Abstract
We have investigated the magnetic susceptibility and magnetocaloric effect of double perovskite oxides Ca\(_2\)FeMoO\(_6\). The magnetic susceptibility measurement shows that Ca\(_2\)FeMoO\(_6\) undergoes a ferrimagnetic to paramagnetic phase transition at around 280 K. The isothermal magnetic entropy change (-\(\Delta S_M\)) of Ca\(_2\)FeMoO\(_6\) is estimated by Maxwell relation and a temperature averaged entropy change (TEC) is adopted to assess the figure of merit of this material. By calculating the exponent n dependence of magnetic entropy change with field, we identify the second order phase transition nature in Ca\(_2\)FeMoO\(_6\).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the keeping development of industry and economy, energy and environmental crisis have become more and more serious. Whether in daily life or scientific research, people rely on refrigeration technology frequently. Most of the commercial refrigeration technologies applied now are traditional gas compression (CGC) refrigeration. However, the gas used in CGC often contains/emisses fluoride/cabon dioxide hence CGC technology is detrimental to air and the Earth. Further, the efficiency of CGC is relatively low, i.e. only 5% - 10% of Carnot cycle efficiency. Therefore, an alternative freezing method is appealing. The magnetic refrigeration technology born from the magnetocaloric effect (MCE) is promising to replace CGC technology as an environment-friendly, high efficiency (it can be reached up to 30%-60% of Carnot cycle) and wide-utilization candidate [1,2,3,4,5].
Due to the extra magnetic entropy change caused by the coupling between crystal structure and magnetism, the first-order magnetic phase transition (FOMT) materials generally show large/giant MCE. Therefore, extensive investigations have been conducted on first-order ferromagnetic phase change materials, such as Gd5Ge\(_{4-x}\)Si\(_x\) [6, 7], MnAs\(_{1-x}\)Sb\(_x\) [8], MnFe(As, P, Si, Ge) [9, 10] and LaFe\(_{13-x}\)Si\(_x\)(H\(_\delta\)) [11,12,13]. However, the FOMT compounds often manifest significant magnetic hysteresis and thermal hysteresis, which would greatly reduce the efficiency of magnetic cooling. Moreover, since the crystal expands and shrinks very fast in the magnetic field generating/degenerating process, these materials will encounter severe mechanical instability [14]. Compared with the FOMT materials, the second-order magnetic phase transition (SOMT) magnetic refrigerants do not have these shortcomings. Therefore, many scientists turn to SOMT compounds with large MCE. Among the SOMT materials, the manganese-based perovskites once caused great interests owing to their large MCE and close to magnetic tricritical point [15, 16]. Additionally, they are robust to air and moisture at high temperatures, give its oxidation nature [17].
Double perovskite oxides are counterpart to manganite. The general formula is A\(_2\)BB\(^{\prime }\)O\(_6\), where A is alkaline-earth or rare-earth element ions, B and B\(^{\prime }\) are different transition metal ions. These materials have been widely studied because of their versatile functionality. Kobayashi et al. found that Sr\(_2\)FeMoO\(_6\) displays a 10% tunneling magnetoresistance effect at room temperature [18], showing promise for development of ambient-temperature- operable magnetoresistive devices. Sugahara et al. systematically studied the structural and thermoelectric properties of Ca\(_2\)FeMoO\(_6\) [19]. The maximum dimensionless figure of merit ZT could reach 0.15 at 1250 K. By performing electrical conductivity and electrochemical measurements, Huan et al. proposed that the A\(_2\)BB\(^{\prime }\)O\(_6\) system is highly promising anode material for solid oxide fuel cells [20]. Although extensive investigations have been carried on, the MCE of these materials remains elusive.
In this paper, we present a study on the magnetic susceptibility and magnetocaloric effect of Ca\(_2\)FeMoO\(_6\). The Curie point is obtained from temperature-dependent magnetization. The magnetic entropy change is evaluated by the Maxwell relation and the figure of merit is determined by analyzing of the temperature averaged entropy change(TEC). Also, we have identified the order of magnetic phase transition according to the exponent n from field dependence of magnetic entropy change near \(T_c\).
2 Experiments
Polycrystal samples were synthesized using conventional solid state reaction method. Firstly, the raw Ca\(_2\)O\(_3\), Fe\(_2\)O\(_3\), MoO\(_3\) powders were weighed according to the appropriate molar ratio Ca: Fe: Mo = 2: 1: 1. Secondly, the weighed powder were ground thoroughly in an agate mortar. Then the mixture was pressed into a pellet and heated at 1200\(^{\circ }\)C for 48 hours in air. To guarantee sample homogeneity, the grinding and heating processes were repeated three times. The X-ray diffraction (XRD) were carried out at room temperature on a D8 ADVANCE X-ray diffractometer in order to determine the crystal structure. The magnetic susceptibility \(\chi\)(T) and the isothermal magnetization measurements were performed on a Quantum Design Magnetic Property Measurement System MPMS-3. During the magnetic susceptibility measurement, the magnetic field was set to 200 Oe and the field cooling(FC) process was adopted. While for the isothermal magnetization, the magnetic field ranged from 0 to 9 T in a temperature interval \(\Delta\) \(T\) = 2 K from 260 to 340 K.
3 Results and Discussion
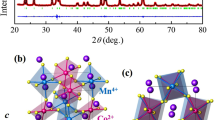
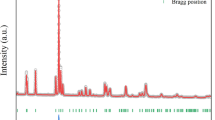
Figure 1 shows the X-ray diffraction pattern of the as-grown Ca\(_2\)FeMoO\(_6\) polycrystal to identify the crystal structure and phase purity. The diffraction data can be well indexed with a monoclinic space group P121/N1 (No. 14) except a few weak peaks, which most likely due to a small amount of Fe\(_2\)O\(_3\). The deduced lattice parameters are a = 5.398 Å, b = 5.520 Å, c = 7.699 Å, and \(\beta\) = 89.95\(^{\circ }\), respectively, which are in good agreement with previous reports [21, 22].
Figure 2 displays the magnetic susceptibility as a function of temperature and its derivative in an applied magnetic field of 200 Oe during an FC process. One can see that below 280 K, the magnetization increases rapidly with cooling and tends to a saturation. While above 280 K, the dependence of magnetization with temperature is relatively weak. The results indicate a temperature-induced ferrimagnetism to paramagnetism phase transition around 280 K in this system. By calculating the first derivative of magnetic susceptibility to temperature (see red line in Fig. 2), it is clearly seen that the slope changes fastest at 280 K. Therefore, the Curie temperature is determined to be 280 K. We note that the \(T_c\) is lower than the published results [18, 21, 22], which may originate from the oxygen vacancy and/or site disorder induced by long-term heating.
To elucidate the MCE in this system, we first present the isothermal magnetization in an applied magnetic field up to 9 T in Fig. 3. The temperatures are from 260 K to 340 K with step \(\Delta\)T = 2 K. At low temperatures, the magnetization increase rapidly at low field and keep increasing with magnetic field in a gentle way at high field. It is seen that the magnetization shows non-signature of full saturation even up to 9 T. The non-saturation behavior is also observed in single crystal sample [21] and the sister compound Sr\(_2\)FeMoO\(_6\) [23], which can be explained by the half-metallic origin of ferrimagnetism. While at high temperatures, the data show typical Brillouin Function behavior, suggesting paramagnetic state is reached.
In equilibrium thermodynamics, a magnet will be warmed up in magnetic field and vise versa in an adiabatic process. This behavior is well known as MCE [24]. Generally, the characteristic parameter of MCE can be expressed by the \(\Delta\) \(S\) \(_M\) according to Maxwell relation
where H, M and T are magnetic field, magnetization and absolute temperature, respectively. In reality, this formula can be further approximated by
where M\(_i\) and M\(_{i-1}\) represent the experimental values of the magnetization at T\(_i\) and T\(_{i-1}\) under the same magnetic field range \(\Delta\)H. Based on Eq. 1, one can calculate the \(\Delta\) \(S_M\) from the discrete M(H) data.
Figure 4 shows the results of -\(\Delta\) \(S_M\) as a function of T and \(\Delta\) \(H\). It is seen that the -\(\Delta\) \(S_M\) peaks at around the Curie temperature and the full width at half maximum (FWHM) is very large, which may suggest a high refrigerant capacity (RC, defined by FWHM times the corresponding -\(\Delta\) \(S_M\)). However, the absolute value of -\(\Delta\) \(S_M\)s are very low which would discount the RC significantly. For example, at \(\Delta\) \(H\) = 5 T, the maximum of -\(\Delta\) \(S_M\) is 1.04 J kg\(^{-1}\) K\(^{-1}\) in Ca\(_2\)FeMoO\(_6\) while it is 9.5 J kg\(^{-1}\) K\(^{-1}\) in the prototype magnetic refrigerant Gd metal [6]. Although the -\(\Delta\) \(S_M\) is much less than the state of the art materials, it is comparable to its sister compound, Sa\(_2\)FeMoO\(_6\). i.e., at \(\Delta\) \(H\) = 1 T, the -\(\Delta\) \(S_M\) is \(\sim\)0.3 J kg\(^{-1}\) K\(^{-1}\) in Ca\(_2\)FeMoO\(_6\), while it is \(\sim\)0.1 J kg\(^{-1}\) K\(^{-1}\) in Sr\(_2\)FeMoO\(_6\) [23].
Since the FWHM is not obtained in our experiment, a temperature average entropy change (TEC) is adopted to determine the refrigerant performance. This caloric material-based figure of merit was proposed by Griffith etal. [25].It is on the ground of -\(\Delta\) \(S_M\) data and calculated over a range of temperatures, \(\Delta\) \(T\) \(_{lift}\), that a material can reasonably support in response to a given field change \(\Delta\) \(H\). The TEC is defined as following
where, \(T\) \(_{mid}\) is chosen by sweeping over -\(\Delta\) \(S_M\)(T, \(\Delta\) \(H\)) data and select the value that maximizes TEC(\(\Delta\) \(T\) \(_{lift}\) for the given \(\Delta\) \(T\) \(_{lift}\). As is shown in Fig. 5, the TEC(12) increase monotonously with magnetic field from \(\sim\)0.3 J kg\(^{-1}\) K\(^{-1}\) at 1 T to 1.65 J kg\(^{-1}\) K\(^{-1}\) at 9 T. When compared with the several well-known materials, the MCE performance is rather weak. i.e., for Gd\(_5\)Si\(_2\)Ge\(_2\), TEC(10) = 6.89 J kg\(^{-1}\) K\(^{-1}\)@1 T; for La(Fe\(_{0.88}\)Si\(_{0.12}\))\(_{13}\), TEC(10) = 9.11J kg\(^{-1}\) K\(^{-1}\)@1 T; for Gd, TEC(10) = 2.91J kg\(^{-1}\) K\(^{-1}\)@1 T [25].
At last, we would like to discuss the order of magnetic phase transition of Ca\(_2\)FeMoO\(_6\) by using the dependence exponent n of magnetic entropy change with field near \(T_c\) proposed by Law et al. [26]. In their arguments, the FOMT and SOMT can be distinguished objectively and straightforwardly by compare an exponent n with a number 2. Where the exponent n can be deduced from the field dependence of magnetic entropy change at various temperatures by
If the exponent n \(\ge\) 2, then the material is a typical FOMT material, otherwise the phase transition would be continuous. As is clearly seen in Fig. 6, the values of n are definitely smaller than 2 around \(T_c\), indicating a second order magnetic phase transition.
4 Conclusions
In summary, by performing magnetic susceptibility and isothermal magnetization measurements, we have investigated the magnetic properties and magnetocaloric effect of a double peroveskite Ca\(_2\)FeMoO\(_6\). A ferrimagnetic to paramagnetic transition at 280 K is identified in the magnetic susceptibility measurement. The magnetocaloric effect is evaluated by calculating the magnetic entropy change as well as the temperature average entropy change. Compared with the well-known magnetic refrigerant candidates, the performance of Ca\(_2\)FeMoO\(_6\) is found rather modest. At last, by deriving the exponent n dependence of magnetic entropy change with field, we verify the second order phase transition nature of Ca\(_2\)FeMoO\(_6\).
References
Gschneidner, K.A., Jr., Pecharsky, V.K., Tsokol, A.O.: Rep. Prog. Phys. 68, 1479 (2005)
Brück, E.: J. Phys. D: Appl. Phys. 38, R381 (2005)
Reis, M.S.: Coordin. Chem. Rev. 417, 213357 (2020)
Zhang, Y.K.: J. Alloy and Compd. 787, 1173–1186 (2019)
Kitanovski, A.: Adv. Engergy Mater. 10, 1903741 (2020)
Pecharsky, V.K., Gschneidner, K.A., Jr.: Phys. Rev. Lett. 78, 4494 (1997)
Pecharsky, V.K., Gschneidner, K.A.: Appl. Phys. Lett. 70, 3299–3301 (1997)
Wada, H., Tanabe, Y.: Appl. Phys. Lett. 79, 3302–3304 (2001)
Tegus, O., Brück, E., Buschow, K.H.J., de Boer, F.R.: Nature 415, 150–152 (2002)
Cam Thanh, D.T., Brück, E., Trung, N.T., Klaasse, J.C.P., Buschow, K.H.J., Ou, Z.Q., Tegus, O., Caron, L.: J. Appl. Phys. 103, 07B318 (2008)
Fujita, A., Fujieda, S., Hasegawa, Y., Fukamichi, K.: Phys. Rev. B 67, 104416 (2003)
Shen, B.G., Sun, J.R., Hu, F.X., Zhang, H.W., Cheng, Z.H.: Adv. Mater. 2, 4545–4564 (2009)
Hu, F.X., Shen, B.G., Sun, J.R., Wu, G.H.: Phys. Rev. B 64, 132412 (2001)
Kuz’min, M.D.: Appl. Phys. Lett. 90, 251916 (2007)
Guo, Z.B., Du, Y.W., Zhu, J.S., Huang, H., Ding, W.P., Feng, D.: Phys. Rev. Lett. 78, 1142 (1997)
Kim, D., Revaz, B., Zink, B.L., Hellman, F., Rhyne, J.J., Mitchell, J.F.: Phy. Rev. Lett. 89, 227202 (2002)
Maiti, T., Saxena, M., Roy, P.: J. Mater. Res. 34, 107–125 (2019)
Kobayashi, K.I., Kimura, T., Sawada, H., Terakura, K., Tokura, Y.: Nature 395, 677–680 (1998)
Sogahara, T., Nong, N.V., Ohtaki, M.: Mater. Chem. Phys. 133, 630–634 (2012)
Huan, Y., Li, Y.N., Yin, B.Y., Ding, D., Wei, T.: J. Power Sources 359, 384–390 (2017)
Moreno, N.O., Barbosa, L.B., Ardila, D.R., Andreeta, J.P.: J. Supercond. Nov. Magn 26, 2501–2503 (2013)
Kim, S.B., Kim, C.S.: J. Radioanal. Nucl. Ch. 330, 439–443 (2021)
Anwar, M.S., Hussain, I., Koo, B.H.: Mater. Lett. 181, 56–58 (2016)
Gschneidner, K.A., Jr., Pecharsky, V.K.: Annu. Rev. Mater. Sci. 30, 387–429 (2000)
Griffith, L.D., Mudryk, Y., Slaughter, J., Pechasky, V.K.: J. Appl. Phys. 123, 034902 (2018)
Law, J.Y., Franco, V., Moreno-Ramírez, L.M., Conde, A., Karpenkov, D.Y., Radulov, I., Skokov, K.P., Gutfleisch, O.: Nat. Commun. 9, 2680 (2018)
Acknowledgements
This project is supported by the Zhengzhou University of Light Industry Foundation of China (Grant No. 2012XJJ008) and the Key Project of Scientific and Technological Research of the Education Department of Henan Province (Grant No. 13B140332).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, X., Li, J. & Wu, J. Magnetocaloric Effect of Ferrimagnetic Double Perovskite Ca\(_2\)FeMoO\(_6\) . J Supercond Nov Magn 35, 909–913 (2022). https://doi.org/10.1007/s10948-021-06133-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-021-06133-0