Abstract
Values of the critical micelle concentration (cmc) and degree of counterion dissociation (α) of four cationic surfactants: cetyltrimethylammonium bromide (CTAB), cetylpyridinium bromide (CPB), cetylpyridinium chloride (CPC) and benzyldimethylhexadecylammonium chloride (BDHAC) in aqueous–glycine medium (concentration of glycine (Gly) varied from 0 to 0.20 mol·dm−3) were determined through conductometric measurements at 303 K. The effect of temperature on the micellization in the presence of 0.10 mol·dm−3 Gly were studied for the surfactants CTAB, CPC and BDHAC. With respect to the concentration of Gly, a decrease in the cmc was observed for CTAB, CPC and CPB whereas an increase was observed for BDHAC. A regular increase in α was obtained for CPB, CPC and BDHAC with respect to the concentration of Gly whereas values were roughly constant in the case of CTAB. Thermodynamic parameters were computed from the temperature dependence of the cmc values and it was found that the micellization process is exothermic. Compensation of enthalpy and entropy was observed for the micellization of CTAB, CPC and BDHAC in the presence of 0.10 mol·dm−3 Gly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Surfactants are amphiphilic molecules that consist of hydrophobic hydrocarbon chains attached to a hydrophilic head group. They organize in water or any suitable medium, forming supramolecular aggregates known as micelles above a characteristic concentration, called the critical micelle concentration (cmc) [1]. The cmc is a distinguishing property of the surfactants, which can vary with various physicochemical conditions. It is well known that the physicochemical and microstructural properties of a particular surfactant can be tuned to desired size, shape and application by varying the structure of surfactant monomers [2, 3], and the solution conditions such as concentration, solvent polarity and type, temperature, pressure as well as by the presence of various additives [4–8].
Amino acids (AA) are zwitterionic biomolecules and are the building blocks of proteins. They have common hydrophilic groups (–COOH, –NH2) and a choice of different hydrophobic groups. They are considered to be strong structure breakers in aqueous solution due to the presence of peripheral charges [9] and generally undergo strong electrostatic interactions with charged species in aqueous solution [10]. Glycine (Gly) is the simplest AA and previous studies have shown that it is solubilized in water by strong electrostatic interactions [11–13].
Amphiphilic molecules and amino acids are present in various natural systems, including biological systems, and are used in medicine, cosmetics and so on [14, 15]. The interaction between surfactant and bioactive molecules is of immense significance to understand various aspects of life processes. Recently, researchers have shown increasing interest in amino acid–surfactant–water systems [10, 16–22]. The interactions between surfactants and amino acids may affect the activity of the amino acids and can also influence the micellization process of surfactants. Furthermore, the investigation of the solution behavior of model compounds such as amino acids, peptides and their derivatives can provide useful information about the interaction which is possible in the proteins and/or about their conformational stability [23–25].
The aim of this work is to investigate the effect of the concentration of Gly on the micellization of four cationic surfactants: cetyltrimethylammonium bromide (CTAB), cetylpyridinium bromide (CPB), cetylpyridinium chloride (CPC) and benzyldimethylhexadecylammonium chloride (BDHAC). All these surfactants carry a hydrocarbon tail of 16 carbon atoms and they differ in their head group (with the exception of CPB and CPC). Besides, Br− is present as counter ion in CTAB and CPB whereas Cl− is in the others. We have varied the concentration of Gly from 0 to 0.20 mol·dm−3 (0, 0.01, 0.025, 0.05, 0.10, 0.15, and 0.20). To see how temperature affects micellization of CTAB, CPC and BDHAC in Gly medium (0.10 mol·dm−3 Gly), conductometric studies were performed at four temperatures 298, 303, 308 and 313 K. Various thermodynamic parameters of these surfactants were also evaluated from the temperature dependence of cmc values and are discussed with reported values available in the literature. The structures of Gly and the surfactants used in the study are given in Scheme 1.
2 Experimental
2.1 Materials
The amino acid glycine (Gly) (Merck, AR) and the surfactants cetyltrimethylammonium bromide (CTAB) (≥ 99 %, Sigma), cetylpyridinium bromide (CPB) (≥ 97 %, Fluka), cetylpyridinium chloride (CPC) (≥ 98 %, Sigma) and benzyldimethylhexadecylammonium chloride (BDHAC) (≥ 97 %, Fluka) were used as received. Milli-Q grade water was used throughout the study.
2.2 Conductometric Study
Conductance measurements were made using a Wayne Kerr 6440B automatic precision bridge and a dip-type cell. Appropriate concentrated stock solutions of surfactants were prepared in water or aqueous–glycine media and kept overnight. Adequate quantities of these solutions were added to the water/aqueous–glycine media in which they were prepared using a calibrated Finnpipette in order to change the concentration of surfactants from well below the cmc to at least 2–3 times higher than the cmc (calibration was done as described in the user manual and the calculated values were found to be within the specified limit). A Haake D8 circulation bath was used for maintaining the temperature of the system at 303 K (± 0.1 K).
3 Results and Discussion
3.1 Effect of Glycine on the cmc and Degree of Counter Ion Dissociation (α)
Representative conductivity (κ) plots obtained in 0.05 mol·dm−3 aqueous–glycine media at 303 K are given in Fig. 1, which show the transition from pre-micellar to post-micellar regions. In the conductivity technique, the cmc of the surfactants is usually determined from the intersection points of the two straight lines in the κ against concentration profile and the degree of counter ion dissociation (α) is calculated as the ratio of the slope of the linear plot in the post-micellar region to that in the pre-micellar region. However, this procedure may introduce some uncertainties in the evaluation of cmc and α values, particularly when the transition from pre- to post-micellar regions is gradual. Therefore, we have applied Carpena’s method [26] to determine the cmc and α values of the surfactant from the conductivity data. This is based on the fitting of conductivity data (κ) as a function of surfactant concentration (c) to the integral of Boltzmann-type sigmoidal equation:
where κ 0, A 1, A 2 and Δc are the conductivity of the solution at zero concentration of the surfactant, pre-micellar slope, post-micellar slope and width of the transition, respectively. The central point on the width of the transition (c 0) corresponds to the cmc and the α is determined as A 2/A 1. The critical micelle concentrations and degrees of counter ion dissociation of the studied surfactants in various compositions of aqueous–glycine media (0, 0.01, 0.025, 0.05, 0.10, 0.15 and 0.20 mol·dm−3) at 303 K as estimated through this method are listed in Table 1.
Ruiz and coworkers have observed a decrease in the cmc with increasing concentration of Gly for the micellization of two non-ionic surfactants octyl-β-thioglucopyranoside [16] and N-decanoyl-N-methylglucamide [21]. Chauhan and coworkers have reported an increase in cmc of CTAB with increase in concentration of lysozyme [27] and decrease in cmc with the increase in concentration of leucine [28] and Gly [22]. The effect of Gly, in the present study, on the cmc of the studied surfactants, can be seen from Fig. 2. As the concentration of Gly is varied from 0 to 0.20 mol·dm−3, a regular decrease in the cmc is obtained for CTAB, CPB and CPC whereas for BDHAC the values increased.
It is well known that micellization substantially depends on the nature of the medium and natures and concentrations of additives, if any. On the addition of Gly, the equilibrium, established between the water molecules and ions of surfactants, changes and now there are changes in the specific interactions: (i) zwitterion-ion interactions (counter ions of surfactants and –NH +3 portion of Gly and COO– of Gly and positive charge on the surfactants or the repulsive interactions between the similarly charged species), (ii) and hydrophobic interactions with respect to the [Gly]. The increase in the cmc of BDHAC could be due to the increased repulsion between benzyldimethylhexadecylammonium ions, which oppose their association thus disfavoring micellization. At the same time, amino acids like Gly are structure breaking solutes and the addition of such substance will decrease the solubility of hydrocarbon tails of surfactants and will increase the interfacial tension between the micelles and aqueous solvents. This will facilitate micellization and will result in decreasing the cmc with respect to increase in concentration of Gly as obtained for CTAB, CPB and CPC. Conductivity data in the presence of 0.10 mol·dm−3 Gly at all the four temperatures (298, 303, 313 and 323 K) were found in the literature [22]. The data were plotted against the concentration of CTAB and the cmc and α values were calculated. These values are also given in Table 1 which shows good agreement with our data.
In ionic micelles, the counter ions bind strongly to the micelle and reside in the Stern layer. The values of degree of counter ion dissociation (α) obtained from Carpena’s method are listed in Table 1 and are also shown in Fig. 3 as a function of concentration of Gly at the studied temperature. A smooth increase was observed for CPB (values change from 0.26 to 0.33), CPC (values change from 0.41 to 0.55) and BDHAC (values change from 0.48 to 0.68), whereas the values are roughly constant for CTAB (values vary between 0.23 and 0.26) with the increase in concentration of Gly. The increase in α obtained in most of the cases could be due the decrease in attractive interaction between the head groups and counter ions caused by the interaction of Gly with the cationic surfactants.
3.2 Effect of Temperature on the cmc and α
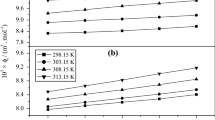
The cmc and α values of CTAB, CPC and BDHAC in the presence of 0.10 mol·dm−3 Gly were determined at four temperatures (298, 303, 308 and 313 K) and the values are given in Table 2. The cmc and α values of CTAB in 0.10 mol·dm−3 calculated from the conductivity data [22] by applying Carpena’s method are also included in Table 1, which shows good agreement. An increase in the cmc values can be seen with increasing temperature in the presence of 0.10 mol·dm−3 Gly. This is due to the disruption of the solvent structure surrounding the hydrophobic tails of the surfactant monomers caused by the increase in temperature. The variation of α of CTAB, CPC and BDHAC with respect to temperature in the presence of 0.10 mol·dm−3 Gly is shown in Fig. 4 and it is apparent from the figure that the value of α is not very sensitive to temperature as there is only a very slight increase in the values.
3.3 Thermodynamics of Micellization
Various thermodynamic parameters viz., Gibbs energy of micellization (ΔG 0m ), enthalpy of micellization (ΔH 0m ) and entropy of micellization (ΔS 0m ) of the ionic surfactants can be deduced from the temperature dependence of the cmc values according to the mass-action model [29] from the equations given below:
where R and T have their usual meaning and x cmc is the cmc in the mole fraction scale. The ratio of the cmc of surfactants to the total concentration of all components in the system was calculated to get the x cmc. The corresponding enthalpy and entropy changes were calculated from Eqs. 3 and 4 respectively:
The values of ln x cmc of CTAB, CPC and BDHAC obtained in the presence of 0.10 mol·dm−3 Gly were plotted against the temperature, T, and linear plots resulted (Fig. 5). The slopes of these plots were taken as the values of ln x cmc/dT.
The ΔG 0m values calculated in the presence Gly are also listed in Table 1. The values in all the cases are negative and become less negative with increase in the concentration of Gly, indicating that the micellization process becomes less favorable (with increase in concentration of Gly) than in pure water. The values of ΔG 0m , ΔH 0m and ΔS 0m obtained in the presence of 0.10 mol·dm−3 Gly at different temperatures are listed in Table 2. It can be seen that the ΔG 0 m values slightly vary with the rise in temperature. The ΔG 0 m , ΔH 0 m and ΔS 0 m values for CTAB in 0.10 mol·dm−3 Gly have been reported in a previous study [22] and it was found that, though there is a good agreement in the ΔG 0m values (values vary from −47.66 to −47.83 kJ·mol−1 in the present study and −47.38 to −46.64 kJ·mol−1 in the literature), ΔH 0m (values vary from −18.70 to −20.10 kJ·mol−1 in the present study and −10.09 to −10.55 kJ·mol−1 in the literature), and the ΔS 0m (values vary from 0.097 to 0.089 kJ·K−1·mol−1 in the present study and 0.125 to 0.115 kJ·K−1·mol−1 in the literature) values slightly differ. However, this difference could be due to difference in the cmc and α values as well as values of the change in slope (d ln x cmc/dT) because the method applied for the deduction of cmc and α values were different in both the studies. To confirm this, the ΔG 0m , ΔH 0m and ΔS 0m values for CTAB in 0.10 mol·dm−3 Gly were re-calculated with the cmc and α values obtained by applying Carpena’s method and these show considerable agreement (the values are given in Table 2). It must be mentioned here that, generally, the determination of ΔH 0m from the temperature dependence of cmc using Eq. 3 is not considered to be very precise as it is based on the assumption that the size and shape of the micelles do not change with temperature and, therefore, directly measured calorimetric values (if available) may differ from the reported values. The enthalpy and entropy values reported here should be treated only as approximate.
From the values of ΔH 0m at different temperatures, it can be seen that the micellization of studied surfactants is exothermic and its magnitude varies with the temperature. The ΔS 0m values of studied surfactants decrease with increase in temperature, i.e., with the rise in temperature, the enthalpy of micellization becomes more negative and the entropy of micellization becomes less positive indicating an enthalpy–entropy compensation for the micellization of CTAB, CPC and BDHAC in the presence of 0.10 mol·dm−3 Gly. To check this point, the enthalpy–entropy compensation (EEC) plots were drawn (Fig. 6).
A linear correlation was obtained for all the surfactants. In general, the micellization process is considered to involve a chemical part and solvation part [30] and the observed linear relationship can be interpreted by the relation, ΔH 0m = ΔH *m + T cΔS 0m [31]. The intercept of the compensation line (ΔH * m ) gives information about solute–solute interaction (chemical part) and the slope (T c, is the compensation temperature) gives some idea about the interaction between solute and solvent (solvation part). The compensation temperature for the studied surfactants fall in the range of 168–220 K in aqueous Gly medium (the values are recorded in Table 3) which shows good interaction between the surfactant and aqueous–Gly medium.
4 Conclusions
The following can be concluded from the present study:
The addition of glycine (Gly) favors the micellization of cetyltrimethylammonium bromide (CTAB), cetylpyridinium chloride (CPC), and cetylpyridinium bromide (CPB) and disfavors the micellization of benzyldimethylhexadecylammonium chloride (BDHAC). During micellization, Gly can either modify the specific interactions with the surfactants or change the solvent nature. On the basis of results obtained, it is reasonable to assume that various interactions/mechanisms are taking place during the micellization of cationic surfactants in the presence of Gly and the net effect of specific interaction will decide the favoring/disfavoring of micellization. With the rise in temperature, an increase in the cmc as well as degree of counter ion dissociation were obtained. The ΔG 0m values become less negative with the increase in the [Gly] and the micellization process is found to be exothermic. With the rise in temperature, the predominance of the enthalpic contribution over the entropic one towards Gibbs energy of micellization (ΔG 0m ) was observed for CTAB, CPC and BDHAC.
References
Rosen, M.J.: Surfactants and Interfacial Phenomena. Wiley, New York (2004)
Haldar, J., Aswal, V.K., Goyal, P.S., Bhattacharya, S.: Role of incorporation of multiple head groups in cationic surfactants in determining micellar properties. Small angle neutron scattering and fluorescence studies. J. Phys. Chem. B 105, 12803–12808 (2001)
Atwood, D., Florence, A.T.: Surfactant Systems: Their Chemistry, Pharmacy and Biology. Chapman & Hall, London (1983)
David S.L., Kumar S., Kabir-ud-Din: Viscosities of cetylpyridinium bromide solutions (aqueous and aqueous KBr) in the presence of alcohols and amines. J. Chem. Eng. Data 42, 198–201 (1997)
Sulthana, S.B., Rao, P.V.C., Bhat, S.G.T., Rakshit, A.K.: Interfacial and thermodynamic properties of SDBS–C12E10 mixed micelles in aqueous media: effect of additives. J. Phys. Chem. B 102, 9653–9660 (1998)
Chakraborty, T., Ghosh, S., Moulik, S.P.: Micellization and related behavior of binary and ternary surfactant mixtures in aqueous medium: cetyl pyridinium chloride (CPC), cetyl trimethyl ammonium bromide (CTAB), and polyoxyethylene (10) cetyl ether (Brij-56) derived system. J. Phys. Chem. B 109, 14813–14823 (2005)
Ray, G.B., Chakraborty, I., Ghosh, S., Moulik, S.P.: Studies on binary and ternary amphiphile combinations of tetradecyltrimethylammonium bromide (C14TAB), tetradecyltriphenylphosphonium bromide (C14TPB), and tetradecylpyridinium bromide (C14PB). A critical analysis of their interfacial and bulk behaviours. J. Phys. Chem. B 111, 9828–9837 (2007)
Das, D., Ismail, K.: Aggregation and adsorption properties of sodium dodecyl sulfate in water–acetamide mixtures. J. Coll. Interface Sci. 327, 198–203 (2008)
Lehninger, A.L., Nelson, D.L., Cox, M.M.: Principles of Biochemistry. Worth Publishers, USA (1993)
Sharma, K.S., Hassan, P.A., Rakshit, A.K.: Surface activity and association behavior of nonaoxyethylene n-dodecylether in aquo amino acid medium: tensiometry, small-angle neutron scattering, dynamic light scattering and viscosity studies. Colloids Surf. A 308, 100–110 (2007)
Leodidis, E.B., Hatton, T.A.: Amino acids in AOT reversed micelles. 1. Determination of interfacial partition coefficients using the phase transfer method. J. Phys. Chem. 94, 6411–6420 (1990)
Leodidis, E.B., Bommarius, A.S., Hatton, T.A.: Amino acids in reversed micelles. 3. Dependence of the interfacial partition coefficient on excess phase salinity and interfacial curvature. J. Phys. Chem. 95, 5943–5956 (1991)
Adachi, M., Harada, M., Shioi, A., Sato, Y.: Extraction of aminoacids to microemulsion. J. Phys. Chem. 95, 7925–7931 (1991)
Adamson, A.W.: Physical Chemistry of Surfaces. John-Wiley, New York (1976)
Luethi, P., Luisi, P.L.: Enzymatic synthesis of hydrocarbon-soluble peptides with reverse micelles. J. Am. Chem. Soc. 106, 7285–7286 (1984)
Ruiz, C.C., M.-Bolivar, J.A., Aguiar, J., P.-Garcia, J.M.: Aggregation behaviour of octyl-β-thioglucopyranoside in the presence of glycine. Colloids Surf. A 249, 35–39 (2004)
Pandey, E., Upadhyay, S.K.: Effect of micellar aggregates on the kinetics of oxidation of α-aminoacids by chloramine-T in perchloric acid medium. Colloids Surf. A 269, 7–15 (2005)
Shukla, R., Upadhyay, S.K.: Tween-80 catalysis in the oxidation of methionine and proline by alkaline hexacyanoferrate(III). Colloids Surf. A 331, 245–249 (2008)
Harutyunyana, N.G., Harutyunyana, L.R., Harutyunyan, R.S.: Volumetric properties of amino acids in aqueous solution of nonionic surfactant. Thermochim. Acta 498, 124–127 (2010)
Ali, A., Tariq, M., Patel, R., Ittoo, F.A.: Interaction of glycine with cationic, anionic, and nonionic surfactants at different temperatures: a volumetric, viscometric, refractive index, conductometric, and fluorescence probe study. Colloid Polym. Sci. 286, 183–190 (2008)
Ruiz, C.C., Hierrezuelo, J.M., M.-Bolivar, J.A.: Effect of glycine on the surface activity and micellar properties of N-decanoyl-N-methylglucamide. Colloid Polym. Sci. 286, 1281–1289 (2008)
Chauhan, S., Sharma, K., Rana, D.S., Kumar, G., Umar, A.: Volumetric and conductance studies of cetyltrimethyl ammonium bromide in aqueous glycine. J. Solution Chem. 42, 634–656 (2013)
Yan, Z., Wang, J., Kong, W., Lu, J.: Effect of temperature on volumetric and viscosity properties of some α-amino acids in aqueous calcium chloride solutions. Fluid Phase Equilib. 215, 143–150 (2004)
Badarayani, R., Kumar, A.: Viscometric study of glycine, l-alanine, glycylglycine in aqueous tetra-n-alkylammonium bromide solutions at 298.15 K. J. Chem. Thermodyn. 36, 983–991 (2004)
Singh, S.K., Kundu, A., Kishore, N.: Interactions of some amino acids and glycine peptides with aqueous sodium dodecyl sulfate and cetyltrimethylammonium bromide at T = 298.15 K: a volumetric approach. J. Chem. Thermodyn. 36, 7–16 (2004)
Carpena, P., Aguiar, J., B.–Galvan, P., Ruiz, C.C.: Problems associated with the treatment of conductivity—concentration data in surfactant solutions: simulations and experiments. Langmuir 18, 6054–6058 (2002)
Chauhan, S., Chauhan, M.S., Sharma, P., Rana, D.S.: Thermodynamics and micellization of cetyltrimethyl ammonium bromide in the presence of lysozyme. J. Mol. Liquids 187, 1–6 (2013)
Chauhan, S., Sharma, K., Rana, D.S., Kumar, G., Umar, A.: Conductance, apparent molar volume and compressibility studies of cetyltrimethylammonium bromide in aqueous solution of leucine. J. Mol. Liq. 175, 103–110 (2012)
Tanford, C.: The Hydrophobic Effect: Formation of Micelles and Biological Membranes. Wiley, New York (1980)
Lumry, R., Rajender, S.: Enthalpy–entropy compensation phenomena in water solutions of protein and small molecules: a ubiquitous property of water. Biopolymers 9, 1125–1227 (1970)
Frank, H.S., Evans, M.W.: Free volume and entropy in condensed systems III. Entropy in binary liquid mixtures; Partial molal entropy in dilute solutions; Structure and thermodynamics in aqueous electrolytes. J. Chem. Phys. 13, 507–532 (1945)
Acknowledgments
T. A. W. gratefully acknowledges fellowship received from the University Grants Commission, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koya, P.A., Wagay, T.A. & Ismail, K. Conductometric Studies on Micellization of Cationic Surfactants in the Presence of Glycine. J Solution Chem 44, 100–111 (2015). https://doi.org/10.1007/s10953-014-0284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0284-y