Abstract
Solubility isotherms of the sparingly soluble salts CaF2(s) and CaSO4·2H2O(s) in their mixed aqueous solutions have been measured at 298.1 K. It was found that the CaF2(s) solubility decreases with increasing CaSO4 concentration in the solution and reaches about 1/3 of the CaF2(s) solubility in pure water in the CaSO4·2H2O(S) saturated solution. A thermodynamic model was developed to predict the CaF2(s) solubility isotherm in this system, in which the short range interactions of the species in the aqueous solution are represented by ion-association constants reported in literature, and the long range interaction, i.e., the electrostatic term, is represented by the well known Davies equation. The predicted solubility isotherm reasonably agrees with the experimental results. The contributions of the long-range term and the short-range term to the calculated solubility isotherm were investigated. It was concluded that the ionic association combining with the Davies equation is sufficient to represent the excess interaction of the CaF2 + CaSO4 aqueous solution at 298.15 K. This model approach could be applicable for other dilute mixed electrolyte systems in which component activity coefficients are lacking and model parameters are difficult to determine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fluoride ion in electrolyte solutions is corrosive for the anode and cathode in the zinc hydrometallurgical process and needs to be removed from the solution to the level of about 50 mg·L−1. Usually, the zinc hydrometallurgical system contains the electrolytes ZnSO4, MnSO4, MgSO4, CaSO4, Fe2(SO4)3 and H2SO4. It is well known that many fluorides (i.e., CaF2, MgF2 and LnF3) possess low solubility in pure water, [1, 2] as is shown in Table 1. If the fluorides’ solubilities in the zinc hydrometallurgical systems were the same as in pure water, then the fluoride ions in the multi-component systems could be expected to be removed by precipitating fluorine ions as sparingly soluble fluorides. To answer this question one need to know the solubility behavior of sparingly soluble fluorides in the systems MF x –NSO4–H2O (MF x = CaF2, MgF2, LnF3; N = Ca, Mg, Zn, Mn, H). However, we are astonished to learn by surveying the literature that no fluoride solubility data in the above systems have been reported in any publication. Because of this, we plan to carry out a series of solubility measurements. The first system is the CaF2 solubility in the CaF2–CaSO4–H2O system at 298.1 K.
2 Experimental
2.1 Materials
CaF2 (Xilong Chemical Co., Ltd., China) of purity of 98.5% was washed three times before use in this work. CaSO4·2H2O(s) (Sinapharm Chemical Reagent Co., Ltd., China) with purity of 99% was used directly in this work without further purification. Deionized water was used after distillation in this work.
2.2 Procedure
Two groups of equilibrium experiments were carried out to determine the solubility isotherms of CaF2(S) and CaSO4·2H2O(S) separately. In the first group of experiments, 5 grams CaF2(s) and about 200 mL deionized water were added to several polytetrafluoroethylene (PTFE) flasks, separately, and then various trace amounts of CaSO4 were added in the flasks to guarantee that the equilibrium solid phase was CaF2(S) only. In the second group of experiments, 5 g CaSO4·2H2O(S), a certain amount of saturation solution of CaF2(S) in pure water at about 298 K, and a certain amount of pure water was added to each flask, so that the solution was in equilibrium with CaSO4·2H2O(S) rather than CaF2(S). All the flasks were placed in a thermostat whose temperature was controlled by a heater (Lauda E200, Germany) and a cooler (Lauda DLK 25, Germany) to 298.1 K with temperature stability of ± 0.01 K. The thermostat temperature was calibrated with a calibrated glass thermometer (Miller & Weber, Inc., USA). The mixture in each flask was stirred by a magnetic stirrer inside the flask driven by a magnetic rotor placed outside the thermostat. Our pre-equilibrium experiments showed that 30–35 days are needed for CaF2(S) to reach equilibrium with aqueous solution; thus 40 equilibrium days were used in the actual equilibrium experiments for CaF2(S). As mentioned in our previous work [3], 120 h is needed for the equilibrium with CaSO4·2H2O(S), which was followed in this work. When equilibrium was reached, the solution was pumped out through a PTFE filtration film with pore diameter of about 5 × 10−7 m. The content of the filtrate was analyzed by the following methods.
2.3 Analysis Methods
The \( {\text{SO}}_{4}^{2 - } \) content in the solution was gravimetrically analyzed by precipitating as BaSO4, Ca2+ content was analyzed by titration with ethylene diamine tetraacetic acid disodium salt (EDTA), while the F− content in the solution was analyzed by a fluoride ions selective electrode (PF-1-01, INESA Scientific Instrument Co., Ltd.). The procedure for the determination of fluoride ions is described in Ref. [4]. Before the sample measurements, the parameter a and b in the equation:
which relates the electro potential and F− concentration, was determined first by the following procedure: (1) a 0.001 mol·dm−3 NaF solution was prepared in 250 mL polytetrafluoroethylene (PTFE) volumetric flasks; (2) 10, 20, 40 and 80 mL NaF solution were pipetted into four 100 mL glass volumetric flasks, respectively; (3) 10 mL total ionic strength adjustment buffer (TISAB: 1 mol·dm−3 NaCl, 0.25 mol·dm−3 acetic acid, 0.75 mol·dm−3 sodium acetate, and sodium citrate 0.001 mol·dm−3) was added to each of the four volumetric flask; (4) finally, pure water was added in each flask to the 100 mL mark line. After thorough mixing, the solution was taken out for potentiometric measurement under magnetic stirring condition (stirring speed: 30 r·m−1). Based on the measurement results, the parameters a and b in Eq. 1 were determined. After equilibrium, a 30 mL sample solution was pipetted in 100 mL volumetric flash and analyzed by the same procedure. According to Eq. 1, the fluorine concentration in the sample solution can be calculated.
3 Experimental Results and Discussions
3.1 Experimental Results
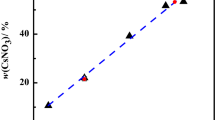
The obtained experimental data of the solubility isotherms for CaF2(s) and CaSO4·2H2O(s) are tabulated in Table 2 and illustrated in Fig. 1.
As shown in Fig. 1, the CaF2(s) solubility decreases with increasing CaSO4 concentration in the solution; when the CaSO4 concentration reaches its saturation limit, the CaF2(s) solubility decreases to about 1/3 of its solubility in pure water.
3.2 Modeling
To understand the CaF2 solubility behavior in aqueous CaSO4 solutions and the essence behind the solubility phenomena, thermodynamic modeling of the solubility was carried out. For this task a thermodynamic model should first be chosen. However, no matter what model is chosen, its binary model parameters should be determined before it can be used for multicomponent system. Since the experimental data of water activity or mean activity coefficient of the system CaF2 + H2O, which are usually necessary for parameterization, are unavailable up to now, a concise thermodynamic model should be developed so that the modeling can be started without these important basic data.
Actually, what any thermodynamic model represents is the excess properties relative to an assumed ideal system. In this work, it is assumed that the aqueous solution consists of ions, association species (ions or neutral molecules) and water molecules. Since the salt concentrations in the systems considered in this work are quite dilute, the contribution of the long range electrostatic interaction to the excess properties should be non negligible and will be expressed by Davies’ equation [5]:
where \( \gamma_{i} \), \( Z_{i} \) and \( I = 0.5\sum\limits_{i} {Z_{i}^{2} m_{i} } \) are ionic activity coefficient, ionic charge and ionic strength of the solution, respectively.
Much evidence shows that there is association between \( {\text{Ca}}_{{ ( {\text{aq)}}}}^{{ 2 { + }}} \) and \( {\text{F}}_{{ ( {\text{aq)}}}}^{ - } \) and between \( {\text{Ca}}_{{({\text{aq}})}}^{2 + } \) and \( {\text{SO}}_{4}^{2 - } \) (aq) that could be the main short-range interaction. In this work, it is assumed that the aqueous system consists of the ions \( {\text{Ca}}_{{({\text{aq}})}}^{2 + } \), \( {\text{F}}_{{({\text{aq}})}}^{ - } \) and \( {\text{SO}}_{4\, (\rm{aq})}^{2 - } \) and the association species \( {\text{CaF}}_{{ ( {\text{aq)}}}}^{ + } \), CaF2(aq) and CaSO4(aq) and water molecules. The species (ions and associated species) concentrations in a certain total salts concentrations of CaF2 and CaSO4 can be calculated using the association constants listed in Table 3 in the following procedure:
The activity \( a_{i} \) of the species \( i \) is the product of its activity coefficient and molality:
The molality \( m_{i} \) and activity \( a_{i} \) of each species \( i \) in a certain solution is obtained by iterative calculations subjected to the association constants in Table 2.
Before the CaF2(s) solubility isotherm in the ternary system CaF2 + CaSO4 + H2O can be calculated, the solubility product constant should first be determined through the solid–liquid equilibrium in the binary system CaF2 + H2O:
The chemical potential (\( \mu \)) of the solid phase CaF2(s) equals the corresponding potentials of the species in aqueous solution, i.e.,
where \( \mu_{i}^{ * } \) and \( a_{i} \) are chemical potential in reference state and activity of the species \( i \), respectively.
In the solution saturated with the CaF2(s) phase, the total CaF2 molality \( m_{{{\text{CaF}}_{ 2} }}^{\text{t}} \) in the solution is \( 2.04 \times 10^{ - 4} \) mol·kg−1 as shown in Table 1. By iterative calculations, we obtain the species molalities \( m_{{{\text{Ca}}^{2 + }_{{ ( {\text{aq)}}}} }} = 1.91 \times 10_{{}}^{ - 4} \), \( m_{{{\text{CaF}}^{ + }_{{ ( {\text{aq)}}}} }} = 8.2 \times 10^{ - 7} \), \( m_{{{\text{CaF}}_{{ 2\,\, ( {\text{aq)}}}} }} = 1.2 \times 10^{ - 5} \) and \( m_{{{\text{F}}^{ - }_{{ ( {\text{aq)}}}} }} = 3.84 \times 10^{ - 4} \) mol·kg−1 in the solution and the solubility product constant \( k_{{{\text{CaF}}_{{ 2 ( {\text{s)}}}} }} { = }10^{ - 10.62} \).
Applying the solubility product as a criterion, one can obtain by calculation the CaF2(s) solubility isotherm, as shown by the solid line in Fig. 2a. It can be seen that the predicted results agree with the experimental data quite well. To investigate the contribution of the long-range term (Eq. 2) on the predicted solubility isotherm, we set all \( \gamma_{i} = 1 \) in Eq. 2 and repeat the above calculation procedure, obtaining the predicted solubility isotherm of CaF2(s), as shown by the dash line in Fig. 2a. It can be observed that the calculated solubility isotherm obviously goes down, deviating from the experimental results.
The association constant for the second-order reaction \( {\text{Ca}}_{{({\text{aq}})}}^{2 + } \, + \,2{\text{F}}_{{ ( {\text{aq}})}}^{ - } \, \rightleftharpoons \,{\text{CaF}}_{{ 2 ( {\text{aq)}}}} \) was unknown for a long time, until Fovet and Gal [7] reported its value to be 105.7, much larger than the that for the first-order reaction \( {\text{Ca}}_{{({\text{aq}})}}^{2 + } \, + \,{\text{F}}_{{({\text{aq}})}}^{ - } \, \rightleftharpoons \,{\text{CaF}}_{{ ( {\text{aq)}}}} \). To test the contribution of the second-order constant on the predicted solubility isotherm, we omit the second-order association constant and keep the first-order association constant for \( {\text{CaF}}_{{ ( {\text{aq)}}}}^{ + } \) and CaSO4(aq), obtaining the calculated results as shown by the solid line in Fig. 2b, which deviates remarkably from the experimental results. The second-order association reaction makes an important contribution to the solubility prediction. Furthermore, we omitted the long-range term by setting all \( \gamma_{i} = 1 \), repeating the above calculation procedure yields the calculated solubility isotherm as shown by the dashed line in Fig. 2b. As expected, the calculated results (dashed line in Fig. 2b) without the long-range term are worse.
Now that the solubility isotherm predicted with the first- and second-order association reaction and the Davies equation agree with the experimental results very well, we suppose that the present thermodynamic model has grasped the essential character of this aqueous system. To understand the structurale change in the system, we calculated the species distribution along the CaF2(s) solubility isotherm and obtained the results shown in Fig. 3. As shown in Fig. 3a, in the CaF2(s) saturated solutions with relatively low CaSO4 concentration, CaSO4 exists in the solution mainly as the dissociated ions \( {\text{Ca}}_{{({\text{aq}})}}^{2 + } \) and \( {\text{SO}}_{4 \, (\rm{aq})}^{2 - } \), and the amount of associated species CaSO4(aq) increases with the increasing total CaSO4 concentration, up to about 50% of the total concentration. Along the CaF2(s) solubility isotherm, the fluoride exists in the aqueous solution mainly as \( {\text{F}}_{{({\text{aq}})}}^{ - } \) and its concentration decreases with decreasing CaF2(s) solubility, while the concentrations of the associated species \( {\text{CaF}}_{{({\text{aq}})}}^{ + } \) and CaF2(aq) remain relatively constant.
4 Conclusions
The solubility isotherms of the solids CaF2(s) and CaSO4·2H2O(s) were measured at 298.1 K. It was found that the CaF2(s) solubility decreases with increasing CaSO4 concentration to about 1/3 of its solubility in pure water in the CaSO4·2H2O(s) saturation solution. A thermodynamic model was developed to represent the properties of the CaF2 + CaSO4 + H2O system, in which the long-range interaction is represented by Davies’ equation and the short-range interaction is represented by ionic association. The CaF2(s) solubility isotherm predicted by the thermodynamic model agrees with the experimental data very well. The contributions of the long-range term (Davies equation) and short-range terms (first and second order of association) to the predicted solubility results have been compared and discussed. A conclusion can be drawn in this work that the excess interaction in the CaF2 + CaSO4 + H2O system can be comprehensively represented by the long-range electrostatic term and the ion association reactions. This model approach could be applicable to the solubility prediction of other similar systems containing sparingly soluble fluoride salts in dilute solution, in which component activities are unavailable and model parameters are difficult to determine.
References
Linke, W.F., Seidell, A. (eds.): Solubility: Inorganic and Metal–Organic Compounds. American Chemical Society, Washington (1965)
Mioduski, T., Gumiński, C., Zeng, D.: IUPAC-NIST solubility data series. 100. Rare earth metal fluordes in water and aqueous systems. Part 1. Scandium group (Sc, Y, La). J. Phys. Chem. Ref. Data 43, 012105-1–012105-48 (2014)
Zeng, D., Wang, W.: Solubility phenomena involving CaSO4 in hydrometallurgical processes concerning heavy metals. Pure Appl. Chem. 83, 1045–1061 (2011)
Light, T.S., Cappuccino, C.C.: Determination of fluoride in toothpaste using an ion-selective electrode. J. Chem. Educ. 52(4), 247–250 (1975)
Davies, L.W.: Ion Association. Butterworths, London (1962)
Martell, A.E., Smith, R.M.: Critical Stability Constants, Inorganic Complexes, vol. 4. Springer, New York (1976)
Fovet, Y., Gal, J.-Y.: Formation constants β 2 of calcium and magnesium fluorides at 25 °C. Talanta 53, 617–626 (2000)
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Nos. 51334008 and 21776316).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Wang, S. & Zeng, D. Solubility and Ionic Interaction of the CaF2–CaSO4–H2O System at 298.1 K. J Solution Chem 46, 1941–1947 (2017). https://doi.org/10.1007/s10953-017-0674-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0674-z