Abstract
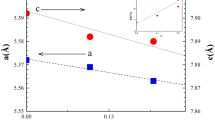
We report on the synthesis and structural and magnetic properties of the new SrLa2FeCuSbO9 perovskite. The compound was prepared by the solid-state reaction method. The crystalline structure was studied by X-ray diffraction experiments using the Rietveld refinement analysis. SrLa2FeCuSbO9 crystallizes in a monoclinic system (P21/n space group). Its unit cell parameters are a = 5.6101(2) Å, b = 5.6125(2) Å, c = 7.9358(3) Å, and β = 90.094(2)°. Sr2+ and La3+, in SrLa2FeCuSbO9, are statistically distributed within the A-perovskite sites. The obtained cationic distribution can be illustrated by the [SrLa2]A[(SbCu0.5)2c(FeCu0.5)2d]BO9 crystallographic formula. SrLa2FeCuSbO9 was found to exhibit a paramagnetic–ferrimagnetic transition around TC = 215 K. At high temperature, the magnetic susceptibility was described by the Curie–Weiss law. The experimental effective moments were explained by only spin contribution for Cu2+ and Fe3+ ions. At low temperature, the compound presents a ferrimagnetic state with signs of frustration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Perovskite compounds of the general formula ABO3, where A is an (s or f) block cation, and B is a (d or p) block cation, have been widely studied because of their very interesting and surprising physical and chemical properties [1]. These compounds represent a large percentage of systems currently studied and continue to interest scientists. In fact, it is due to the remarkable flexibility of this structure which can accommodate a wide variety of chemical elements with different oxidation states, which gives a large number of compounds with different fundamental and interesting chemical and physical properties such as electronic structures ranging from insulating to metallic [2], superconductivity [3], ferromagnetic [4], ferroelectric [5], magnetoresistance [6], and multiferroic [7]. In ABO3 perovskite phases, if two or three different types of atoms are introduced into A and/or B sites, more complex structures, such as double perovskite AA′BB′O6 and triple perovskite A2A′B2B′O9, can be adopted [8]. In recent years, A3B2B′O9 triple perovskites have been of great interest, because they present new promising physical properties such as relaxor ferromagnetic [9]. Recently, SrLa2FeCoSbO9 compounds have been synthesized and their structural and magnetic properties have been studied [10]. It has been found that this compound presents a relative height magnetic transition to a ferrimagnetic phase (TC = 215 K). This result was related to the presence of two different magnetic (B, B′) cations in the octahedral sites. Indeed, this compound adopts a monoclinic perovskite structure (P21/n space group) with two distinct octahedral sites; one of these sites is occupied by (2/3Co2+, 1/3Fe3+) and the other by (2/3Sb5+, 1/3Fe3+). Similar compounds such as La2SrCo2FeO9 [11], La2SrFe2CoO9 [12], and Sr3CrFeMoO9 [13] have been investigated. The earlier three compounds were shown to present a ferrimagnetic state below 123 K, 280 K, and 400 K, respectively. The high magnetic transition temperature observed in these compounds seems to be linked to the presence of Fe3+ ions which imposes strong magnetic interactions. Several results show that the compounds which contain the same magnetic cations in two octahedral sites present a relatively low magnetic transition temperature TC such as the compounds La3Ni2SbO9 (TC = 105 K) [9], La3Co2SbO9 (TC = 55 K) [14], LaSr2Cr2SbO9 (TC = 150 K) [15], SrLa2Ni2TeO9 (spin glass below 35 K) [16], and Sr3Fe2TeO9 (spin glass below 80 K) [17]. It should be noted that in this type of compounds the crystal structure, the nature of the chemical elements which are in A, B, and B′ sites, and the cation distribution between the two B and B′ sites of the perovskite structure play a determinant role on their magnetic properties. Indeed, these parameters can modulate the competition between the different types of magnetic interactions which lead to particular effects, such as magnetic frustration or high Tc ferromagnetic properties. In our previous structural characterization study, the structure of the [CaLa2]A[CaTi2]BO9 phase (P21/n space group) is shown to adopt a partial 1:1 ordering between cations in the B site whereas in the case of [CaLn2]A[CuTi2]BO9 (Ln = Pr, Nd, Sm) and [CaLn2]A[ZnTi2]BO9 (Ln = La, Pr, Nd, Eu) (Pbnm space group) the cationic distribution within the A and B sites is statistical [18,19,20]. In this work, we present the synthesis and structural properties of the SrLa2FeCuSbO9 compound, and their cationic distribution is obtained by using different models in the Rietveld refinement. We also present a study of the magnetic properties using magnetization measurements. The detailed relationship between the structural and magnetic properties was also investigated.
2 Experimental
The polycrystalline sample of SrLa2FeCuSbO9 was prepared by conventional solid-state reaction at high temperature from the mixture, in stoichiometric proportions, of La2O3, SrCO3, Sb2O3, CuO, and Fe2O3 powders (purity > 99.95%). The mixture was ground, pressed, and annealed at 900 °C for 24 h, 1100 °C for 48 h, and 1200 °C for 48 h. The obtained sample is finally ground, pressed, and sintered at 1300 °C for 48 h. X-ray diffraction (XRD) experiment was performed at room temperature by means of a diffractometer Panalytical X′Pert Pro (θ-2θ) equipped with detector x’celerator radiance (CuKα = 1.5406 Å) (45 kV, 40 mA), slope of divergence of 1°, 0.10 mm receiving slot, and anti-dispersion slot of 1°. Data were collected from 10 to 110° (2θ), in increments of 0.02°, with a count time of 15 s per step. The refinement of the crystal structure was performed by the Rietveld method [21] using the FullProf program [22]. The changes of magnetization as function of temperature (2–300 K) through zero-field cooling (ZFC) and field cooling (FC) procedures and under a fixed value of applied magnetic field of 50 Oe were performed using the Quantum Design MPMS-XL5 SQUID magnetometer. Magnetization measurements as a function of applied magnetic field (hysteresis loops) were recorded at 10 K from − 50 kOe up to + 50 kOe.
3 Results and Discussion
3.1 Structural Study
Obtained XRD pattern indicates that SrLa2FeCuSbO9 crystallizes in monoclinic symmetry (P21/n space group). The pattern also indicates the presence of a secondary phase with a small amount (about 7% by weight) which is attributed to La3SbO7 (JCPDS PDF#23-1138). The structural refinements were carried out by the Rietveld method using the FullProf program. The most intense peak region due to La3SbO7 was excluded from the refinement. The structural model used in our refinements was based on those reported for SrLa2FeCoSbO9 (P21/n space group) [10]. Note that in SrLa2FeCoSbO9, 1/3 of the Sr2+ ions and 2/3 of the La3+ ions are statistically distributed in the (4e) positions of A-perovskite sites. This structure contains two distinct (2c) and (2d) positions of the octahedral B-sites, which must be occupied by the smallest (Fe, Co, and Sb) elements. The oxygen ions are located in three different (4e) positions.
The first step of refinement was to determine the correct cell parameters using the P21/n space group. In structural refinement, three models were tested and the quality of the refinements was judged based on the reliability factors (RP, Rwp, RB, RF-factor, and Chi2). The refined parameters were the lattice parameters, the oxygen atomic coordinates, the occupancy of the different sites, and the atomic displacement parameters of all atoms. The peak shapes were described using a pseudo-Voigt function.
The three tested models for the SrLa2FeCuSbO9 compound can be illustrated by the three crystallographic formula: SrLa2(CuSb0.5)2c(FeSb0.5)2dO9 in the first model (M1), SrLa2(CuFe0.5)2c(SbFe0.5)2dO9 in the second model (M2), and SrLa2(SbCu0.5)2c(FeCu0.5)2dO9 in the third model (M3). Figure 1 represents the observed and calculated shape of the selected peak region which is more sensitive to the cationic distribution, for the three models.
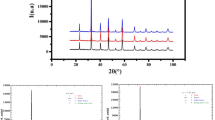
The M1 model, where the Sb5+ ions are distributed between the two (2c) and (2d) sites, gives unsatisfactory refinement results, in good agreement with reported work for the isotypic SrLa2FeCoSbO9 compound [10]. According to obtained reliability factors, the two M2 and M3 models seem to be the best. However, for the M2 model, where the Fe3+ ions are distributed between the two sites, the refinement leads to negative values for the parameters of displacement of oxygen and cations in the (2c) and (2d) sites. The M3 model, where the Cu2+ ions are distributed between the two (2c) and (2d) sites, gives satisfactory refinement results (RB = 3.5%). Figure 2 shows the good agreement between the observed and calculated profiles obtained for the SrLa2(SbCu0.5)2c(FeCu0.5)2dO9 crystallographic formula. Table 1 displays details of the crystal structures, cell parameters, oxygen atomic coordinates, cationic distribution, and reliability factors.
SrLa2FeCuSbO9 adopts a crystal structure with monoclinic P21/n space group. In this structure, 1/3 of the Sr2+ ions and 2/3 of the La3+ are statistically distributed in the A-perovskite sites. The two distinct (2c) and (2d) octahedral sites are occupied by (2/3Sb5+, 1/3Cu2+) and (2/3Fe3+, 1/3Cu2+), respectively. Table 2 groups the (La/Sr)–O, (Sb/Cu)–O and (Fe/Cu)–O bond distances and the different bond angles, and Fig. 3 shows the crystal structure of SrLa2FeCuSbO9. The way in which the octahedra are connected indicates that the crystal structure is typical of a perovskite with octahedral tilt distortion and that the tilt system is a+a+c− [23]. We also note that the angles in the (Sb/Cu)O6 and (Fe/Cu)O6 octahedra are all far from those of the ideal structure of perovskite. This can be explained by the tolerance factor of Goldschmidt [24]. Taking into account the ionic distribution obtained by the Rietveld refinement and based on the effective Shannon’s ionic radii [25] (r(O2−) = 1.26 Å, rVI(Cu2+) = 0.87 Å, rVI(Fe3+) = 0.785 Å, r(La3+) = 1.356 Å, rIV(Sb5+) = 0.74 Å, r(Sr2+) = 1.45 Å), the value of the calculated tolerance factor of Goldschmidt (t) is t = 0.90. This last value is low enough to give rise to significant deviations from the ideal perovskite. Note that the presence of Cu2+(3d9) ions in the two (2c) and (2d) sites can reinforce the distortion of octahedra by Jahn–Teller effect, but the amount of Cu2+ in every B site is not in favor of their appearance. The comparison of the experimental A–O and B–O band distance values with those calculated from Shannon’s ionic radii (i.e., (Sr/La)–O = 2.64 Å, (Sb/Cu)–O = 2.04 Å, and (Fe/Cu)–O = 2.09 Å) agrees well with the obtained cationic distribution (Table 2).
The bond valence model [26] can give an estimate of the valences of cations and anions in a crystal structure. The value of calculated bond valence sum for each (Sb/Cu)2c and (Fe/Cu)2d sites are shown in Table 2. Note that a relativity good agreement between expected and calculated bond valence sums for the two octahedral (2c) and (2d) sites is obtained. The slight divergence observed for the (Sr/La) site, can be related to the disordered distribution of the two atom types within the same crystallographic position.
3.2 Magnetic Study
Figure 4 a shows the temperature dependence of zero field cooled (ZFC) and field cooled (FC) magnetization for SrLa2FeCuSbO9 recorded in an applied magnetic field of 50 Oe in the temperature range of (2–300) K. A clear transition from the paramagnetic to ferrimagnetic state is observed at TC. The magnetic transition temperature TC is extracted from the derivative dM(T)/dT (see Fig. 4b); both ZFC and FC magnetization curves show the same transition temperature TC = 215 K. Fig. 5 displays the inverse magnetic susceptibility in the temperature ranges from 2 to 300 K. At high temperature (T > TC), the inverse magnetic susceptibility is linear and can be described using Curie–Weiss law:
Here, C is the Curie constant and θ is the Curie–Weiss temperature. The results of the fit using Curie–Weiss law are plotted in Fig. 5 with a solid line. The obtained Curie constant C and Weiss constant θ are 3.81 emu K/mol and + 226 K, respectively. Experimental effective magnetic moment (μeff) was extracted from the obtained value of the Curie constant (μeff = 5.51 μB/f.u). μeff was compared with the theoretical moment obtained using spin-only approximation for both Cu2+ and Fe3+ ions. The magnetic moments μS (Cu2+) and μS (Fe3+) can be expressed as \( {\mu}_{\mathrm{s}}\left({M}^{n+}\right)=g\sqrt{S\left(S+1\right)} \), with g (g = 2) is the Landé factor, S (Cu2+) = 1/2 and S (Fe3+) = 5/2, which give μS (Cu2+) = 1.73 μB and μS (Fe3+) = 5.91 μB. The total theoretical moment was calculated using the following formula: \( {\mu}_{\mathrm{theo}}=\sqrt{\mu_s^2\left({\mathrm{Cu}}^{2+}\right)+{\mu}_s^2\left({\mathrm{Fe}}^{3+}\right)} \) (μB/formula unit), the obtained theoretical moment is μtheo = 6.15 μB, which is slightly higher than the experimental one (μeff = 5.51 μB) with a difference Δμ = |μeff-μtheo| of 10.4%. This difference could be attributed to the presence of impurities (La3SbO7) detectable by XRD. On the other hand, the Curie–Weiss temperature value (θ = + 226 K) is high and positive which indicates the dominance of strong ferromagnetic interactions at low temperature. In addition, the transition temperature TC = 215 K is very close to the Curie–Weiss temperature θ with a ratio of f = θ/TC = 1.05. This obtained value of the f parameter is characteristic of a ferromagnetic behavior [27]. At low temperature (T < TC), the magnetization curves ZFC and FC increase rapidly and stabilize at low temperature (see Fig. 4a), indicating the establishment of a ferrimagnetic order. The magnetization curves ZFC and FC show the existence of irreversibility over a wide temperature range (T < TC). A similar difference in ZFC and FC magnetization data has been reported in disordered double and triple perovskite compounds La3Ni2-xCuxB′O9 (B′ = Sb, Ta, Nb) [28], La2BMnO6 (B = Ni or Co) [29]. This behavior has been explained by a disordered magnetic system such as spin glasses and clusters glasses [9, 12].
Figure 6 displays the magnetization as a function of the applied magnetic field at 10 K. The magnetization curve shows a weak hysteresis loop typical of a ferrimagnetic system with an unsaturated character up to 50 kOe, weak coercive field (HC = 195 Oe) and remanent magnetization Mr = 0.45 μB/f.u. (see inset in Fig. 6). The first magnetization as a function of the magnetic field shows a rapid increase for low magnetic fields, followed by a linear increase as a function of the applied magnetic field without saturation, which indicates blocked magnetic moments due to the inhomogeneity in the distribution of magnetic moments caused by cationic disorder. The spontaneous magnetization Ms was deduced by extrapolating to H = 0 Oe the linear part of the magnetization curve; Ms is found to be close to 1.46 μB/f.u.
It is of interest to note that the Mr/Ms ratio can give an idea on the structure of magnetic domains in ferromagnetic or ferrimagnetic materials. As well, it is known that for polycrystalline samples with uniaxial and cubic magnetocrystalline anisotropy, the Mr/Ms ratios are 0.50 and 0.83, respectively [30, 31]. Furthermore, the Mr/Ms ratio equal to or greater than 0.5 indicates that the sample can be in the form of nanoparticles with a single magnetic domain, and for Mr/Ms less than 0.5 it indicates the formation of a multidomain structure [32, 33]. In our case, the calculated Mr/Ms ratio, at T = 10 K, is approximately 0.30. This last value is less than 0.50, which could indicate the formation of a multidomain structure, in accordance with the structure of our compound as a bulk material. Otherwise, this low value of Mr/Ms can also be related to the inhomogeneity in the distribution of magnetic moments caused by the cationic disorder.
Assuming a G-type collinear ferrimagnetic arrangement, where Fe3+(S = 5/2), Cu2+(S = 1/2), and Sb5+ (diamagnetic) ions are distributed in a disordered manner in the two different (2c) and (2d) crystallographic sites, the spontaneous magnetization Ms can be estimated by the Néel model assuming a mean magnetization in each site. Considering the cationic distribution formula, the mean values of magnetizations M(2c) in the (2c) and M(2d) in the (2d) sites are 3.66 μB and 0.33 μB, respectively. Therefore, the net magnetization Ms can be calculated by the Néel model Ms = M(2c) − M(2d). The magnitude of the net magnetization Ms per formula unit should be 3.33 μB/f.u. However, the experimental value of 1.46 μB/f.u. is considerably less than the theoretical value, which indicates that the compound is far from saturated. This behavior has already been observed on triple-perovskite compounds [9, 15, 34, 35] which might be attributed to the following reasons. First, the cationic disorder imposed by non-magnetic ions such as Sb5+ ions disrupts the magnetic interactions, which prevents the establishment of a long-range magnetic order and leads to the formation of magnetic domains. Therefore, the low magnetization measured at low temperature is attributed to the spontaneous magnetization present in each domain. This behavior was observed on the partially disordered La3Ni2SbO9 compound, which behaves as a ferrimagnetic relaxant below 110 K [9]. Secondly, the weak magnetization measured at low temperature is linked to the weakening of the efficiency and the dominance of the magnetic NN interactions by the presence of the diamagnetic Sb5+ ions. NN interactions are magnetic superexchange intercations between the nearest neighboring cations [10]. In this case, the long-range magnetic order is maintained, but the NN coupling is weakened. This is the case for the SrLa2FeCoSbO9 compound which has a magnetic transition temperature (TC = 215 K) higher than that of La3Ni2SbO9 [9, 10].
This second model seems to give an explanation of the magnetic behavior observed at low temperature in our compound which presents a cationic disorder with a relative high transition temperature (TC = 215 K) similar to that observed for SrLa2FeCoSbO9. The positive Curie–Weiss temperature θ = +229 K, the shape of the ZFC/FC magnetization curves and the presence of a hysteresis loop are characteristic of a ferromagnetic behavior. However, the existence of a high irreversibility at low temperatures between the ZFC and FC magnetization curves and the low value of the spontaneous magnetization at H = 50 kOe indicates the presence of inhomogeneity in magnetic interactions caused by the cationic disorder. In order to improve the understanding of the magnetic interactions in this compound, neutron diffraction and electronic microscopy will be useful.
4 Conclusion
In summary, we have investigated the structural and magnetic properties of SrLa2FeCuSbO9. The crystal structure was refined by X-ray powder diffraction data in the monoclinic P21/n space group. The A sites are statistically occupied by 1/3 of Sr2+ ions and 2/3 of La3+. The two distinct octahedral (2c) and (2d) sites are occupied by (2/3Sb5+, 1/3Cu2+) and (2/3Fe3+, 1/3Cu2+), respectively. The result of the structural analysis indicates that the crystal structure of our compound is typical of a perovskite with octahedral tilt distortion and that the tilt system is a+a+c−. Magnetization measurements indicate a paramagnetic–ferrimagnetic transition around TC = 215 K. In the paramagnetic region, the magnetic susceptibility was described by Curie–Weiss law. The experimental effective moments were explained by only spin contribution for the Fe3+ and Cu2+ ions. The effective magnetic moment obtained experimentally is μeff = 5.51 μB/f.u. The positive and high Curie–Weiss temperature (θ = +226 K) indicates the dominance of strong ferromagnetic interactions at low temperature. The obtained value of the frustration parameter is characteristic of a ferromagnetic behavior. In the ordered magnetic regime, the shape of the ZFC/FC magnetization curves and the presence of a hysteresis loop are characteristic of a ferrimagnetic behavior. However, the existence of a high irreversibility at low temperatures between the ZFC and FC magnetization curves and the low value of the spontaneous magnetization at H = 50 kOe indicates the presence of inhomogeneity in magnetic interactions caused by the cationic disorder. Finally, these results will be useful to understand the magnetic properties in SrLa2FeCuSbO9.
References
Tilley, R.J.: Perovskites: structure-property relationships. John Wiley & Sons, New York (2016)
Torrance, J., Lacorre, P., Nazzal, A., Ansaldo, E., Niedermeyer, C.: Systematic study of insulator-metal transitions in perovskites RNiO3 (R= Pr, Nd, Sm, Eu) due to closing of charge-transfer gap. Phys. Rev. B. 45, 8209 (1992)
Maeno, Y., Hashimoto, H., Yoshida, K., Nishizaki, S., Fujita, T., Bednorz, J., Lichtenberg, F.: Superconductivity in a layered perovskite without copper. Nature. 372, 532 (1994)
Park, J.H., Vescovo, E., Kim, H.J., Kwon, C., Ramesh, R., Venkatesan, T.: Direct evidence for a half-metallic ferromagnet. Nature. 392, 794–796 (1998)
Ronald, E.C.: Origin of ferroelectricity in perovskite oxides. Nature. 358, 136–138 (1992)
Kobayashi, K.I., Kimura, T., Sawada, H., Terakura, K., Tokura, Y.: Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature. 395, 677–680 (1998)
Fernandez-Posada, C.M., Castro, A., Kiat, J.-M., Porcher, F., Pena, O., Alguero, M., Amorin, H.: A novel perovskite oxide chemically designed to show multiferroic phase boundary with room-temperature magnetoelectricity. Nat. Commun. 7, 12772 (2016)
Anderson, M.T., Greenwood, K.B., Taylor, G.A., Poeppelmeier, K.R.: B-cation arrangements in double perovskites. Prog. Solid State Chem. 22, 197–233 (1993)
Battle, P.D., Evers, S.I., Hunter, E.C., Westwood, M.: La3Ni2SbO9: a relaxor ferromagnet. Inorg. Chem. 52, 6648 (2013)
Tang, Y., Hunter, E.C., Battle, P.D., Hendrickx, M., Hadermann, J., Cadogan, J.M.: Ferrimagnetism as a consequence of unusual cation ordering in the perovskite SrLa2FeCoSbO9. Inorg. Chem. 57, 7438 (2018)
Albornoz, J.C., Landínez Téllez, D.A., Roa-Rojas, J., Munévar, J.A., Baggio-Saitovich, E.: Structural and magnetic properties of the new La2SrCo2FeO9 triple perovskite. J. Supercond. Nov. Magn. 26, 2313–2317 (2012)
Casallas, F., Vera, E., Landínez, D., Parra, C., Roa, J.: Structural properties, electric response and magnetic behaviour of La2SrFe2CoO9 triple complex perovskite. Phys.: Conf. Ser. 687, 012047 (2016)
Li, Z., Jiao, L., Ji, W.J., Xu, J., Wang, J.F., Gu, Z.B., Zhou, J., Yao, S.-H., Chen, Y.B., Zhang, S.-T.: Yan-Feng: triple perovskite Sr3CrFeMoO9 ultrathin films with ferromagnetism above room temperature. Scr. Mater. 69, 590 (2013)
Franco, D.G., Fuertes, V.C., Blanco, M.C., Fernandez-Diaz, M.T., Sanchez, R.D., Carbonio, R.E.: Synthesis, structure and magnetic properties of La3Co2SbO9: a double perovskite with competing antiferromagnetic and ferromagnetic interactions. J. Solid State Chem. 194, 385–391 (2012)
Hunter, E.C., Battle, P.D., Paria Sena, R., Hadermann, J.: Ferrimagnetism as a consequence of cation ordering in the perovskite LaSr2Cr2SbO9. J. Solid State Chem. 248, 96–103 (2017)
Paria Sena, R., Hadermann, J., Chin, C.M., Hunter, E.C., Battle, P.D.: Structural chemistry and magnetic properties of the perovskite SrLa2Ni2TeO9. J. Solid State Chem. 243, 304–331 (2016)
Tang, Y., Hunter, E.C., Battle, P.D., Paria Sena, R., Hadermann, J., Avdeev, M., Cadogan, J.M.: Structural chemistry and magnetic properties of the perovskite Sr3Fe2TeO9. J. Solid State Chem. 242, 86–95 (2016)
Aatiq, A.: Crystal structure of the new perovskite CaLa2CaTi2O9(=(Ca1/3La2/3)A (Ca1/3Ti2/3)BO3). Solid State Sci. 5, 745–749 (2003)
Aatiq, A., Boukhari, A.: Synthesis and structural study of the new perovskite series CaLn2ZnTi2O9(= (Ca1/3Ln2/3)A (Zn1/3Ti2/3)BO3) (Ln = La, Pr, Nd, Eu). Mater. Lett. 58, 2406–2411 (2004)
Iturbe-Zabalo, E., Igartua, J.M., Aatiq, A., Pomjakushin, V.: A structural study of the CaLn2CuTi2O9 (Ln = Pr, Nd, Sm) and BaLn2CuTi2O9 (Ln = La, Pr, Nd) triple perovskite series. J. Mol. Struct. 25, 205–401 (2013)
Rietveld, H.M.: A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65–71 (1969)
Rodriguez-Carvajal, J.: Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter. 192, 55–69 (1993)
Woodward, P.M.: Octahedral tilting in perovskites. I. Geometrical Considerations. Acta Cryst. B. 53, 32–43 (1997)
Goldschmidt, V.M.: Die Gesetze der Krystallochemie. Naturwissenschaften. 14, 477–485 (1926)
Shannon, R.D.: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A. 32, 751–767 (1976)
Brown, I.D., Altermatt, D.: Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. B. 41, 244–247 (1985)
Ramirez, A.P.: Strongly geometrically frustrated magnets. Annu. Rev. Matter. Sci. 24, 453–480 (1994)
Chin, C.M., Battle, P.D., Hunter, E.C., Avdeev, M., Hendrickx, M., Hadermann, J.: Stabilisation of magnetic ordering in La3Ni2-xCuxB’O9 (B’ = Sb, Ta, Nb) by the introduction of Cu2+. J. Solid State Chem. 276, 164–172 (2019)
Mao, Y., Parsons, J., McCloy, J.S.: Magnetic properties of double perovskite La2BMnO6 (B = Ni or Co) nanoparticles. Nanoscale. 5, 4720 (2013)
Chikazumi, S., Graham Jr., C.D.: Physics of ferromagnetism. Oxford Clarendon Press, Oxford (1997)
Xie, X., Che, H., Wang, H., Lin, G., Zhu, H.: Negative zero-field-cooled magnetization in YMn0.5Cr0.5O3 due to giant coercivity and trapped field. Inorg. Chem. 57, 175–180 (2018)
Ali, I., Islam, M.U., Awan, M.S., Ahmad, M., Ashiq, M.N., Naseem, S.: Effect of Tb3+ substitution on the structural and magnetic properties of M-type hexaferrites synthesized by sol–gel auto-combustion technique. J. Alloy. Compd. 550, 564–572 (2013)
Almessiere, M.A., Slimani, Y., Sertkol, M., Nawaz, M., Sadaqat, A., Baykal, A., Ercan, I., Ozçelik, B.: Effect of Nb3+ substitution on the structural, magnetic, and optical properties of Co0.5Ni0.5Fe2O4 nanoparticles. Nanomaterials. 9, 430 (2019)
Tang, Y., Paria Sena, R., Avdeev, M., Battle, P.D., Cadogan, J.M., Hadermann, J., Hunter, E.C.: Magnetic properties of the 6H perovskite Ba3Fe2TeO9. J. Solid State Chem. 253, 347–354 (2017)
Wang, J.F., Hu, B., Zhang, J., Gu, Z.B., Zhang, S.T.: Composition dependent magnetism in novel triple perovskite Sr3MFeMoO9 (M=Mn, Co, Ni). Ceram. Int. 40, 8753–8759 (2014)
Funding
This study was financially supported by the Moroccan Ministry of Higher Education, Scientific Research and Training (MESRSFC) managerial staff, National Center for Scientific and Technical Research (CNRST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boudar, N., Mehdaoui, B. & Aatiq, A. Structural and Magnetic Properties of the New SrLa2FeCuSbO9 Perovskite Phase. J Supercond Nov Magn 33, 2829–2836 (2020). https://doi.org/10.1007/s10948-020-05538-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-020-05538-7


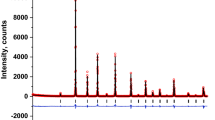

 ) XRD patterns, in the 2θ = 18–26° range of SrLa2FeCuSbO9, in the three models of cationic distribution
) XRD patterns, in the 2θ = 18–26° range of SrLa2FeCuSbO9, in the three models of cationic distribution
 ) and difference (
) and difference ( ) profile of the XRD pattern of SrLa2FeCuSbO9 (*La3SbO7)
) profile of the XRD pattern of SrLa2FeCuSbO9 (*La3SbO7)


