., Liu, N.: Liquid–solid metastable equilibria of a quinary system Na+, K+//Cl−,\({\text{SO}}_{4}^{2 - }\),\({\text{NO}}_{3}^{ - }\)–H2O at 298 K. J. Chem. Eng 29, 510–515 (2015)
Song, P.S., Dong, Y.P., Wu, L.: The phase diagram of Li+, Na+, K+//Cl−,\({\text{SO}}_{4}^{2 - }\)–H2O quinary system at 25 °C and its application. J. Salt Lake Res. 25, 9–17 (2017)
Zhang, X., Huang, X.L.: Study on the phase equilibria of the quaternary system Na+//Cl−, \({\text{NO}}_{3}^{ - }\), \({\text{SO}}_{4}^{2 - }\)–H2O at low temperatures. Chemistry 78, 337–341 (2015)
Liao, L., Huang, X.L., Song, H.: Phase equilibria of quinary system Na+, K+//Cl−, \({\text{SO}}_{4}^{2 - }\), \({\text{NO}}_{3}^{ - }\) –H2O at 258.15 K. J. Chem. Eng. 30, 7–12 (2016)
Zhu, Q.L., Huang, X.L.: Liquid-solid phase equilibrium of Na+, K+, Mg2+//Cl−, \({\text{SO}}_{4}^{2 - }\)–H2O system at −15°C. J. Chem. Ind. Eng. 66, 1252–1257 (2015)
Ham, F.V.D., Witkamp, G.J., Graauw, J.D., Van Rosmalen, G.M.: Eutectic freeze crystallization simultaneous formation and separation of two solid phases. J. Cryst. Growth 198, 744–748 (1999)
Kim, D.H.: A review of desalting process techniques and economic analysis of the recovery of salts from retentates. Desalination 270, 1–8 (2011)
Mahdavi, M., Mahvi, A.H., Nasseri, S., Yunesian, M.: Application of freezing to the desalination of saline water. J. Sci. Eng. 36, 1171–1177 (2011)
Hasan, M., Rotich, N., John, M., Louhi-Kultanen, M.: Salt recovery from wastewater by air-cooled eutectic freeze crystallization. Chem. Eng. J. 326, 192–200 (2017)
Rich, A., Mandri, Y., Mangin, D., Rivoire, A., Abderafi, S.: Sea water desalination by dynamic layer melt crystallization: parametric study of the freezing and sweating steps. J. Cryst. Growth 342, 110–116 (2012)
Vaessen, R., Seckler, M., Witkamp, G.J.: Eutectic freeze crystallization with an aqueous KNO3−HNO3 solution in a 100-L cooled-disk column crystallizer. Ind. Eng. Chem. Res. 42, 4874–4880 (2003)
Hasan, M., Filimonov, R., Chivavava, J., Sorvari, J., Louhi-Kultanen, M., Lewis, A.E.: Ice growth on the cooling surface in a jacketed and stirred eutectic freeze crystallizer of aqueous Na2SO4 solutions. Sep. Purif. Technol. 175, 512–526 (2017)
Christov, C.: Chemical equilibrium model of solution behavior and bishofite (MgCl2·6H2O(cr)) and hydrogen-carnallite (HCl·MgCl2·7H2O(cr)) solubility in the MgCl2+H2O and HCl·MgCl2+H2O systems to high acid concentration at (0 to 100) °C. J. Chem. Eng. Data 54, 2599–2608 (2009)
Christov, C.: Isopiestic determination of the osmotic coefficients of an aqueous MgCl2 + CaCl2 mixed solution at (25 and 50)° C. Chemical equilibrium model of solution behavior and solubility in the MgCl2 + H2O and MgCl2 + CaCl2 + H2O systems to high concentration at (25 and 50)°C. J. Chem. Eng. Data 54, 627–635 (2008)
Pabalan, R.T., Pitzer, K.S.: Thermodynamics of concentrated electrolyte mixtures and the prediction of mineral solubilities to high temperatures for mixtures in the system Na−K−Mg−Cl−SO4−OH−H2O. Geochim. Cosmochim. Acta 51, 2429–2443 (1987)
Spenser, R., Moller, N., Weare, J.: The prediction of mineral solubilities in natural waters: a chemical equilibrium model for the Na−K−Ca−Mg−Cl−SO4−H2O system at temperatures below 25 °C. Geochim. Cosmochim. Acta 54, 575–590 (1990)
Drebushchak, V.A., Ogienko, A.G., Yunoshev, A.S.: Metastable eutectic melting in the NaCl−H2O system. Thermochim. Acta 647, 94–100 (2017)
Dubessy, J., Audeoud, D., Wilkins, R., Kosztolanyi, C.: The use of the Raman microprobe MOLE in the determination of the electrolytes dissolved in the aqueous phase of fluid inclusions. Chem. Geol. 37, 137–150 (1982)
Samson, I.M., Walker, R.T.: Cryogenic Raman spectroscopic studies in the system NaCl–CaCl2–H2O and implications for low temperature phase behavior in aqueous fluid inclusions. Can. Mineral. 38, 35–43 (2000)
Acknowledgements
The authors greatly acknowledge the financial support from the program of the National Natural Science Foundation of China (Grant No. 21766033) and the foundation of Key Laboratory of Cleaner Transition of Coal & Chemicals Engineering of Xinjiang University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, XY., Huang, XL., Wang, XF. et al. Solid–Liquid Phase Equilibria in the Quinary System Na+, K+, Mg2+//Cl−, \({\text{NO}}_{3}^{ - }\)–H2O and Its Subsystems at 258 K. J Solution Chem 49, 885–901 (2020). https://doi.org/10.1007/s10953-020-00978-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-00978-0