Abstract
Solid–liquid equilibria in the quaternary systems NaBr–KBr–MgBr2–H2O and KBr–NaBr–CaBr2–H2O at 348.15 K were determined using the method of isothermal solution saturation. The solubilities of the equilibrium solutions were determined. According to the experimental data, the equilibrium phase diagrams and water content diagrams of the two quaternary systems were plotted. The solubility diagram of the quaternary NaBr–KBr–MgBr2–H2O system has two invariant points, five univariant curves, and four crystallization fields (which are saturated with MgBr2·6H2O, KBr·MgBr2·6H2O, NaBr and KBr, respectively, where KBr·MgBr2·6H2O is a double salt). The solubility diagram of the quaternary system KBr–NaBr–CaBr2–H2O includes one invariant point, three univariant curves, and three crystallization fields (CaBr2·2H2O, KBr and NaBr). The dissolution equilibrium constant K of NaBr, KBr, MgBr2·6H2O, KBr·MgBr2·6H2O and CaBr2·2H2O at 348.15 K were fitted using Pitzer’s equation. A chemical model based on the Pitzer equation was constructed to calculate the solubilities of salts in the two quaternary systems at 348.15 K. The calculated solubilities are in agreement with experimental results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The studies of phase equilibria in multicomponent salt–water systems at different temperatures are fundamental for the development and utilization of brine resources. Bromide rich brines are widely distributed in the Qinghai–Tibet plateau and the Sichuan basin in China. In recent decades, some scholars have investigated the phase equilibria of parts of the salt–water systems [1, 2]. In particular, the underground brines in the western Sichuan basin contain very abundant liquid mineral resources. The contents of boron, potassium, and bromine in these brines are far above the grades necessary for industrial utilization [3, 4]. The underground brines have stable chemical properties, and have high concentrations of potassium, sodium, boron, bromine, as well as accompanying lithium, strontium, magnesium, calcium, and many other useful components.
Underground brines are very complex salt–water systems, whose formation often involve solid–liquid equilibria. Furthermore, the phase diagrams at different temperatures play an important role in exploiting the brine resources and understanding the geochemical behavior of these brine–mineral systems. Therefore, it is necessary and urgent to investigate the thermodynamics (especially the phase equilibrium properties) of Br-rich brine–mineral systems at multiple temperatures.
A number of experimental and modelling studies on brine–salt mineral system have been carried out in recent decades. Aiming at the seawater system, Harvie et al. [5–8] carried out research into the prediction of mineral solubilities in natural waters of the Na–K–Mg–Ca–H–Cl–SO4–OH–HCO3–CO3–CO2–H2O systems from zero to high concentration and as a function of temperature using Pitzer’s equation. Felmy and Weare [9] predicted the borate mineral equilibria in Searles salt lake in the USA. In view of the abundant bromide resource in potash salt deposits, the phase equilibria and phase diagrams of the ternary KCl–KBr–H2O system was determined at 298, 313, 333, 323 and 373 K, and the LiI–LiBr–H2O and NH4Br–LiBr–H2O systems at 298 K [10–13]. In our previous work, we reported the phase diagrams of some bromide-rich ternary and quaternary systems, i.e., K2B4O7–KBr–H2O and Na2B4O7–NaBr–H2O at 298 K [14, 15], Na–K–Br–SO4–H2O at 323 K [16], NaCl–NaBr–Na2SO4–H2O at 348 K [17], Na2B4O7–NaBr–Na2SO4–H2O at 348 K [18], and KCl–KBr–K2SO4 at 323 K [19].
Recently, Christov reported predictions of the solubilities of bromide salts and solution behavior in solid–liquid equilibrium systems [20–24]. The thermodynamic modeling was carried out to high concentration and temperature with Pitzer’s equation. Due to the lack of solubility data involving bromide salts at different temperatures, the calculations were mainly focused on binary and ternary systems such as CaBr2–H2O, MgBr2–H2O, NaBr–H2O, KBr–H2O, NaBr–KBr–H2O, NaBr–Na2SO4–H2O, and KBr–K2SO4–H2O [20–24].
Therefore, this paper has three objectives: (1) measure the solubilities of salts in the aqueous solutions of the quaternary systems NaBr–KBr–CaBr2–H2O and NaBr–KBr–CaBr2–H2O at 348.15 K, identify the equilibrium solid phases and construct the experimental phase diagrams and water content diagrams of the two quaternary systems; (2) use Pitzer’s equation to fit the dissolution equilibrium constant of salts; and (3) use these thermodynamic data to develop a thermodynamic model for the prediction of bromide salt solubilities in the two quaternary systems.
2 Experimental Sections
2.1 Reagents and Instruments
Distilled water with conductivity less than 10−4 S·m−1 and pH = 6.6 was used for the phase equilibrium experiments and chemical analysis of samples. A HZ–9613Y type thermostated shaker with a precision ±0.1 K was used in the solid–liquid equilibrium measurements. NaBr, 99.0 % pure, was from Tianjin Zhiyuan Chemical Reagent Factory, China; MgBr2·6H2O and CaBr2·2H2O, 98 % pure, were from Shanghai Xinbao Fine Chemical Plant, China; KBr, 99.0 % pure, was from the Chengdu area of the industrial development zone, Xindu Mulan, China.
2.2 Experimental Methods
The experiments were conducted by the method of isothermal solution saturation. Samples of the quaternary systems were prepared by gradual addition of the third salt to the saturated solution of the ternary subsystems at 348.15 K. The solid reagents and water were prepared in 100 mL glass bottles. Then, all bottles were sealed and placed in the thermostated shaker. The solid–liquid mixtures in the bottles were agitated for 1 week to accelerate equilibration. Experimental results show that equilibrium can be attained in 10–15 days under continuous shaking, and the time for the clarification of an aqueous solution is about 4–6 days. The clarified solutions were taken out periodically for chemical analysis. If the solution composition did not change, the system can be considered to have reached thermodynamic equilibrium. In this state, the solution composition should represent that of the saturated solution. Liquid samples were then taken for chemical analysis.
2.3 Analytical Methods
The concentration of K+ was obtained by a gravimetric method using sodium tetraphenylborate (uncertainty: 0.5 %). The concentration of Mg2+ was determined by titration with an EDTA standard solution in the presence of the indicator eriochrome black T; an ammonium chloride buffer solution was used to maintain the high pH (10–11) of solutions in the complexometric titration process (uncertainty, 0.3 %). The concentration of Ca2+ was determined by titration with a standard EDTA solution in the presence of the indicator eriochrome K–B, while a sodium hydroxide solution was used for maintaining the high pH (11–12) of solutions in the complexometric titration (uncertainty: 0.5 %). The concentration of Br− was measured by titration with silver nitrate solution (the Mohr method, uncertainty: ±0.3 %). The concentration of Na+ was evaluated according to the charge balance of ions. The details of the above analytical methods can be found in the literature [25].
3 Results and Discussions
3.1 The KBr–NaBr–MgBr2–H2O System
The experimental solubilities of salts in the quaternary system KBr–NaBr–MgBr2–H2O at 348.15 K are given in Table 1. According to the solubilities, the corresponding dry salt compositions were calculated and are also tabulated in Table 1. Based on the dry salt composition, the phase diagram and water content diagram are presented in Figs. 1 and 2, respectively.
It can be seen from Table 1 and Fig. 1 that the double salt (KBr·MgBr2·6H2O) is formed in the quaternary system. The phase diagram (Fig. 1) is comprised of five univariant curves (A1E1, E1F1, B1E1, C1F1 and D1F1) and two co-saturated points (E1 and F1). The four crystallization fields are MgBr2·6H2O, KBr·MgBr2·6H2O, NaBr and KBr, respectively. The crystallization field of MgBr2·6H2O is smaller, which indicates that its solubility is greater in this system. The experimental results show that MgBr2 has a strong salting-out effect on KBr and NaBr.
Figure 2 shows the water content diagram of the quaternary system KBr–NaBr–MgBr2–H2O at 348.15 K and the abscissa is the Jänecke index of J (KBr). Figure 2 shows that the water content increases at the univariant curves D1F1, and decrease at the univariant curves A1E1 with an increase of the Jänecke index values of J (KBr).
3.2 The KBr–NaBr–CaBr2–H2O System
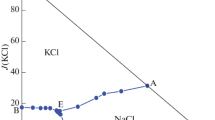
The measured values of salt solubilities of the quaternary system KBr–NaBr–CaBr2–H2O at 348.15 K are presented in Table 2, where ion concentrations are expressed in mass fractions w, J is the Janeäcke index, with J(KBr) + J(NaBr) + J(CaBr2) = 100 g. Based on the data in Table 2, a stable equilibrium phase diagram of the system at 348.15 K is given in Fig. 3.
The quaternary system KBr–NaBr–CaBr2–H2O at 348.15 K has no complex salt or solid solution. There is one invariant point, three univariant curves, and three regions of crystallization in this system. The three crystallization fields corresponding to KBr, NaBr and CaBr2·2H2O. The crystallization area of potassium bromide (KBr) is larger than that of other salts. This means that KBr has a lower solubility than the other salts in the quaternary NaBr–KBr–CaBr2–H2O system.
Three univariant curves are A2E2, B2E2, and C2E2. Point E2 is the invariant point for the system KBr–NaBr–CaBr2–H2O at 348.15 K, which is saturated with KBr, NaBr, and CaBr2·2H2O, and the composition of the corresponding liquid phase is w (KBr) = 0.0421, w (NaBr) = 0.0929, and w (CaBr2) = 0.5708.
Figure 4 shows the water content diagram of the quaternary system KBr–NaBr–CaBr2–H2O at 348.15 K, and the abscissa is the Jänecke index values of KBr. Figure 4 shows that the water content increases at the univariant curves E2C2, and remains unchanged at the univariant curves A2E2 and B2E2 with an increase of the Jänecke index values of J (KBr). It reaches its biggest value at the point C2.
4 Solubility Predictions
4.1 Model Parametrization
Pabalan [26] proposed a high temperature thermodynamic model for the concentrated electrolyte solution system Na–K–Mg–Ca–SO4–OH–H2O over a temperature range (from 273.15 to 473.15 K). In order to obtain thermodynamic properties and parameters of the ternary solutions, such as osmotic coefficients ϕ, binary and ternary ionic interaction parameters, and dissolution equilibrium constant K, the following equations were applied to the phase equilibria and chemical equilibria of a given solution [27]:
where Par(T) refers to temperature functions for the model parameters as described in Møller (1988); the temperature T is in Kelvin. The Debye–Hückel constant \( A^{\phi } \) was taken from the literature [28]. The parameters \( a_{1} ,\;a_{2} ,\;a_{5} \) and \( a_{6} \) are based on the results of Christov [20]. In Eqs. 2–5, \( \gamma_{i} \) and m i are the activity coefficient and molality of solute species i, respectively.
4.2 Solubility Calculation
Based on Pitzer’s ion-interaction model for aqueous electrolyte solutions and the corresponding temperature-dependent parameter expressions, the dissolution equilibrium constant (K) of solid salts (MgBr2·6H2O, KBr·MgBr2·6H2O, NaBr, KBr and CaBr2·2H2O) were fitted by multiple linear regression method. The constants in Eq. 1 for the binary and ternary interaction parameters are taken from the literature [20] (see Table 3). Table 4 shows the calculated dissolution equilibrium constant (ln K) values at 348.15 K.
Harvie and Weare [6] showed that the Pitzer approach could be extended to accurately calculate salt solubilities in complex brines. The solubilities of salts in two quaternary systems at 348.15 K were calculated with the Pitzer model. As shown in Figs. 5 and 6, the experimental and predicted phase diagrams (dashed line) of the two quaternary systems at 348.15 K are in general agreement. Tables 5 and 6 give a comparison of the calculated and experimental solubilities at the invariant points of the two quaternary systems and the saturated solution compositions of the relevant quaternary subsystems, respectively. As can be seen from Figs. 5 and 6, there are some deviations between the calculated and experimental solubilities. The sources of deviations include (1) experimental uncertainty, and (2) uncertainties of the ion-interaction parameters of Pitzer’s equation due to the regression and the experimental data used in the regression, although the regression equation is applicable only in a certain temperature range (the accuracy of the ion-interaction model requires further validation at high temperatures and high concentrations). Overall, the above results indicate that the model used in this work is capable of a reasonable prediction of the salt solubilities for the quaternary systems in question.
5 Conclusion
The solubilities of salts in the systems KBr–NaBr–MgBr2–H2O and KBr–NaBr–CaBr2–H2O at 348.15 K were determined with the method of isothermal solution saturation. According to the experimental data, the stable equilibrium phase diagrams and water content diagrams of the two quaternary systems were constructed. Based on Pitzer’s model and temperature-dependent parameter expressions, the dissolution equilibrium constant of the salts are represented. The solubilities of salts in the quaternary systems were predicted at 348.15 K. The results indicate that the Pitzer model based on the temperature-dependent parameter expressions is applicable for the prediction of solubilities in the quaternary systems.
References
Sun, B., Li, B., Fang, C.H., Du, X.H., Song, P.S.: Lithium salt lake brine system related research. J. Salt Lake Res. 3, 34–39 (1995). (in Chinese)
Zhang, Z.L., Sang, S.H., Li, M.: Study on metastable phase equilibrium of quaternary system K2B4O7–K2SO4–KCl–H2O at 298 K. Chem. Eng. 5, 46–48 (2009). (in Chinese)
Shu, S.B., Xu, Y.L., Xu, E.X., Xiao, C.W.: Study on the occurrence of potassium-rich brine in a geological structure in West Sichuan and the analytical patterns. Chin. Well Rock Salt 2, 2–26 (2003). (in Chinese)
Lin, Y.T., Cao, S.X.: New discovery rare gas field with rich potassium and boron in West Sichuan Basin. Chin. Geogr. 7, 45–47 (2001). (in Chinese)
Harvie, C.E., Weare, J.H., Hardie, L.A., Eugster, H.P.: Evaporation of seawater: calculated mineral sequences. Science 208, 498–500 (1980)
Harvie, C.E., Weare, J.H.: The prediction of mineral solubilities in natural waters: the Na–K–Mg–Ca–Cl–SO4–H2O system from zero to high concentration at 25 °C. Geochim. Cosmochim. Acta 44, 981–997 (1980)
Harvie, C.E., Eugster, H.P., Weare, J.H.: Mineral equilibriain the six-component seawater system, Na–K–Mg–Ca–SO4–Cl–H2O at 25 °C. II: compositions of the saturated solutions. Geochim. Cosmochim. Acta 46, 1603–1618 (1982)
Harvie, C.E., Møller, N., Weare, J.H.: The prediction of mineral solubilities in natural waters: the Na–K–Mg–Ca–H–Cl–SO4–OH–HCO3–CO3–CO2–H2O system from zero to high concentration at 25 °C. Geochim. Cosmochim. Acta 48, 723–751 (1984)
Felmy, A.R., Weare, J.H.: The prediction of borate mineral equilibria in natural waters: application to Searles Lake. California. Geochim. Cosmochim. Acta 50, 2771–2783 (1986)
Weng, Y.B., Wang, J.K., Yin, Q.X., Wang, Y.F.: Phase diagram for the ternary system of K+/Cl−, Br−–H2O at 298 K, 313 K and 333 K. Petrochem. Technol. 4, 358–361 (2007). (in Chinese)
Zhang, K.J., Sang, S.H., Wang, D., Zhang, J.J.: Phase equilibrium in the ternary system KCl–KBr–H2O at 323 K. J. Salt Chem. Ind. 6, 5–7 (2011). (in Chinese)
Hu, Y.X., Sang, S.H., Cui, R.Z., Zhong, S.Y.: Phase equilibrium in the ternary system KCl–KBr–H2O at 373 K. Chin. Sci. Pap. 9, 847–850 (2013). (in Chinese)
Tang, Z.X., Zhang, F.X., Guo, Z.Z., Huang, J.Y.: Potassium lithium halide water system research. IV. The solubilities of ternary systems NH4I–LiI–H2O, LiI–LiBr–H2O and NH4Br–LiBr–H2O 298 K. J. Salt Lake Res. 3, 9–13 (1993)
Sang, S.H., Yin, H.A., Ni, S.J., Zhang, C.J.: A study on equilibrium solubilities and properties of solutions in the ternary system K2B4O7–KBr–H2O at 298 K. J. Chengdu Univ. Techol Ed. 4, 414–416 (2006) (in Chinese)
Sang, S.H., Yu, H.Y.: Study on the phase equilibrium of Na2B4O7–NaBr–H2O ternary system at 298 K. Sea Lake Salt Chem. Ind. 2, 4–5 (2006). (in Chinese)
Sang, S.H., Sun, L.M., Li, H., Zhang, X., Zhang, K.J.: Study on phase equilibria for quaternary system of Na+, K+/Br−, \( {\text{SO}}_{4}^{2 - } \)–H2O at 323 K. Chin. J. Inorg. Chem. 5, 845–849 (2011) (in Chinese)
Zhang, Y.G., Sang, S.H., Zhang, K.J., Hu, F.M., Cui, R.Z.: Study on phase equilibria for quaternary system of NaCl–NaBr–Na2SO4–H2O at 348 K. J. Salt Chem. Ind. 2, 12–15 (2013). (in Chinese)
Ning, H.Y., Sang, S.H., Wang, D., Zeng, X.X.: Study on phase equilibria in Na2B4O7–NaBr– Na2SO4–H2O quaternary system at 348 K. Chem. Eng. 4, 27–30 (2012). (in Chinese)
Wang, D., Sang, S.H., Zeng, X.X., Ning, H.Y.: Phase equilibrium of KCl–KBr–K2SO4–H2O quaternary system at 323 K. Petrochem. Techol. 3, 285–288 (2011). (in Chinese)
Christov, C.: Study of bromide salts solubility in the (m1KBr + m2CaBr2)(aq) system at T = 323.15 K. Thermodynamic model of solution behavior and (solid + liquid) equilibria in the ternaries (m1KBr + m2CaBr2)(aq), and (m1MgBr2 + m2CaBr2)(aq), and in the quinary (Na + K + Mg + Ca + Br + H2O) systems to high concentration and temperature. J. Chem. Thermodyn. 55, 7–22 (2012)
Christov, C.: An isopiestic study of aqueous NaBr and KBr at 50 °C: chemical equilibrium model of solution behavior and solubility in the NaBr–H2O, KBr–H2O and Na–K–Br–H2O systems to high concentration and temperature. Geochim. Cosmochim. Acta 71, 3557–3569 (2007)
Christov, C.: Isopiestic investigation of the osmotic coefficients of MgBr2(aq) and study of bromide salts solubility in the (m1KBr + m2MgBr2)(aq) system at T = 323.15 K. Thermodynamic model of solution behaviour and (solid + liquid) equilibria in the MgBr2(aq), and (m1KBr + m2MgBr2)(aq) systems to high concentration and temperature. J. Chem. Thermodyn. 43, 344–353 (2011)
Christov, C.: Study of bromide salts solubility in the (m1NaBr + m2MgBr2)(aq) system at 323.15 K. Thermodynamic model of solution behavior and (solid–liquid) equilibria in the (Na + K + Mg + Br + H2O) system to high concentration and temperature. J. Chem. Thermodyn. 47, 335–340 (2012)
Christov, C.: Isopiestic investigation of the osmotic coefficients of aqueous CaBr2 and study of bromide salt solubility in the NaBr–CaBr2–H2O system at 50 °C: thermodynamic model of solution behavior and solid–liquid equilibria in the CaBr2–H2O, and NaBr–CaBr2–H2O systems to high concentration and temperature. CALPHAD 35, 42–53 (2011)
Institute of Qinghai Salt-Lakes, Chinese Academy of Sciences: Analytical Methods of Brines and Salts, 2nd edn. Science Press, Beijing (1988). (in Chinese)
Pabalan, R., Pitzer, K.S.: Thermodynamics of concentrate electrolyte mixtures and the prediction of mineral solubilities to high temperatures for mixtures in the system Na–K–Mg–Cl–SO4–OH–H2O. Geochim. Cosmochim. Acta 51, 2429–2443 (1987)
Christov, C., Møller, N.: Chemical equilibrium model of solution behavior and solubility in the H–Na–K–OH–Cl–HSO4–SO4–H2O system to high concentration and temperature. Geochim. Cosmochim. Acta 68, 1309–1331 (2004)
Ananthaswamyt, J., Atklnson, G.: Thermodynamics of concentrated electrolyte mixtures: 4. Pitzer–Debye–Hückel limiting slopes for water from 0 to 100 °C and from 1 atm to 1 kbar. J. Chem. Eng. Data 29, 81–87 (1984)
Acknowledgments
This project was supported by the National Natural Science Foundation of China (41373062, U1407108), the Specialized Research Fund (20125122110015) for the Doctoral Program of Higher Education of China, and the Scientific Research and Innovation Team in Universities of Sichuan Provincial Department of Education (15TD0009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, J., Sang, S. & Liu, Q. Measurements and Calculations of the Solid–Liquid Equilibria in the Quaternary Systems NaBr–KBr–MgBr2–H2O and NaBr–KBr–CaBr2–H2O at 348.15 K. J Solution Chem 44, 1963–1975 (2015). https://doi.org/10.1007/s10953-015-0377-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0377-2