Abstract
Protonation constants of methyl/nitro substituted 1,10-phenanthrolines {(m/n-sphen): 4-methyl-phenanthroline (4-mphen), 5-methyl-1,10-phenanthroline (5-mphen), 4,7-dimethyl-1,10-phenanthroline (dmphen), 3,4,7,8-tetramethyl-1,10-phenanthroline (tmphen) and 5-nitro-1,10-phenanthroline (5-nphen)] and the amino acids (aa) l-tyrosine (tyr) and glycine (gly), and their corresponding binary and ternary stability constants with Cu(II), were determined in aqueous 0.1 mol·L−1 KCl ionic media at 298.15 K. The protonation constants of the ligands and the stability constants of the binary and ternary complexes of Cu(II) with the ligands were calculated from the potentiometric data using the “BEST” software package. The species distribution diagrams were obtained using the “SPE” software package under the experimental conditions described. The order of stability of the ternary complexes in terms of the primary ligands is [Cu(tmphen)(aa)]+ > [Cu(dmphen)(aa)]+ > [Cu(4-mphen)(aa)]+ > [Cu(5-mphen)(aa)]+ > [Cu(5-nphen)(aa)]+. The stability constants of the ternary complexes decrease in the following order: [Cu(m/n-sphen)(gly)]+ > [Cu(m/n-sphen)(tyr)]+, which is identical to the sequence found for the binary complexes of Cu(II) with gly and tyr.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
There is a growing interest in studying the properties of amino acids and 1,10-phenanthroline derivatives. Copper is a bio-essential transition metal ion that is known to form very stable binary as well as ternary complexes with various compounds [1]. 1,10-Phenanthroline derivatives are nitrogen-donor bidentate ligands with relatively high affinities for copper [2–4]. Their extended aromatic ring system allows these ligands to bind to DNA by intercalative and non-intercalative interactions, either as free ligands or in metal complexes [5, 6]. The amino acids are the chemical units of proteins that constitute the structure for all living organisms and are essential for various biochemical processes that support the maintenance of life in individual organisms [7]. They are good chelating agents and can coordinate with transition metals, either through their amino or carboxyl groups.
Quite recently, we reported that water soluble binary and ternary copper(II) complexes of l-tyrosine and substituted 1,10-phenanthrolines (1,10-phenanthroline, 4,7-dimethyl-1,10-phenanthroline, 5-nitro-1,10-phenanthroline) bind to and cleave DNA and also exhibit cytotoxic activity that is greater than that of cisplatin [5, 6]. We have also reported that a ternary copper(II) complex of l-leucine and substituted 1,10-phenanthroline (4,7-diphenyl-1,10-phenanthroline) binds DNA/BSA and also displays antioxidant/radical scavenging activities [8]. It is essential to investigate the formation of ternary complexes of copper(II) with substituted 1,10-phenanthrolines with amino acids as a contribution to the knowledge of the speciation of copper(II) complexes in biological systems. For this reason, accurate determination of the protonation and stability constant values is fundamental to understanding the behavior of ligands and their interaction with metal ions in aqueous solution. Potentiometric titration is accepted as a powerful and simple electroanalytical technique for the determination of stability constants. Our previous potentiometric titration studies in aqueous solution include the study of biologically important ligands such as ATP [9], amino acids [9, 10], catechols [11], catecholamines [12, 13].
Considering the facts mentioned above, in this paper we investigate the stability constants of binary and ternary copper(II) complexes with methyl/nitro-substituted 1,10-phenanthroline {[m/n-sphen]: 4-methyl-phenanthroline (4-mphen), 5-methyl-1,10-phenanthroline (5-mphen), 4,7-dimethyl-1,10-phenanthroline (dmphen), 3,4,7,8-tetramethyl-1,10-phenanthroline (tmphen) and 5-nitro-1,10-phenanthroline (5-nphen)] and selected amino acids {[aa]: l-tyrosine (tyr) and glycine (gly)} in aqueous solution (Fig. 1). A thorough literature review showed that no similar work has been published on systems involving copper(II) with 4-mphen, 5-mphen, dmphen, tmphen and 5-nphen as primary ligands and tyr and gly as a secondary ligand. Additionally, no work seems to have been reported on the protonation constant of 4-mphen or study of complexation between copper(II) and 4-mphen in aqueous solutions. In this study, the protonation constants of ligands. and the stability constants of the binary and the mixed ligand complexes formed with copper(II), have been determined by potentiometric methods in 0.1 mol·L−1 KCl media at 298.15 K. The protonation constants of the ligands and the stability constants of the complexes have been calculated with the BEST software package [14]. In addition, the distribution of complex species in these systems is graphically presented using the SPE software package [14]. The tendency of these metals and ligands to form binary or ternary complexes was also evaluated and was analyzed by calculating their Δlog10 K values.
2 Experimental
2.1 Materials and Reagents
All ligands were purchased from Sigma–Aldrich (99% purity) except dmphen (Alfa Aesar). All ligands were treated with excess HCl in aqueous solution to yield the protonated species (H2L2+). Copper(II) chloride was purchased from Merck (>98% purity). A copper(II) chloride solution was prepared in concentrated HCl (Merck, 37% assay). The stock solution of copper(II) chloride was standardized complexometrically with the disodium salt of ethylenediaminetetraacetic acid (Na2EDTA, 99%) using a suitable indicator [15]. Excess acid in the Cu(II) stock solution was determined by potentiometric titration as described previously [9–12]. A carbonate-free KOH (Fluka, 86% purity) solution was prepared and standardized potentiometrically against the primary standard potassium hydrogen phthalate (Merck, 99.9%) [16, 17]. A hydrochloric acid solution (0.1 mol·L−1) was prepared and standardized by titration against the standard potassium hydroxide. Potassium chloride (Merck) was of p.a. reagent grade and was used as a background electrolyte. The ionic strength of each solution was adjusted to 0.1 mol·L−1 by the addition of KCl as supporting electrolyte. All solutions were prepared with analytical grade water (R = 18 MΩ cm) using grade A glassware.
2.2 Procedure, Measurements and Data Processing
Potentiometric titrations were carried out using a Schott TitroLine Alpha Plus automatic titrator equipped with a Schott combined pH electrode. A double-walled titration cell with inlet and outlet connections providing water circulation was used to carry out the pH titrations. The titration cell was thermostatted at 298.2 ± 0.1 K using a VWR 11405 circulating thermostat. The volumes were made up to 50 mL with deionized water, prior to the titrations being performed. The overall experimental procedure involved potentiometric titrations of the following solutions:
-
(a)
5 mL of 0.1 mol·L−1 HCl + 5 mL of 1 mol·L−1 KCl (for cell calibration).
-
(b)
Solution from (a) + 0.1 mmol dmphen/nphen + 0.1 mmol HCl (for the determination of the protonation constants of the dmphen/nphen),
-
(c)
Solution from (a) + 0.1 mmol gly + 0.1 mmol HCl (for the determination of the protonation constants of gly).
-
(d)
Solution from (b) + 0.1 mmol copper(II) (for the determination of the stability constants of the MA binary complexes).
-
(e)
Solution from (c) + 0.1 mmol copper(II) (for the determination of the stability constants of the MB binary complexes).
-
(f)
Solution from (b) + (0.1 mmol gly + 0.1 mmol HCl) + 0.1 mmol copper(II) (for the determination of stability constants of MAB+ ternary complexes).
The solutions were stirred magnetically under a continuous flow of N2 gas. The ionic strength was adjusted with 0.1 mol·L−1 KCl. The ligand/metal + ligand solutions were titrated against 0.1 mol·L−1 KOH in an atmosphere of pure N2 gas, and the potential difference values were recorded in mV. The potentiometric cell was calibrated before each experiment to allow measurement of the hydrogen ion concentration rather than its activity. For this purpose, the standardized HCl solution was titrated with KOH solution. From the titration data in the acidic region, an equation was obtained \( ({{E(mV)}}{\kern 1pt} {\kern 1pt} = {\kern 1pt} {{E}}_{\text{cell}}^{\text{o}} \, + \,{{S}}\log [{\text{H}}^{ + } ]\, + \,{{E}}_{\text{j}} ) \). In this equation, \( {{E}}_{\text{cell}}^{\text{o}} \) is the standard potential in this cell, S is the electrode slope and E j is the liquid junction potential value, which depends only on the ionic strength. When the potential values were measured for the ligand or metal + ligand solutions, pH values were calculated from the potential value using the above equation. From the titration data in the basic region, K w values were calculated from several separate [H+] and [OH−] measurements in 0.1 mol L−1 KCl [14, 15]. The daily-calculated pK w values varied between 13.60 and 13.70. Potentiometric titrations were carried out using three different metal (or ligand) concentrations (between 1.0 × 10−3 and 4.0 × 10−3 mol·L−1).
The stability constant, β pqrs , is defined by Eqs. 1 and 2 (charges are omitted for simplicity), where p, q, r, and s are the moles of Cu, A (m/n-sphen), B (amino acids), and proton (H), respectively, in the complex Cu p (A) q (B) r H s . The log10 β pqrs values were calculated by using the BEST [14] software package. The species-distribution diagrams were obtained with the SPE software package [14] using the experimental conditions employed.
The overall experimental procedure involving potentiometric titrations was carried out as previously reported [9–13]. The protonation constants of dmphen and nphen, and stability constants of binary [Cu(dmphen)]2+ and [Cu(5-nphen)]2+ systems were taken from our previous study [10].
3 Results and Discussion
3.1 Protonation Constants of the Ligands
Potentiometric titrations of solutions containing amino acid in excess HCl were performed with 0.1 mol·L−1 KOH solution in 0.1 mol·L−1 KCl ionic media at 298.15 K. The observed pH values were plotted against m (mmol base/mmol ligand). The potentiometric titration curves are labeled as “I” in Figs. 2 and 3. The protonated gly (H2B+) has acidic protons on both the amine and carboxyl termini of the molecules. Tyr can be written as H2BH+ due to the –OH group in the aromatic ring, where the “H” on the right side of B is the phenolic hydroxyl proton. In the titrations of the amino acid ligands, an inflection point was observed at m = 1.0. The proton of the α–COOH moiety is neutralized in the acidic region of the titrations, while the second proton, bound to the amine nitrogen (\( - {\text{NH}}_{3}^{ + } \)), is neutralized in the basic region (pH = 8.0–10.0). Only for tyr, with the third proton dissociating above pH = 10, is the proton of the –OH group connected with the aromatic ring.
Potentiometric titration curves of the systems studied in 0.1 mol·L−1 KCl ionic media at 298.2 K, TCu = 1.5 × 10−4 mol·L−1: a I, tyr alone; II, 4-mphen alone; III, Cu(II)–tyr (1:1); IV, Cu(II)–4-mphen (1:1); V, Cu(II)–4-mphen–tyr (1:1:1). b I, tyr alone, II, 5-mphen alone; III, Cu(II)–tyr (1:1); IV, Cu(II)–5-mphen (1:1); V, Cu(II)–5-mphen–tyr (1:1:1)
Potentiometric titration curves of the systems studied in 0.1 mol·L−1 KCl ionic media at 298.2 K, TCu = 1.5 × 10−4 mol·L−1: a I, gly alone; II, tmphen alone; III, Cu(II)–gly (1:1); IV, Cu(II)–tmphen (1:1); V, Cu(II)–tmphen–gly (1:1:1). b I, gly alone; II, dmphen alone; III, Cu(II)–gly (1:1); IV, Cu(II)–dmphen (1:1); V, Cu(II)–dmphen–gly (1:1:1)
Potentiometric titrations of solutions containing protonated m/n-sphen and excess HCl (5 equivalents with respect to the ligand) were performed with 0.1 mol·L−1 KOH solution in 0.1 mol·L−1 KCl ionic media at 298.15 K. The resulting titration curves are labeled as “II” in Figs. 2 and 3. In this study, protonated 4-mphen, 5-mphen, dmphen, tmphen and 5-nphen are shown as H2A2+, and the two protons were neutralized during the titrations. The first nitrogen-bound acidic proton is neutralized in the buffer zone (m = 0.0–1.0) in the pH range of 2.0–3.0. The second acidic proton is neutralized at higher pH. The 4-mphen, 5-mphen, dmphen, tmphen and 5-nphen ligands have two nitrogens but we could only measure one protonation constant. It was impossible to measure the second protonation constant because it occurs in a very acidic region that cannot be measured with a glass electrode due to the high contribution of the liquid junction potential [18]. Protonation constants of the ligands in this study were calculated with the BEST software applied to the potentiometric titration data. From the experimental data shown in Tables 1 and 2, it is noted that the protonation constants of ligands obtained in this work are consistent with those reported in the literature within a very reasonable difference range [10, 19–22].
3.2 Binary Complexes of Amino Acids
The potentiometric titrations of the Cu(II)–aa systems (aa: amino acid, B: tyr and gly) were performed at 1:1 molar ratios (Figs. 2 and 3, curves III). In each of the 1:1 Cu(II):aa systems, two inflection points were observed at m = 2.0 and 3.0. In the m = 0.0–2.0 buffer zone, CuB+ (for gly) and Cu(BH)+ (for tyr) complexes are observed along with initial formation of the hydroxo complex at m = 2.0. In Figs. 2 and 3, curve III, the hydrolysis of CuB+ and Cu(BH)+ complexes are shown by the dashed line. In all of the amino acid systems, the difference between the titration curves of the ligand alone and metal + ligand solutions (ΔpH) is approximately 5.0. The observed decrease in the binary CuB+ and Cu(BH)+ curves in comparison to the free ligand solution curve indicates formation of the binary complexes in solution. The equilibria involved in the Cu(II):gly system can be described by the following equations, where, β is the overall stability constant of the complex, K is the stepwise stability constant, and H2B+ refers to the protonated gly. (In the equations below, the ligand and the complexes for the Cu–tyr system can be represented as H2BH+, Cu(BH)+ and CuB(OH), respectively):
Taking into account all the possible species, the stability constants of the binary complexes were calculated from the potentiometric titration data using the BEST software package. The experimental data is reported in Table 3. The stability constants found in our study are in good agreement with the data reported in the literature [10, 22]. The results show that the stability order for the binary complexes, in terms of the amino acids, is [Cu(gly)]+ > [Cu(tyr)]+. It is suggested that the absence of an aromatic ring in tyr is responsible for the lower stability of its complex.
3.3 Binary Complexes of Methyl/Nitro Substituted 1,10-Phenanthrolines
The potentiometric titrations of the Cu(II):m/n-sphen systems (m/n-sphen (A): 4-mphen, 5-mphen, dmphen, tmphen and 5-nphen) were performed in 0.1 mol·L−1 KCl media at 298.15 K. Initially, the solid ligand and excess HCl solution were added to the potentiometric cell. The titration curves drawn for Cu(II):m/n-sphen systems at a 1:1 molar ratio are shown as curve IV in Figs. 2 and 3. Two inflection points are observed at m = 2.0 and 3.0. The observed decrease in the binary CuA2+ curve in comparison to the free ligand solution curve indicates formation of binary complexes in solution. The observation of an inflection point at m = 2.0 indicates the existence of a CuA2+ complex. A CuA(OH)+ hydroxo complex begins to form after m = 2.0. Hydrolysis of the CuA2+ complex is shown by the dashed line in Figs. 2 and 3, curve IV. The equilibria involved in the Cu(II):m/n-sphen systems are described by the following equations, where β is the overall stability constant of the complex, and K is the stepwise stability constant:
In the 1:1 molar ratio potentiometric titrations, the stability constants of the binary complexes (CuA2+ and CuA(OH)+) were calculated using the BEST software package. The stepwise and overall stability constants of the binary complexes are listed in Table 3. The calculated log10 β ML values are 8.89, 7.57, 6.96, 6.66 and 5.27, respectively, for [Cu(tmphen)]2+, [Cu(dmphen)]2+, [Cu(4-mphen)]2+, [Cu(5-mphen)]2+ and [Cu(5-nphen)]2+. Last of all, the log10 β ML values found here are in considerable disagreement with those in the literature [20, 23, 24]. The disagreement appears to be due to the fact that the literature data were obtained with different physical techniques (spectrophotometric, polarography, calorimetry, partition method, etc.) and at different temperatures, making comparison difficult. In addition, the data have often been obtained in different solvent media and at different concentrations.
3.4 Ternary Complexes
The potentiometric titrations of the 1:1:1 Cu(II):(m/n-sphen):(aa) systems were performed in 0.1 mol·L−1 KCl ionic media at 298.15 K. In all systems, only one inflection point was observed at m = 4.0 (Figs. 2 and 3, curve V). The protonated m/n-sphen and amino acid ligands each have two acidic protons. Thus, there are four total protons in a mixed-ligand environment involving both species.
The potentiometric titration curves of the Cu(II):(m/n-sphen):(aa) systems show a buffer zone at a lower pH than the curves of the ligands alone and indicate the titration of a total of four protons; thus, two ligands bind to the each metal ion. The formation reaction (Eq. 9) and the overall stability constant equation of the MAB+ ternary complexes, reaction (Eq. 10) are given by:
The stability constants of the ternary MAB-type complexes formed by Cu(II) with m/n-sphen and the amino acids have been calculated (log10 β MAB). In all systems, analysis of the mixed ligands results in the formation of only the MAB+ complex as shown in Table 4. The log10 β MAB values indicate that the stability of the MAB+ ternary complexes do not show which ligand (m/n-sphen or aa) binds more strongly with the Cu(II) ion. To identify which of the ligands are primary or secondary, the \( \log_{10} K_{\text{MAB}}^{\text{MA}} \) and \( \log_{10} K_{\text{MAB}}^{\text{MB}} \) constants were calculated with the following two equations:
The \( \log_{10} K_{\text{MAB}}^{\text{MA}} \) and \( \log_{10} K_{\text{MAB}}^{\text{MB}} \) values are listed in Table 4. The fact that the \( \log_{10} K_{\text{MAB}}^{\text{MA}} \) constants are higher in all studied systems indicates that the m/n-sphen ligand is the primary one in these complexes. The stabilities of the binary and ternary complexes can also be compared in another way. For example, the Δlog10 K parameter expresses the effect of the bound primary ligand on an incoming secondary ligand. The Δlog10 K parameters were calculated with the equation below and are listed in Table 4:
This reaction represents the following overall equilibrium, \( {\text{MA}}\, + {\text{MB}}\, \to {\text{MAB}}\, + \,{\text{M}} \); thus,
According to Sigel, when the ∆log10 K values are positive the ternary complexes are more stable than the corresponding binary complexes, while if the values for ∆log10 K are negative, then the reverse is true [25]. In all studied systems the Δlog10 K values are found to be positive, indicating that the ternary complexes are more stable than the binary complexes (Table 4). This was foreseen, as this stability order has been observed before when the primary ligand is m/n-sphen and the secondary ligand has an oxygen or nitrogen donor atom [26]. The reason for the extra stability of these complexes may be due to interactions outside the coordination sphere, such as the formation of hydrogen bonds between the coordinated ligands, charge neutralization, chelate effect and stacking interactions [27–29].
The protonation constants of the m/n-sphen and amino acids, along with their complex stability constants with Cu(II) in the binary systems, were introduced into the BEST software package program for determination of the stability constants of complexes in the ternary systems. The results are presented as the overall formation constants (log10 β MAB) in Table 4. The order of stability of the ternary complexes in terms of primary ligands is tmphen > dmphen > 4-mphen > 5-mphen > 5-nphen, and also the order of stability of the ternary complexes in terms of secondary ligands has been found to be gly > tyr. This behavior may reflect the nature of the electronic interaction of both the m/n-phen (tmphen, dmphen, 4-mphen, 5-mphen, 5-nphen) and amino acids (gly and tyr) with the Cu(II) ion.
Depending upon the nature of the ligands, and based on basic chemical knowledge, the structures of the ternary complexes are proposed as shown in Fig. 4. Substituted 1,10-phenanthrolines are strong bidentate ligands that form stable chelates with many transition metals. The amino acids act as bidentate ligands with coordination involving the carboxylic oxygen and nitrogen atom of the amino group. In this study, the Cu(II) ion can display square-pyramidal coordination where the water molecule is in the axial position and the base is defined by the N and one of the O atoms from the gly/tyr ligand, and both substituted 1,10-phenanthrolines N atoms, such as [Cu(gly)(dmphen)(H2O)]+. Additionally, Cu(II) can show octahedral geometry by two N-donor atoms of one, chelating substituted 1,10-phenanthrolines ligand, the carboxylate oxygen and the amino nitrogen atoms of gly/tyr, and two O donors from the coordinated water, such as [Cu(gly)(dmphen)(H2O)2]+.
3.5 Distribution Diagrams
Assessment of the concentration distribution of various complex species as a function of pH provides a useful description of metal ion binding in biological system. The concentration distribution of species occurring in all the binary and mixed-ligand systems was calculated using the SPE software. The distribution diagrams of the all mixed-ligand systems with Cu(II) are shown in Figs. 4, 5 and 6.
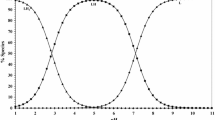
The species distribution curves for the [Cu(m/n-sphen)(tyr)]+ system are shown in Figs. 5a and 6a. The mixed ligand species [Cu(m/n-sphen)(tyr)]+ starts to form at pH = 2.0 and, with increasing pH, its concentration increases reaching a maximum of 99.0% at pH = 5.5. In other words, the [Cu(m/n-sphen)(tyr)]+ complex species is the main species in the physiological pH range. The predominant species are CuA2+, H2(BH)+, H(BH), Cu2+, HA+, CuA(BH)+ at pH = 2.0; CuA(BH)+, CuA2+, H(BH), HA+ at pH = 3.0; CuA(BH)+, CuA2+, H(BH) at pH = 4.0; and CuA(BH)+ at pH = 5.0 (Figs. 5b and 6b).
The concentration distribution curves for the [Cu(dmphen)(gly)]+ system are shown in Fig. 7a. At pH = 2.0, in the [Cu(dmphen)(gly)]+ system, the percentage concentration of CuA2+ are ca. 66% and, above pH = 2.0, the CuAB+ complex begins to form with its concentration reaching ~98% of the total Cu species between pH = 4.0 and 5.0. The predominant species are CuA2+, H2B+, HB, Cu2+, HA+ and CuAB+ at pH = 2.0; CuAB+, CuA2+, HB and HA+ at pH = 3.0; CuAB+, CuA2+ and HB at pH = 4.0; and CuAB+ at pH = 5.0 (Fig. 7b).
In the ternary systems, the [Cu(m/n-sphen)(tyr)]+ and [Cu(dmphen)(gly)]+ species occur at higher concentrations than their binary analogues, indicating preference for formation of ternary species, which agrees with predictions made from the Δlog10 K values.
4 Conclusions
In this study, protonation and complex formation equilibria of Cu(II) with m/n-sphen and amino acids were investigated using potentiometric methods in aqueous 0.1 mol·L−1 KCl ionic media at 298.15 K. The protonation constants of the ligands and the stability constants of the binary and ternary complexes of Cu(II) with the ligands were calculated from the potentiometric data using the “BEST” software package. The concentration distribution curves of each complex species in solution were also evaluated with the “SPE” software package. The species distribution curves show the formation of ternary complexes. The stabilities of the binary and ternary Cu(II) complexes formed by tmphen are higher than those of m/n-sphen; tmphen is strong Lewis base and has electron-donating methyl groups at positions 3, 4, 7 and 8. The order of stability of the ternary complexes in terms of amino acids is gly > tyr. It is proposed that the absence of an aromatic ring and steric effect in try is responsible for the lower stability of its complex. The experimental Δlog10 K parameters were calculated, showing the effect of the bound primary ligand toward an incoming secondary ligand. ∆log10 K values are positive; this indicates that the ternary complexes are more stable than the corresponding binary ones. The reported data in this study will make an important contribution to the literature. Thus, it is hoped that these data will be a significant contribution to workers carrying out mechanistic studies in biological media.
References
Kaim, W., Schwederski, B.: Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life. Wiley, New York (1996)
McBryde, W.A.E., Brisbin, D.A., Irving, H.: 1004. The stability of metal complexes of 1,10-phenanthroline and its analogues. Part III. 5-Methyl-1,10-phenanthroline. J. Chem. Soc., 5245–5253 (1962)
Irwing, H., Mellor, D.H.: 1003. The stability of metal complexes of 1,10-phenanthroline and its analogues. Part II. 2-Methyl- and 2,9-dimethyl-phenanthroline. J. Chem. Soc. 5237–5245 (1962)
Irwing, H., Mellor, D.H.: 1002. The stability of metal complexes of 1,10-phenanthroline and its analogues. Part I. 1,10-Phenanthroline and 2,2′-bipyridyl. J. Chem. Soc. 5222–5237 (1962)
İnci, D., Aydın, R., Yılmaz, D., Gençkal, H.M., Vatan, O., Çinkılıç, N., Zorlu, Y.: New water-soluble copper(II) complexes including 4,7-dimethyl-1,10-phenanthroline and l-tyrosine: synthesis, characterization, DNA interactions and cytotoxicities. Spectrochim. Acta A. 136(Part B), 761–770 (2015)
İnci, D., Aydın, R., Vatan, O., Yılmaz, D., Gençkal, H.M., Zorlu, Y., Cavaş, T.: Binary and ternary new water soluble copper(II) complexes of l-tyrosine and substituted 1,10-phenanthrolines: effect of substitution on DNA interactions and cytotoxicities. Spectrochim. Acta A 145, 313–324 (2015)
Garret, R.H., Grisham, C.M.: Biochemistry. Sanders, New York (1995)
İnci, D., Aydın, R., Zorlu, Y.: Affinity of a new copper(II) complex to DNA/BSA andantioxidant/radical scavenging activities: crystal structure of [Cu(4,7-diphenyl-1,10-phenanthroline)(leucine)(NO3)(H2O)]. J. Coord. Chem. 69, 2677–2696 (2016)
Aydın, R., Yırıkoğulları, A.: Potentiometric study on complexation of divalent transition metal ions with amino Acids and adenosine 5′-triphosphate. J. Chem. Eng. Data 55, 4794–4800 (2010)
İnci, D., Aydın, R.: Stabilities of the ternary complexes of copper(II) with substituted 1,10-phenanthrolines and some amino acids in aqueous solution. J. Solution Chem. 43, 711–726 (2014)
Aydın, R., Serbest, Z., Özer, U.: Formation of the complexes between lanthanum(III) ion and 5-sulfosalicylate,5-nitrosalicylate. Rev. Inorg. Chem. 25, 271–283 (2005)
Aydın, R.: Study on the interaction of yttrium(III) with adrenaline, noradrenaline, and dopamine. J. Chem. Eng. Data 52, 2400–2404 (2007)
Aydın, R., İnci, D.: Potentiometric and spectrophotometric studies of the complexation of lanthanum(III) with adrenaline, noradrenaline, and dopamine. J. Chem. Eng. Data 57, 967–973 (2012)
Martell, A.E., Motekaites, R.J.: Determination and Use of Stability Constants. VCH Publishers, New York (1989)
Schwarzenbach, G., Flaschka, A.: Complexometric Titrations. Methuen, New York (1969)
Gran, G.: Determination of the equivalent point in potentiometric titrations. Acta Chem. Scand. 4, 559–577 (1950)
Rossotti, F.J.C., Rossotti, H.: Potentiometric titrations using Gran plots. J. Chem. Educ. 42, 375–378 (1965)
Soliman, A.A., El-Sherif, A.A., Amin, M.A.: Thermodynamics, chemical speciation and complex formation equilibria studies of binary and mixed ligand complexes of Cu(II) with 2,2′-bipyridyl and some aromatic diamines. J. Solution Chem. 44, 77–99 (2015)
Brandt, W.W., Gullstrom, D.K.: Studies on some ferrous complexes of substituted 1,10-phenanthrolines. J. Am. Chem. Soc. 74, 3532–3535 (1952)
The stability of metallic complexes of two dimethyl-phenanthrolines: Brisbin, D.A., MCbryde, W.A.E. Can. J. Chem. 41, 1135–1141 (1963)
Schilt, A.A., Smith, G.F.: Acid dissociation constants of substituted 1,10-phenanthrolines. J. Phys. Chem. 60, 1546–1548 (1956)
Demirelli, H., Köseoğlu, F.: Equilibrium studies of Schiff bases and their complexes with Ni(II), Cu(II) and Zn(II) derived from salicylaldehyde and some α-amino acids. J. Solution Chem. 34, 561–577 (2005)
Tanaka, M., Tabata, M.: Stability constants of metal(II) complexes with amines and aminocarboxylates with special reference to chelation. Bull. Chem. Soc. Jpn. 82, 1258–1265 (2009)
Banks, C.V., Bystroff, R.I.: Stability orders in transition metal-1,10-phenanthroline complexes. J. Am. Chem. Soc. 81, 6153–6158 (1959)
Sigel, H.: Ternary Cu2+ complex: stability, structure and reactivity. Angew. Chem. Int. Ed. Engl. 14, 394–402 (1975)
Patel, P.J., Patel, V.K., Bhattachanya, P.K.: Effect of two ligands on ternary complex stability. Inorg. Chem. 21, 3163–3166 (1982)
Watanabe, S., Saito, T.: Circular dichroism and ultraviolet studies of the cupric(II) and nickel(II)–3,4-dihydroxyphenylalanine complex. J. Inorg. Biochem. 58, 147–155 (1995)
Yamauchi, O., Sakurai, T., Nakahara, A.: Stereo selectivity in mixed ligand copper(II) complexes with electrostatic ligand–ligand interactions. Application to optical resolution of α-amino acids with a charged side chain. Bull. Chem. Soc. Jpn. 50, 1776–1779 (1977)
Sakurai, T., Yamauchi, O., Nakahara, A.: Mixed ligand copper(II) complexes of α-amino acids with ligand–ligand interactions. Bull. Chem. Soc. Jpn. 49, 169–173 (1976)
Acknowledgements
We thank the Research Fund of Uludag University for the financial support given to the research project (Project Numbers OUAP (F)-2015/14) and the Scientific and Technological Research Council of Turkey (TUBITAK) for a Domestic PhD Scholarship intended for priority areas of the first author (Code: 2211-C). This study is a part of a doctoral thesis in progress of the first author at the Graduate School of Natural and Applied Sciences of Uludag University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
İnci, D., Aydın, R. Potentiometric Studies on Complexation of Cu(II) Ion with Methyl/Nitro-Substituted 1,10-Phenanthrolines and Selected Amino Acids. J Solution Chem 46, 124–138 (2017). https://doi.org/10.1007/s10953-016-0551-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0551-1