Abstract
The stable phase equilibria of the ternary system MgCl2 + SrCl2 + H2O and the two quaternary systems NaCl + MgCl2 + SrCl2 + H2O and KCl + MgCl2 + SrCl2 + H2O at 373 K were investigated using an isothermal method. Based on the experimental results, the isothermal solubility diagram of the ternary system MgCl2 + SrCl2 + H2O, and the solubility diagrams and water content diagrams of the two quaternary systems were constructed. The results show that the ternary system MgCl2 + SrCl2 + H2O at 373 K belongs to the simple co-saturated type without complex salt or solid solution. There are two invariant points, three univariant curves and three crystallization fields corresponding to SrCl2·2H2O, SrCl2·H2O and MgCl2·6H2O. The quaternary system NaCl + MgCl2 + SrCl2 + H2O at 373 K is of the simple co-saturated type and no complex salt or solid solution are found. The phase diagram contains two invariant points, five univariant curves and four crystallization fields (where the solids are NaCl, MgCl2·6H2O, SrCl2·H2O and SrCl2·2H2O). The system KCl + MgCl2 + SrCl2 + H2O at 373 K has a double salt named carnallite (KCl·MgCl2·6H2O) without solid solution formation. The phase diagram contains three invariant points, seven univariant curves and five crystallization fields (where the solids are KCl, MgCl2·6H2O, SrCl2·H2O, SrCl2·2H2O and KCl·MgCl2·6H2O).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
China is a country with abundant underground brine resources that are distributed in many oil and gas bearing sedimentary basins. Due to the recharge of underground brine from multiple sources, and through sedimentary metamorphism during burial, most of the underground brine is at high temperature, high salinity, and under high pressure. Therefore, there are obvious distinctions in the chemical composition compared with the shallow groundwater, seawater and even saline lake brine. The underground brine in the Sichuan basin, whose brine salinity reaches 190–290 g·L−1, is not only rich in Na+, Ca2+, Mg2+, K+, Cl− and other common elements, but also rich in Sr2+, Br−, I−, Ba2+ and other minor components. The content of these components often can meet or exceed the industrial grade, and what’s more, they are important or indispensable materials in light industry, the chemical industry, nuclear engineering, aerospace and other high-tech fields [1, 2]. Consequently, with the gradual depletion of solid mineral resources, the comprehensive utilization of underground brine has an economic value and broad social prospects that cannot be overestimated.
In the process of mineralization and the exploitation of mineral resources, there is a dynamic solid–liquid phase equilibrium. The comprehensive utilization of brine resources usually needs to be guided by the multi-temperature phase equilibrium, and it can be realized by a series of phase separation techniques, such as evaporation, crystallization, salting out, heating, cooling or dissolution [3, 4]. Therefore, the studies of the solid–liquid phase equilibria of the underground brine can reveal the metallogenic contents of the liquid mineral and guide the development and utilization of brine resources.
The underground brine of Sichuan basin, which is rich in the elements strontium and potassium, can be simplified to the multi-component system NaCl + KCl + MgCl2 + SrCl2 + CaCl2 + H2O. The phase equilibria of the subsystems of this complex multi-component system at different temperatures have been investigated by many workers. The ternary systems NaCl + SrCl2 + H2O, KCl + SrCl2 + H2O [5], MgCl2 + SrCl2 + H2O [6], CaCl2 + SrCl2 + H2O [7], as well as the quaternary system NaCl + KCl + SrCl2 + H2O and the multi-component systems NaCl + KCl + SrCl2 + CaCl2 + H2O [8] and NaCl + KCl + SrCl2 + CaCl2 + MgCl2 + H2O [9] have been studied. The quaternary system KCl + CaCl2 + MgCl2 + H2O and the three ternary systems it embodies, at 273, 308 and 348 K, have also been studied [10,11,12,13,14,15]. The solubilities in the ternary systems NaCl + SrCl2 + H2O [16], KCl + SrCl2 + H2O [17], and CaCl2 + SrCl2 + H2O [18] at 298 K have been explored. Studies of some brine systems containing potassium and strontium have also been carried out by our group, they are the ternary systems MgCl2 + SrCl2 + H2O, NaCl + SrCl2 + H2O, KCl + SrCl2 + H2O and quaternary system SrCl2 + KCl + NaCl + H2O [19,20,21]. The purpose of this article is to report the solubilities and identify the equilibrium solid phases of the ternary system MgCl2 + SrCl2 + H2O and the two quaternary systems NaCl + MgCl2 + SrCl2 + H2O and KCl + MgCl2 + SrCl2 + H2O at 373 K. The latter two systems have not been published yet. Although the phase equilibria on the ternary system MgCl2 + SrCl2 + H2O had been reported [6], this study only presented the invariant point data, and there is very little solubility data in the literature. So, the present work is a continuation of the previous project. Simultaneously, our identification has promising application in exploiting the brine resources and understanding the geochemical behavior.
2 Experimental Section
2.1 Reagents and Instruments
2.1.1 Reagents
The starting materials NaCl, KCl, MgCl2·6H2O and SrCl2·6H2O used in the experiment are all produced in Chengdu Kelong Chemical Reagent Factory, and they are all analytically pure agents. The purity of the reagents is more than 99.5%, with the exception of MgCl2·6H2O, whose purity is not less than 98%. The water for preparation of sample solution and analytical water is deionized water, whose pH is nearly 6.6 and conductivity is less than 1 × 10−5 S·m−1.
2.1.2 Experimental Instruments
A standard electronic balance of 110 g capacity made by the Mettler Toledo Instruments Co., Ltd., whose precision is 0.0001 g, was used to weigh the samples. An ultrapure water apparatus (UPT-II-20T) was used to produce deionized water.
The constant temperature oil bath oscillator (HZ-9613Y, ± 0.1 K) supplied by the Jintan Jieruier Instrument Co., Ltd. was used to control the equilibrium temperature.
X-ray diffraction (DX-2700) instrument manufactured by the Dandong Fangyuan Instrument Co., Ltd. was used to identify the solid phases.
2.2 Experimental Method
The isothermal method [4] was adopted to study the ternary system MgCl2 + SrCl2 + H2O and quaternary systems NaCl + MgCl2 + SrCl2 + H2O and KCl + MgCl2 + SrCl2 + H2O. Based on the solubility data of each single salt and the existing invariant point solubility in each ternary system, a certain proportion of blend salts and 30 mL water were mixed in the ground glass bottles (3 cm in diameter and 12 cm in height), the sealed bottles were placed in the constant temperature oil bath oscillator (HZ-9613Y). The oscillator was used to promote the establishment of equilibrium. The temperature was controlled at 373 ± 0.1 K for the isothermal dissolution equilibrium. All the samples were fully shaken for 3 days and then kept standing for 2–4 days at 373 K. The supernatant solution was taken out regularly for chemical analysis until the contents of the chemical components remained constant, which indicated that the samples had reached equilibrium. The required time for equilibration was found to be 5–7 days. Sampling correctly was the key to the success of the experiment. We only took the supernatant solution during the sampling, thus avoiding particles from entering the samples. Also the solid phases were large grains and precipitated at the bottom at 373 K in the experiment, the height of the solution is much larger than the diameter of the bottle, and it is also more than 2/3 the height of the bottle, so that it can avoid solid phase entering the sampling pipette. The pipette was preheated, then it was inserted into the supernatant layer to suck up 2–3 mL of liquid quickly. The removed samples were put into weighing bottles with known, constant weight, and then the weighing bottles were accurately weighed and diluted with distilled water in a 100 mL volumetric flask and then analyzed. The mixtures of solids were taken out to be checked by X-ray diffraction.
2.3 Analytical Methods
The concentration of potassium ion (K+) was analyzed by a sodium tetra-phenyl borate(STPB)–hexadecyltrimethyl ammonium bromide (CTAB) back titration (uncertainty of 0.5%).The concentration of magnesium ion (Mg2+) was evaluated with an EDTA standard solution and eriochrome black T as the indicator. The strontium ion (Sr2+) concentration was titrated in a strongly alkaline solution with an EDTA standard solution in the presence of the calcium indicator (uncertainty: 0.5%). The chloride ion (Cl−) concentration was determined with Mohr’s method using a silver nitrate standard solution (with a precision of 0.3%) in near neutral solution. The concentration of sodium ion (Na+) was obtained according to the ion charge balance and assisted by atomic absorption spectrometry (uncertainty, 0.3%). Equilibrium solid phases were identified by a chemical reaction method combined with XRD (X-ray diffraction).
3 Results and Discussion
3.1 The Ternary System MgCl2 + SrCl2 + H2O at 373 K
The measured data of the ternary system MgCl2 + SrCl2 + H2O at 373 K are listed in Table 1, where the concentration of the liquid is expressed in mass fraction w(B). The graphical representation shown in Fig. 1 is also plotted based on the determined data. As will be seen from Fig. 1, the isothermal solubility diagram contains two invariant points (E1 and F1) and three solubility curves: B1F1, corresponding to the crystallization of SrCl2·2H2O; F1E1, corresponding to the crystallization of SrCl2·H2O; E1A1, the MgCl2·6H2O curve. The invariant point F1 is saturated with SrCl2·2H2O and SrCl2·H2O and the composition of the point is w(MgCl2) = 34.3%, w(SrCl2) = 6.40%. The other co-saturated point E1 is saturated with MgCl2·6H2O and SrCl2·H2O and the composition of the point is w(MgCl2) = 41.68%, w(SrCl2) = 1.68%.
Figure 2 is the X-ray diffraction pattern at the invariant point E1, where equilibrium solids MgCl2·6H2O and SrCl2·H2O were identified. Figure 3 is the X-ray diffraction of the invariant point F1, at which SrCl2·2H2O and SrCl2·H2O coexist. The tie-lines joining the composition points of a solution and its corresponding “dry” residue meet at the composition points of the coexisting solid phase. At the same time, MgCl2 acts as a salting-out agent with respect to SrCl2. The strontium chloride dihydrate loses one molecule of water and is converted into strontium chloride monohydrate in the presence of the increasing amount of magnesium chloride hexahydrate.
The Swedish scholar Assarsson studied the solubility of the ternary system MgCl2 + SrCl2 + H2O at 291–373 K [6]. Their salt solubility data and the phase diagrams of this system at different temperatures are very few, and thus incomplete. For example, the compositions of each salt and each univariant curve are not given at 373 K [6]. In this paper, a complete study of this system at 373 K is carried out, and the solubility data and solid crystallization regions associated with the univariant curves that are missing in the literature are added.
Our team also carried out the solubility of the ternary system MgCl2 + SrCl2 + H2O at 348 and 323 K [19] earlier. The contrast chart at these three temperatures is shown in Fig. 4. It can be seen that the phase diagrams at 323 and 348 K are similar in shape, and both contain two single variable curves, one invariant point and two crystalline regions, where SrCl2·2H2O has the larger crystallization region, and it reveals that the solubility of SrCl2 in the equilibrium solution is very small. However, the phase diagram at 373 K consists of three single variable curves, two co-saturated points and three crystallization regions. The equilibrium solid phases at 323 and 348 K are MgCl2·6H2O and SrCl2·2H2O, whereas when the temperature increases to 373 K, the equilibrium solids are MgCl2·6H2O, SrCl2·2H2O and SrCl2·H2O. The mass fraction of MgCl2 in the co-saturated liquid phase increases and the mass fraction of SrCl2 increases slightly with the increase of temperature.
Equilibrium phase diagram of the ternary system MgCl2 + SrCl2 + H2O at 373, 348 and 323 K [19]
3.2 The Quaternary System NaCl + MgCl2 + SrCl2 + H2O at 373 K
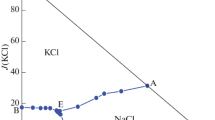
The quaternary system NaCl + MgCl2 + SrCl2 + H2O at 373 K was brought into equilibrium by the isothermal method. The liquid composition (mass percent) and the corresponding equilibrium solids are shown in Table 2 (J is the dry salt index of the quaternary system, J(NaCl) + J(MgCl2) + J(SrCl2) = 100). According to the experimental results, the corresponding phase diagram and water diagram are drawn (Figs. 5 and 6). As can be seen from Table 2 and Fig. 5, the quaternary system NaCl + MgCl2 + SrCl2 + H2O at 373 K is a simple co-saturated phase diagram, no double salt formation or solid solution is formed, and there are two invariant points E2 and F2. As can be seen from Figs. 7 and 8 XRD diffraction patterns that the position of the characteristic peaks present are in accordance with those of the reference compounds, hence the invariant points are saturated with salts NaCl + MgCl2·6H2O + SrCl2·H2O and NaCl + SrCl2·2H2O + SrCl2·H2O. The composition of the invariant point E2 is w(NaCl) = 0.13%, w(MgCl2) = 41.53%, w(SrCl2) = 1.51%, and that of the invariant point F2 is w(NaCl) = 1.04%, w(MgCl2) = 34.71%, w(SrCl2) = 6.68%.
Additionally, the quaternary system NaCl + MgCl2 + SrCl2 + H2O at 373 K is composed of five univariate curves: A2E2, B2E2, C2E2, D2F2 and E2F2, four solid phase crystallization areas (where the solids are NaCl, MgCl2·6H2O, SrCl2·H2O and SrCl2·2H2O). The crystalline zone of MgCl2·6H2O is much smaller than those of NaCl, SrCl2·H2O and SrCl2·2H2O, indicating that the solubility of MgCl2·6H2O is the largest and that it is difficult to crystallize from the solution. This quaternary system comprises three ternary systems, those are NaCl + MgCl2 + H2O, NaCl + SrCl2 + H2O and MgCl2 + SrCl2 + H2O. Each invariant point of the ternary systems is reflected in the phase diagram. Point A2 and D2 are the invariant points of the ternary system MgCl2 + SrCl2 + H2O, point B2 is the invariant point of the ternary system NaCl + MgCl2 + H2O, at the same time point C2 is the invariant point of the ternary system NaCl + SrCl2 + H2O.
Figure 6 shows the relationship between the liquid water content J(H2O) and the liquid composition J(NaCl). On the univariant curve F2C2, the water content of the solution decreases with the increase of J(NaCl), however, the water content of the solution on the other curves does not change obviously with the increase of J(NaCl). Therefore, the water content reaches its minimum at the point C2 (J = 93.05), which indicates that the total salt concentration reaches its maximum at the point C2.
3.3 The Quaternary System KCl + MgCl2 + SrCl2 + H2O at 373 K
The method for the identification of the quaternary system KCl + MgCl2 + SrCl2 + H2O at 373 K is the same as above. All the measured values are given in Table 3. The dry salt phase diagram is plotted based on the experimental results in Fig. 9. As shown in Fig. 9, the quaternary system KCl + MgCl2 + SrCl2 + H2O embodies three ternary systems, which are as follow: MgCl2 + SrCl2 + H2O, KCl + MgCl2 + H2O and KCl + SrCl2 + H2O. A3 and H2 are the invariant points of the ternary system MgCl2 + SrCl2 + H2O, B3 and C3 are the invariant points of the ternary system KCl + MgCl2 + H2O, and D3 is the invariant point of the ternary system KCl + SrCl2 + H2O. This quaternary system that generates a potassium and magnesium chloride double salt named carnallite (Car), is made up of three invariant points (E3, F3 and G), seven univariant curves and five crystallizing fields. The invariant point E3 is saturated with MgCl2·6H2O + SrCl2·H2O + KCl·MgCl2·6H2O, and the content of the saturated solution is w(KCl) = 0.52%, w(SrCl2) = 1.36%, w(MgCl2) = 41.40%. The invariant point F3 is saturated with SrCl2·2H2O + SrCl2·H2O + KCl·MgCl2·6H2O, and the composition of the equilibrium solution is w(KCl) = 4.54%, w(SrCl2) = 6.47%, w(MgCl2) = 33.53%. The last invariant point G is at w(KCl) = 6.18%, w(SrCl2) = 10.85%, w(MgCl2) = 29.76%, where KCl, SrCl2·2H2O and KCl·MgCl2·6H2O coexist. The seven univariant curves are namely A3E3, B3E3, C3G, D3G, H2F3, E3F3, and F3G. The five crystallization zones are MgCl2·6H2O, SrCl2·H2O, SrCl2·2H2O, KCl·MgCl2·6H2O and KCl, respectively. As can be seen from Fig. 9, the crystalline region of KCl is the largest, however, that of MgCl2·6H2O is the smallest, which indicate that KCl has the lowest solubility and MgCl2·6H2O has the largest.
The water content diagram of the quaternary system KCl + MgCl2 + SrCl2 + H2O at 373 K is constructed in Fig. 10. It illustrates the relationship between the water content J(H2O) and the liquid composition J(KCl). On the univariant curve GC3, the water content of the solution increases with increasing J(KCl) and reaches its maximum at the point C3, which shows that the total salts concentration reaches its minimum at the point C3. On the contrary, the relationship on curve GD3 is opposite to that of the curve GC3, hence the total salts concentration at the point D3 reaches its maximum.
Figure 11 is the X-ray diffraction pattern of the saturated point E3, where the equilibrium solids MgCl2·6H2O, SrCl2·H2O and KCl·MgCl2·6H2O are all identified. Figure 12 shows that the invariant point F3 is saturated with solid phases SrCl2·2H2O, SrCl2·H2O and KCl·MgCl2·6H2O.
3.4 Method Verification
The isothermal method is a classical method which has been used to study isothermal phase diagrams for a long time. The reliability and repeatability of the experimental results have been verified by a large number of related studies, which ensure the reliability of the experimental data. In the process of the experiments, we selected at least three groups of parallel samples to verify the repeatability of the experiment, especially the boundary points and the invariant points. For example, in the first point (A1) of Table 1, the mass fraction of MgCl2 measured is 42.29%, while that reported in the literature [4] is 42.2%, the standard deviation of these two results is 0.064%. And the mass fraction of SrCl2 measured in the seventh point (B1) is 50.25%, while that in literature [5] is 50.8%, the standard deviation of these two results is 0.39%. The results of the determination and the results reported in the literature agree within the experimental error.
Some other systems at 373 K had also been carried out by our group using the isothermal method, such as the ternary system KCl + KBr + H2O [22], where the mass fraction of KCl measured in the boundary point is 36.04%, while that reported in the literature [4] is 35.90% and in the ternary NaCl + ZnCl2 + H2O [23], the mass fraction of NaCl measured in the boundary point is 28.25%, while that reported in the literature [4] is 28.20%. In addition, the invariant point data of the ternary system NaCl + Na2SO4 + H2O at 373 K [24] studied by another group are w(NaCl) = 26.19%, w(Na2SO4) = 4.38%, while that reported in the literature [4] are w(NaCl) = 25.9%, w(Na2SO4) = 4.40%. These data agree within the experimental error. Therefore, our experimental method at 373 K is reliable.
4 Conclusions
The ternary system MgCl2 + SrCl2 + H2O and quaternary systems NaCl + MgCl2 + SrCl2 + H2O and KCl + MgCl2 + SrCl2 + H2O were brought into equilibrium at 373 K by means of the isothermal method. Solubilities and the corresponding solid phases were determined. The results show that the ternary system MgCl2 + SrCl2 + H2O and quaternary system NaCl + MgCl2 + SrCl2 + H2O belong to simple co-saturated systems; however, there is a complex salt KCl·MgCl2·6H2O in the quaternary system KCl + MgCl2 + SrCl2 + H2O. The ternary system MgCl2 + SrCl2 + H2O at 373 K has two invariant points, three univariant curves and three crystallization areas (SrCl2·2H2O, SrCl2·H2O and MgCl2·6H2O). The quaternary system NaCl + MgCl2 + SrCl2 + H2O at 373 K contains two co-saturated points, five univariant curves and four crystallization regions corresponding to NaCl, MgCl2·6H2O, SrCl2·H2O and SrCl2·2H2O. The quaternary system KCl + MgCl2 + SrCl2 + H2O at 373 K is made of three invariant points, seven univariant curves and five crystallization fields, which are KCl, MgCl2·6H2O, SrCl2·H2O, SrCl2·2H2O and KCl·MgCl2·6H2O, respectively.
References
Zhou, X.: Hydrogeochemical characteristics and formation of subsurface brines of deep aquifers in Longnv temple brine-bearing structure Sichuan Basin (in Chinese). Geoscience 7, 83–92 (1993)
Lin, Y.T.: Study on sustainable development of potassium boron lodine and bromine in brine of Sichuan Basin (in Chinese). J. Salt Lake Res. 9, 56–60 (2001)
He, F.M., Liu, S.C.: Handbook of Methods for the Identification of Mineral Salts (in Chinese). Chemical Industry Press, Beijing (1985)
Niu, Z.D., Cheng, F.Q.: Phase Diagram of Water Salt System and Its Application (In Chinese). Tianjin University Press, Tianjin (2002)
Assarsson, G.O.: Equilibria in aqueous systems containing Sr2+, K+, Na+ and Cl−. J. Phys. Chem. 57, 207–210 (1953)
Assarsson, G.O., Balder, A.: Equilibria between 18 °C and 100 °C in the aqueous systems containing Sr2+, Mg2+and Cl−. J. Phys. Chem. 58, 416–417 (1954)
Assarsson, G.O., Balder, A.: Equilibria between 18° and 114° in the aqueous ternary systems containing Sr2+, Ca2+and Cl−. J. Phys. Chem. 57, 717–722 (1953)
Assarsson, G.O., Balder, A.: Equilibria in aqueous systems containing Ca2+, Sr2+, K+, Na+ and Cl− between 18 °C and 114 °C. J. Phys. Chem. 58, 253–255 (1954)
Assarsson, G.O., Balder, A.: The poly-component aqueous systems containing the chlorides of Ca2+, Mg2+, Sr2+, K+ and Na+ between 18 °C and 93 °C. J. Phys. Chem. 59, 631–633 (1955)
Igelsrud, I., Thompson, T.G.: Equilibria in the saturated solutions of salts occurring in sea water. I. The ternary systems MgCl2–KCl–H2O, MgCl2–CaCl2–H2O, CaCl2–KCl–H2O and CaCl2–NaCl–H2O at 0 °C. J. Am. Chem. Soc. 2, 318–322 (1936)
Igelsrud, I., Thompson, T.G.: Equilibria in the saturated solutions of salts occurring in sea water. II. The quaternary system MgCl2–CaCl2–KCl–H2O at 0 °C. J. Am. Chem. Soc. 58, 2003–2009 (1936)
Lightfoot, W.J., Prutton, C.F.: Equilibria in saturated solutions. I. The ternary systems CaCl2–MgCl2–H2O, CaCl2–KCl–H2O, and MgCl2–KCl–H2O at 35 °C. J. Am. Chem. Soc. 68, 1001 (1946)
Lightfoot, W.J., Prutton, C.F.: Equilibria in saturated solutions. II. The quaternary system CaCl2–MgCl2–KCl–H2O at 35 °C. J. Am. Chem. Soc. 70, 4112 (1948)
Lightfoot, W.J., Prutton, C.F.: Equilibria in saturated salt solutions. II The ternary systems CaCl2–MgCl2–H2O, CaCl2–KCl–H2O, and MgCl2–KCl–H2O at 75 °C. J. Am. Chem. Soc. 68, 1001–1002 (1946)
Lightfoot, W.J., Prutton, C.F.: Equilibria in saturated salt solutions. IV. The quaternary system CaCl2–MgCl2–KCl–H2O at 75 °C. J. Am. Chem. Soc. 71, 1233–1235 (1949)
Ding, X.P., Sun, B., Shi, L.J., Yang, H.T., Song, P.S.: Study on phase equilibria in NaCl–SrCl2–H2O ternary system at 25 °C (in Chinese). Inorg. Chem. Ind. 42, 9–10 (2010)
Shi, L.J., Sun, B., Ding, X.P., Song, P.S.: Phase equilibria in the ternary system KCl–SrCl2–H2O at 298 K (in Chinese). Chin. J. Inorg. Chem. 26, 333–338 (2010)
Bi, Y.J., Sun, B., Zhao, J., Song, P.S., Li, W.: Phase equilibrium in ternary system SrCl2–CaCl2–H2O at 25 °C (in Chinese). Chin. J. Inorg. Chem. 27, 1765–1771 (2011)
Li, D.W., Sang, S.H., Cui, R.Z.: Phase equilibria in the ternary system MgCl2–SrCl2–H2O at 323 K and 348 K (in Chinese). J. Sichuan Univ. (Nat. Sci. Ed.) 52, 638–644 (2015)
Li, D.W., Sang, S.H., Cui, R.Z., Wei, C.: Solid–liquid equilibria in the ternary systems NaCl–SrCl2–H2O and KCl–SrCl2–H2O at 348 K. J. Chem. Eng. Data 60, 1227–1232 (2015)
Zhang, X., Sang, S.H., Zhong, S.Y., Huang, W.Y.: Equilibria in the ternary system SrCl2 − KCl − H2O and the quaternary system SrCl2 − KCl − NaCl − H2O at 323 K. Russ. J. Phys. Chem. A 89, 2322–2326 (2015)
Hu, Y.X., Sang, S.H., Cui, R.Z., Zhong, S.Y.: Phase equilibria in the ternary system KBr–KCl–H2O at 373 K (in Chinese). Chin. Sci. Paper. 8, 847–850 (2013)
Zhang, X.P., Zhang, W.Y., Wang, D., Zhang, H., Sang, S.H.: Measurement of mineral solubilities in the ternary systems NaCl–ZnCl2–H2O and MgCl2–ZnCl2–H2O at 373 K. Russ. J. Inorg. Chem. 62, 995–1002 (2017)
Lu, H.J., Wang, J.K., Yu, J., Wu, Y.F., Wang, T., Bao, Y., Ma, D., Hao, H.X.: Phase equilibria for the pseudo-ternary system NaCl–Na2SO4–H2O of coal gasification wastewater at T = 268.15 to 373.15 K. Chin. J. Chem. Eng. 25, 955–962 (2017)
Acknowledgements
This project was supported by the National Natural Science Foundation of China (41373062, U1407108) and scientific research and innovation team in Universities of Sichuan Provincial Department of Education (15TD0009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Gao, YY., He, XF., Zhang, WY. et al. Phase Equilibria in the Ternary System MgCl2 + SrCl2 + H2O and the Quaternary Systems NaCl + MgCl2 + SrCl2 + H2O and KCl + MgCl2 + SrCl2 + H2O at 373 K. J Solution Chem 47, 1157–1171 (2018). https://doi.org/10.1007/s10953-018-0781-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-018-0781-5