Abstract
The current report elaborates on the fabrication and evaluation of magnetic and electrical properties of technologically beneficial composites of spinel ferrite and graphene oxide. The synthesis of spinel ferrite with composition Mg0.6Ni0.4Al0.1Fe1.9O4 is done by using the sol–gel auto combustion technique. Modified Hummers method is adopted for the preparation of graphene oxide. The nanocomposites of spinel ferrite-GO (C1, C2, C3) with appropriate ratios of GO are fabricated by sonication of GO and ferrite in water. XRD patterns confirmed the successful synthesis of single-phase cubic ferrite and GO. The dielectric constant of all the prepared samples decreased with increasing the applied frequency, obeying Maxwell–Wagner model. The decrease in temperature-dependent resistivity with increasing temperature indicates semiconducting nature of all samples. The M-H loops of all samples are narrow. The squareness ratio of all the samples is less than 1, which suggests that the present investigated samples are multi-domain. The sample C1 (90%ferrite, 10% GO) with high saturation magnetization, high coercivity, low dielectric constant, and high resistivity made it suitable for use in high-frequency applications like microwave absorbers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Graphene and graphene composites are vital materials nowadays due to versatile properties of graphene. Graphene consists of only carbon atoms bonded together in a single-layered sheet with a honeycomb arrangement of the lattice [1]. Graphene is a 2D layer allotrope of sp2 hybridized carbon atoms. The sp2 hybridization between one s-orbital and two p-orbitals leads to a trigonal planar structure with the formation of a σ-bond between carbon atoms with a separation of 1.42 Å [2]. Graphene possesses startling properties, e.g., intrinsic flexibility, excellent optical transparency, large electrical and thermal conductivity high charge mobility greater than Si, and the large specific area, the ultra-high surface-to-volume ratio. Graphene-based nanomaterials (GBNS) can be classified depending upon the number of layers stacked. When GBNS is in the oxidized state, the structure can be classified into two types, graphene oxide (GO) and reduced graphene oxide (rGO) [3,4,5]. The vital functional groups of GO are alcohols and epoxides whereas ketone groups lie in the corners. Hydroxyl (–OH), carbonyl (–CO¼), carboxyl (–COOH), and epoxide (–O–) are the four functional groups in graphene oxide [6]. Magnetic functionalization of carbon materials with magnetic particles reveals novel GO/magnetic composite materials for high-frequency applications and EMI shielding [3, 7]. It is reported that the Mg-ferrite/GO composites exhibit high adsorption abilities for organic pollutants [8, 9]. Magnesium ferrite is considered to be the most suitable material for microwave absorbing materials due to its high resistivity and low dielectric loss. Its magnetic and electrical properties may be enhanced by substituting transition metal cations [10, 11]. The present study aims to synthesize and characterize Mg0.6Ni0.4Al0.1Fe1.9O4 and Mg ferrite/GO nanocomposites for high-frequency applications.
1.1 Experimental Procedure
1.1.1 Preparation of Spinel Ferrites
Synthesis of spinel ferrite is accompanied by the sol–gel method. Chemicals used for the preparation of the sample were magnesium nitrate, nickel nitrate, aluminum nitrate, iron nitrate, and citric acid. All the chemicals were taken in stoichiometric amounts and dissolved in 100 ml deionized water separately. All these solutions were poured down in a 500-ml beaker and stirred for 20–30 min on a hot plate for making a homogeneous solution. Drops of ammonium hydroxide were added to the solution to neutralize it. The solution was heated with continuous stirring to maintain the temperature of the solution at 60–80°. With continuous stirring and heating, the solution was first converted into a colloidal solution and then finally into a gel. After the formation of gel, the stirring is stopped and heated to 300 ℃ to convert the gel into ashes. This ash was ground and then sintered at 900 ℃ for 5 h to get a fine powder.
1.1.2 Preparation of Graphene Oxide by Hummer’s Method
Graphene oxide will be prepared by Hummer’s method [12]. An appropriate amount of graphite powder was added to a 1000-ml beaker. The solution was continuously stirred for 1 h. After that, 2 g of NaNO3 was added to the solution and stirring continued for a couple of hours. Then, the beaker was removed from the stirrer and placed in a tub filled with ice cubes to cool down the solution up to 5℃. The beaker was again placed on a stirrer. KMnO4 (12 g) was added slowly within 2 h. After adding a small number of KMnO4 (2 g), the sample was continuously stirred for 3 to 5 min and then removed from the stirrer and again placed in an ice tub to keep the sample cool due to exothermic reaction. After adding KMnO4 completely, the temperature of the stirrer was set between 25 and 35 ℃, and 250 ml deionized water was added to the sample solution. The sample was continuously stirred for 3 to 5 h for complete oxidation. In the last step, the reaction was terminated by adding 10 ml of hydrogen peroxide and the sample solution was stirred for half-hour. Here, the reaction was completed and GO was in solution form. The solution was centrifuged to remove impurities. Finally, the paste of GO was dried at 80° for 4–5 h and ground to get a fine powder.
1.1.3 Composites Preparation
The weighed amount of ferrite and GO samples were transferred into three different beakers of 100 ml, labeled as C1, C2, C3. Ethanol was taken as a solvent in these three beakers separately. Then, these beakers were placed in the sonicator for 20 min at room temperature. These samples were poured into the pattery dishes and kept in a drying oven for 3 h at 60 ℃ for drying the composites and after grinding the dried samples, the final product (fine powder) is prepared.
2 Results and Discussions
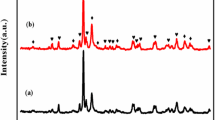
The XRD patterns of Mg0.6Ni0.4Al0.1Fe1.9O4, graphene oxide, and their composites are shown in Fig. 1 The characteristic reflections of spinel ferrite were observed at 30.30°, 33.24°, 35.70°, 43.40°, 54.03°, 57.32°, 62.97° with respective hkls (111), (311), (400), (422), (511), and (400) are observed in X-ray spectrum. Single-phase spinel ferrite is obtained, which belongs to space group Fd3m. An additional peak at 33.242° also appeared, which indicates the presence of α-Fe2O3 [14, 15]. The XRD pattern of GO shows the characteristic peaks at 2θ = 10.11° with interlayer spacing d = 0.89 nm, at 2θ = 26.61°, and at 43° which confirms the formation of graphene oxide. The peaks of GO depend on the level of oxidation. As the oxidation level increases, the rest of the peaks disappears except 2θ = 10–11° [13]. The main peaks of pure ferrite sample and GO appeared in XRD spectra of all composites indicating their successful synthesis [16]. The suppression of GO characteristic peaks in the composites C1, C2, and C3 is an indication of exfoliation in spinel ferrite between interlayers of GO sheets. The variation in d-values also confirmed the exfoliation of GO (Table 1).
2.1 Electrical Resistivity
Keithly source meter model 2400 is used to measure the DC resistivity. From Fig. 2, it is noted that at room temperature, the conductivity of GO is higher than ferrite particles. The electrical properties of graphene oxide are based on its oxidation level. The sp2/sp3 ratio has a direct impact on oxidation levels. The sp2/sp3 ratio can be varied by reduction or addition of oxygen functionalized groups in GO’s structure. In this way, the electrical properties of GO can be tuned. The ferrite particles are expected to enter into a hexagonal honey comb–like structure that results in a slight increment in resistivity. Further by increasing the GO content and decreasing the ferrite content, the number of GO charge carriers contributing to the conduction mechanism is increased and the number of ferrite particles that occupy the honeycomb structure of GO is decreased, resulting in an increase in density of GO charges as well as conduction mechanism. Ultimately, the resistivity decreased. The hopping mechanism is supported by the enhanced charge contents of GO [17].
Figure 3 shows the Arrhenius plots of the samples C1, C2, and C3. It is observed that the resistivity of all investigated samples decreases by increasing temperature, which is an indication of semiconducting behavior. Figure 4 shows the activation energy vs GO content. It is observed that the activation energy is decreased as GO is substituted. The activation energy follows the resistivity versus GO contents. It is noted that the greater the resistivity of the sample, the higher will be its activation energy and vice versa [18], whereas the resistivity of GO depends on the level of oxidation and hopping of charges.
2.2 Dielectric Properties
Frequency-dependent dielectric properties of Mg0.6Ni0.4Al0.1Fe1.9O4, GO and their ferrite/GO composites (with 10%Wt, 20%Wt, 30% wt of GO) are discussed below:
Figure 5 shows the dielectric constant vs frequency for all the samples. The graphs show the typical behavior of ferrites following the Maxwell–Wagner-type model [4]. It assumes a two-layered heterostructure of ferrites, that is, conducting grains, separated by highly resistive grain boundaries. The high value of ɛ′ at low frequency is due to interfacial polarization and at intermediate high frequency, the dipoles exhibit dispersion and at further high frequency, the ɛ′ is almost independent of frequency because the dipoles don’t follow the applied frequency. From Fig. 5, it can be observed that the magnitude of the ɛ′ increases as GO content increases. The GO content has a significant effect on the dielectric constant, and it increases the conduction due to the hopping of charge carriers (Fe2+ to Fe3+ + e−). Figure 5 shows the increasing trend of ɛ′. The dielectric constant of samples is in the following trend: ɛ′ of ferrite < C1 < C2 < C3 < GO. The combined graph ɛ′ vs frequency for all samples is shown in Fig. 5. If figures of the dielectric constant of samples are compared with Fig. 2 of room temperature resistivity, these two parameters ɛ′ and ɛ″ behave oppositely. The values of \(\sqrt{\rho }\). ɛ′ for composites is constant as listed in Table 2 [17].
Figure 6 depicts the dielectric loss as a function of frequency for all the samples. The ɛ″ shows similar behavior as that of ɛ′ and follows Maxwell–Wagner model [4]. The magnitude of ɛ″ is lesser than ɛ′. The dielectric loss shows the loss of energy due to defects, voids, or dislocations in the sample whereas ɛ′ shows the capability of a dielectric to store energy by weakening the internal electric field. Figure 6 shows the value of ɛ″ at 20 Hz that shows the increasing value of ɛ″ from ferrite with the lowest value and GO with the highest value. Figure 6 shows the stacked graph of ɛ″ vs frequency for all prepared samples. The dielectric loss is found to decrease with the increasing concentration of GO. The addition of GO in spinel ferrite caused a decrease in electrical conductivity; this decrease in electronic conduction may be due to fact that the defect sites created on pure graphene oxide might have been occupied by spinel ferrite and they effectively block the electron hopping [19]. The conductivity of GO is larger than pure ferrite. By increasing GO concentration in ferrite, the conductivity of composites increases due to an increase in charge content taking part in the hopping mechanism.
Figure 7 depicts ac conductivity vs frequency for ferrite, C1, C2, C3, and GO respectively. At low-frequency values, the functional groups of GO supported enhanced conduction. At an elevated frequency range, the charge carriers become active and actively support the conduction mechanism. But in the intermediate high-frequency range, the charge carriers at functional groups do not follow the applied frequency leading to a reduction in ac conductivity [20]. The conductivity of GO is larger than pure ferrite. By increasing GO concentration in ferrite, the conductivity of composites increases due to an increase in charge content taking part in the hopping mechanism.
2.3 Magnetic Properties
Figure 8 shows the M-H loops of Mg0.6Ni0.4Al0.1Fe1.9O4, GO, and Mg-Ferrite/GO nanocomposites C1, C2, C3 with a different weight percentage of ferrite and GO. M-H loops of all samples are shown in Fig. 8. The addition of GO content affects the Ms, Mr, and HC values of pure ferrite. The saturation magnetization of pure ferrite is highest among all samples, which is 21.11 emu/g. By increasing the wt% of graphene oxide in composites, the Ms and Mr value decreases, as shown in Fig. 9. This is due to the non-collinear magnetic moment of surface spins. Another reason for a decrease in Ms value is that the magnetic moment of pure ferrite is greater than GO which resulted in weak A-B magnetic interaction between them [21, 22]. The remanence value of all samples is very small ranging from 0 to 5 emu/g. The squareness ratio is less than 1, which is indicative of single-domain samples. Mr vs GO concentration shows the same trend as that of Ms, as shown in Fig. 9. The Mr, Ms, and Hc values are listed in Table 3. Figure 10 shows coercivity vs GO content; it is observed that ferrite samples have the lowest coercivity value (146.73 Oe) and highest average crystallite size (44.86 nm) among all prepared samples, whereas C1 has the highest coercivity (186.71 Oe) and lowest average crystallite size (40.021 nm). C2 and C3 also follow the same trend as that of ferrite and C1 [23]. The drastic increase in the coercivity values for composites as compared to ferrite samples may be due to a change in the microstructure of the composites where the possibility of defect formation is maximum on the graphene oxide sites. It is expected that domain wall pinning [24] may occur at these defect sites, thereby increasing coercivity.
3 Conclusions
The spinel ferrite Mg0.6Ni0.4Al0.1Fe1.9O4 was synthesized by the sol–gel method and the Hummers method was used for preparing graphene oxide. XRD patterns revealed the formation of a single-phase cubic structure of spinel ferrite and successful synthesis of GO. The preparation of nanoparticles was confirmed by crystallite size calculation by using the Sherrer formula which was in the range 31–48 nm. The observed value of room temperature electrical resistivity of spinel ferrite was 1.013 × 106 Ω-cm. The observed ac conductivity of GO was higher than that of the ferrite sample which in turn decreased the resistivity value from 2.64 × 106 to 7.02 × 103 Ω-com for C1 to C3 respectively. At 10-kHz frequency, the dielectric constant varies from 8 × 103 (C1) to 7 × 104 (C3) and dielectric loss ranges from 5 × 103 (C1) to 6 × 104(C3). The saturation magnetization decreases from 17.8 (C1) to 13 (C3) emu/g due to weakening of AB interactions and coercivity decreases from 187 (C1) to 184 (C3) Oe due to defects in the composites. It is recommended that these samples may be used as microwave absorbing material.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Fatemeh, et al.: Recent developments in graphene and graphene oxide: properties, synthesis, and modifications: a review. Chem. Select 5, 1–21 (2020)
Castro Neto, A.H., Guinea, F., Peres, N.M.R., Novoselov, K.S., Geim, A.K.: The electronic properties of graphene. Rev. Modern Phys. 81(1), 109–162 (2009)
Durmus, Z., Durmus, A., Kavas, H.: Synthesis and characterization of structural and magnetic properties of graphene/hard ferrite nanocomposites as microwave-absorbing material. J. Mater. Sci. 50, 1201–1213 (2015)
Ameer, S.: Iftikhar Hussain Gul, Nasir Mahmood, Muhammad Mujahid, Synthesis, characterization and optical properties of in situ ZnFe2O4 functionalized nano hybrids through modified solvothermal approach. Opt. Mater. 45, 69–75 (2015)
Tan, T. L., Huang, J.: Carbon nanotubes and graphene (Second Edition). 341–374 (2014)
Nawaz, M., Islam, M. U., Aamir Nazir, M., Bano, I., Gul, I. H., Ajmal, M.: Transport properties in spinel ferrite/graphene oxide nanocomposites for electromagnetic shielding. Ceram. Int. 47, 25505–25513 (2021)
Chung, D.D.L.: Materials for electromagnetic interference shielding. Mater. Chem. Phys. 255, 123587 (2020)
Ateia, E.E., Mohammad, A.T., Elsayed, K.: Impact of Gd3+/graphene substitution on the physics properties of magnesium ferrite nanocomposites. JMMM 452, 169–178 (2018). https://doi.org/10.1016/j.jmmm.2017.12.053
McAllister, M.J., et al.: Single sheet functionalized graphene by oxidation and thermal expansion of graphene. Chem. Mater. 19, 4396–4404 (2007)
Kong, L.B., Li, Z. W., Lin, G. Q., Gan, Y. B.: Magneto – dielectric and magnetic properties of Mg- Cu- Co ferrite ceramics, electrical, dielectric, and magnetic properties, J. Am. Ceram. Soc. 90, 2014–2112 (2007)
Konseglu, Y., Kavas, H., Aktas, B.: Surface effects on magnetic properties of superparamagnetic nanoparticles. Phys. Status Solidi A 203, 1595–1601 (2006)
Hummers Jr W.S., Offeman R.E.: Preparation of graphitic oxide. J. Am. Chem. Soc. 80(6), 1339–1339 (1958)
Krishnamoorthy, K., Veerapandian, M., Yun, K., Kim, S.-J.: The chemical and structural analysis of graphene oxide with different degrees of oxidation. CARBON 53, 38–49 (2013)
Ali, R., Mahmood, A.: Muhammad Azhar Khan. Adeel Hussain Chughtai, Muhammad Shahid, Imran Shakir, Muhammad Farooq Warsi, journal of Alloys and compounds 584, 363–368 (2014)
Sumangala, T.P., Mahender, C., Sahu, B.N., Venkataramani, N.: Shiva Prasad, Study of magnesium ferrite nanoparticles with excess iron content. Phys. B 448, 312–315 (2014)
Khurana, G., Kumar, N., Kooriyattil, S., Katiyar, R.S.: Structural, magnetic, and dielectric properties of graphene oxide/Zn x Fe1− x Fe2O4 composites. J. Appl. Phys. 117(17), 17E106 (2015)
Ameer, S.: Iftikhar Hussain Gul, Nasir Mahmood, Muhammad Mujahid, Semiconductor-to-metallic flipping in a ZnFe2O4–graphene based smart nano-system: temperature/microwave magneto-dielectric spectroscopy. Mater. Charact. 99, 254–265 (2015)
Muhammad Ajmal, M.U.: Islam, Absar Ali, Structural, Electrical and dielectric properties of hexa- ferrite-polyaniline nano-composites. J. Supercond. Nov. Magn. 31, 1375–1382 (2018)
Mohsin Saeed, M., Ajmal, M., Islam, M. U., Aamir Nazir, M.: Synthesis and characterization of Y-type ferrite reinforced graphene oxide composites. J. Supercond. Nov. Magn. 33, 2705–2713 (2020)
Waqas Nafees, M., Islam, M. U., Ajmal, M., Hussain, M.: Aisha Iftikhar and Shahzad Naseem, Effect of Al3+substitution on the electrical and magnetic properties of Li-base ferrites. Mater. Res.Exp. 6(12), 126121 (2019)
Rana, M.U., Islam, M.U., Ahmad, I., Abbas, T.: Determination of Magnetic properties and Y-K angles in Cu-Zn-Fe-O system. J. of Mang. and Mag. Mat. 187(2), 242–246 (1998)
Smit, J., Wijn, H.P.J.: Ferrites, Philips Technical Library, Eindhoven, 150 (1959)
Saravanan, M., Sabari Girisun, T.C.: Enhanced nonlinear optical absorption and optical limiting properties of superparamagnetic spinel zinc ferrite decorated reduced graphene oxide nanostructures. Appl. Surf. Sci. 392, 904–911 (2017)
Ali, I., Shakoor, A.: M U Islam, Muhammad Saeed, Synthesis and characterization of hexagonal ferrite Co2Sr2Fe12O22 with doped polypyrrole composites. Curr. Appl. Phys. 13(6), 1090–1095 (2013)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasrullah, F., Islam, M.U., Nazir, A. et al. Electromagnetic Properties of Mg0.6Ni0.4Al0.1Fe1.9O4-Graphene Oxide Nanocomposites. J Supercond Nov Magn 35, 2821–2829 (2022). https://doi.org/10.1007/s10948-022-06321-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-022-06321-6