Abstract
Pre-reacted powders of M-type strontium hexaferrites with chemical composition Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19 (x = 0.1, 0.2, 0.3 0.5, 0.6) were obtained from the conventional solid state reaction method. The final consolidation of these powders were performed by using the spark plasma sintering (SPS) technique. The influence of the La3+ content on the crystal structure and the magnetic properties of the samples were investigated firstly under the framework of a phenomenological model that predicted the cation substitution/distribution in the Sr and/or Fe sites. The theoretical results indicated that La3+ ion mostly occupies the Sr2+ site. It was also found that as La content increased, a certain amount of Fe2+ ions appear as a consequence of the Fe3+ to Fe2+ reduction. However, the Fe2+ occupied preferably the 12k and 4f1 iron sites. The magnetic properties of the obtained samples were assessed by measurements performed in a vibrating sample magnetometer. Also, X-ray diffraction (XRD) measurements were presented as a complementary characterization. We have found that the magnetic properties of the samples degraded as the La3+ content increased and the increase of the Fe2O3 impurity phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
M-type hexaferrites are used in applications such as loudspeakers, windscreen wiper motors, and other small electric motors, as permanent magnets in refrigerator seal gaskets, small motors for cordless appliances, and in automobile applications, etc [1]. One of the most commonly used materials for hard magnets is the M-type strontium hexaferrite (SrM), whose chemical formula is SrFe12O19, having excellent magnetic properties, high chemical stability, and relatively low cost [1].
Several investigations have been carried out to study the influence of various dopants on its physical properties of M-type hexaferrites. In particular, the influence on the structural and magnetic properties of the rare earth as dopant element in SrM has been extensively studied. Buzinaro et al. [2] reported that the strontium hexaferrite doped with samarium (Sm3+) in the Sr2+ site causes the decrease in the saturation magnetization from about 68.8 emu/g at x = 0 to about 60.1 emu/g at x = 0.10 may be related to the changes in the valence state of iron from Fe3+ to Fe2+ in the 12k and 2a sites. Others works also discussed that the partial substitution of Sr2+ cations by rare earth elements has been carried out Pr3+ [3], Nd3+ [3, 4], Eu3+ [5], Gd3+ and Dy3+ [6], and La3+ [5,6,7,8,9,10,11]. According to the presented studies, the replacement of divalent Sr by trivalent rare earth elements in SrM hexaferrites is associated with a valence change of the Fe3+ by Fe2+ ions in the different iron sites. Therefore, the physical properties’ behavior can be influenced. The valence changes in the preferential sites were supported by the results of the Mössbauer experimental technique. However, to our knowledge, only few theoretical studies about M-type strontium hexaferrites have been reported regarding in relation with the cation distribution in the different crystallographic sites of this crystal structure [12,13,14].
Therefore, the aim of this work is to investigate the influence of the partial substitution of the Sr2+ by La3+ ions on the structural and magnetic properties of the Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19 compounds. The framework of the phenomenological model was reported in our previous work [15], which allow the theoretical prediction of the cation substitution distribution in M-type hexaferrites.
2 Experimental Procedure
Polycrystalline samples of Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19 (x = 0.1, 0.2, 0.3, 0.5, and 0.6) were prepared by the conventional solid–state reaction method. Powders of SrCO3, La2O3, and Fe2O3 were mixed at the desired stoichiometric ratios. The mixed powders of each composition were milled for 8 h and calcined in air at 1000 °C for 8 h. The calcined powders were then ground for 4 h and pressed into pellets at 2.6 ton/cm2. The obtained pellets were sintered at 1200 °C in air for 45 min. After that, the pellet samples were again reground and these powders will be hereafter referred to as pre-reacted powders.
The final consolidation of the samples was performed in a SPS 1050 Dr SinterⓇ apparatus manufactured by Sumitomo Coal Mining Co. Ltd., Japan. Pre-reacted powders of Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19 (∼ 3 g) were placed inside a cylindrical graphite die with dimensions of 50 mm (outer diameter) and 20 mm (inner diameter). Two graphite plungers of ∼ 40 mm in height and 20 mm in diameter were placed between the sample, in which the pressure was applied. In order to protect the die and facilitate the sample release after consolidation, graphite foils were inserted between the internal surfaces of the die and between the top and the bottom surfaces of the sample and the graphite plungers. The graphite die was then placed inside the chamber of the SPS apparatus and sintering was conducted under vacuum (from 10 to ∼30 Pa). The consolidation temperature, TD, the holding time, tD, and the heating rate, TD/tr, for all samples were 900 °C, 5 min, and ∼ 175 °C/min, respectively. The temperature was measured during the consolidation process by using a (K-type) thermocouple, which was inserted into a small hole in one side of the graphite die. The maximum uniaxial compacting pressure used to synthesize all samples experiments was 50 MPa reached by a gradual increase during the first 3 min. After the sintering process, each sample was first naturally cool for about 50 min and then removed from the die. The density of all consolidated pellets was determined by using the Archimedes method.
The phase identification of the samples was evaluated from X-ray diffraction patterns obtained in a Bruker-AXS D8 Advance diffractometer. These measurements were performed at room temperature using CuKα radiation in the 3°≤ 2𝜃 ≤ 80° range with a 0.05° (2𝜃) step size, and 5-s counting time.
The magnetization measurements as a function of applied magnetic field M(H) were carried out in a vibrating sample magnetometer (Lakeshore 7404, USA). M(H) data were taken at 300 K and under an applied magnetic field range -14 ≤ H ≤ 14 kOe.
3 Phenomenological Model
The SrM crystal structure has a great number of similar crystallographic sites that can be occupied by the cationic dopants. In this structure, the O2− ions form a hexagonal closed packed lattice and every five oxigen layers, one O2− ion is replaced with Ba2+, Sr2+ or Pb2+ in BaM, SrM or PbM respectively [16]. Fe3+ occupies the interstitial positions of the oxygen lattice [16]. In the unit cell of the SrM, Fe3+ ions occupy three octahedral sites (2a, 4f2, and 12k), one tetrahedral (4f1) site, and one bipyramidal (2b) site [16], according to Wyckoff’s notation. Also, there are six different sites: one polyhedral site for Sr2+ ions (2d) and other five for oxygen ions [17].
The process can be modeled as an interaction process between the cation and the site to be occupied. The model is only valid for low dopant concentrations per unit formula because possible effects due to the distortion of the lattice were not considered [15, 18]. Considering that the doping process obeys statistical laws , the central parameter of the model is the substitution index per site, SI [18]. This parameter can be interpreted as a measure of the site occupation probability of the dopant cation on the i th site and can be defined as [15]:
where i is the i th-site number

In (2a), σj = \(k_{e} m^{\text {hcat}}_{j}\) is the standard deviation, and ke ∼ 0.15 is a value obtained according to Pauling’s rules and the specific experimental conditions [19]. Also, \({\Delta }m_{j} = m^{\text {dop}}_{j}-m^{\text {hcat}}_{j}\) and mj can be any of the following factors (see Table 1):
- 1)
the cationic radius, \(R_{\text {cat}_{\text {i}}}= \alpha _{e_{i}}R_{\text {ion}}\), where \(\alpha _{\text {e}_{\text {i}}}\) is a coefficient that depends on the coordination number at the i th site, nci, and Rion is the ionic radius;
- 2)
the electron’s valence, q;
- 3)
the ionic potential, \(P_{\text {ion}_{\text {i}}}=q/R_{\text {cat}_{\text {i}}}\);
- 4)
the electric charge per site, Qi = q/nci, where nci is the coordination number at the i th site (see Table 1) and
- 5)
the effective atomic number Zeff = (Zcat − q) + 2nci, where Zcat is the atomic number of the cation;
- 6
the electronegativity, χ.
Notice that (2a) quantifies the differences between the dopant cation (dop) and the host cation (hcat) that may be Sr2+ or Fe3+. Additionally, in (2b) and (2c), a binomial statistical distribution function was introduced to consider the relation between the occupation number of the ion in each site, Ni, and the fraction of dopant concentration, ηdop, and the relation between the coordination number of the ion in each site, nci, and the fraction of dopant electronic density, ρdop.
In the same manner as in (1) and (2a–c), it is possible to define a substitution index per site for the host cation (hcat), \(SI^{\text {hcat}}_{i}\). In this case, the “dopant” cation and the host one are the same ion, i.e., Δ mj = 0 [18].

Taking into account all the above considerations makes it possible to define the occupation probability of the dopant and the host cation, respectively, as:

Also, the site occupation number of the dopant cation can be calculated as:
where Q\(^{\text {hcat}}_{i}\) is the occupation number of the host cation, which is defined as:

In all above-mentioned equations, the host cation is referring to Sr2+ or Fe3+ according to the crystallographic site that occupies the dopant cation.
4 Results and Discussion
Let us first inspect the influence of La3+ content on the structural and the phase formation of the M-type strontium ferrite within the framework of the phenomenological model presented above. Figure 1a and b show the occupation probability of La3+ in the Sr and Fe sites, respectively. The obtained results indicate that \(S_{i}^{\text {La}^{3+}}\) is eleven orders of magnitude higher in Sr site than in Fe; therefore, the La3+ should occupy the Sr2+ site in the crystal structure. This is not an unexpected result by considering the similitude between Sr2+ (host cation) and La3+ (dopant cation). However, values of the \(S_{i}^{\text {La}^{3+}}\) in Sr site are also low mostly due to the difference between the valences of the La3+ and Sr2+. Such a difference also affect the charge neutrality of the samples as a whole, triggering the reduction of Fe3+ ions to Fe2+, as reported elsewhere [7, 9]. Under the above result, it is also necessary to investigate the occupation probability of the Fe2+ on both the Fe and the Sr sites.
Figure 2a and b show the occupation probability of the Fe2+ in the Fe3+ and Sr2+ sites, respectively. The results indicate that the order of \(S_{i}^{\text {Fe}^{2+}}\) is ∼ 10− 4 for the iron sites, which is higher than the values of the \(S_{i}^{\text {Fe}^{2+}}\) in the Sr2+ site (see Fig. 2a), even higher than the occupation probability of the La3+ in the Sr site (see Fig. 1a). Also, according to the results showed in Fig. 2b, \(S_{i}^{\text {F}e^{2+}}\) increases with increasing of the La3+ content being more marked in the 12k site, followed by the 4f1 and 4f2 sites. The above findings suggest that the Fe2+ cation occupy the iron sites and not the strontium site inside the crystal structure of the SrM, and that when the SrM is doped with La3+ may occur the valence reduction of Fe3+ by Fe2+ in the 2a and 4f2 iron sites [7, 9].
We have also studied the influence of the La3+ content on the reticular energy, UPOT, of the SrM ferrite. In a previous work, the UPOT was calculated by using the Eqs. (4)–(7) of Ref. [15]. Additionally, our estimations of the reticular energy were conducted by assuming two physical scenarios: (A) considering only the La3+ dopant and (B) taken into account both La3+ and Fe2+ ions. As observed in the Fig. 3, if we consider the case A, then the reticular energy of the compound tends to increase with the La content. However, in the case B, the values of UPOT are lower than in the case B and slightly decreases with increasing the La content. The above result can be related to the difference of the ionic radius of Fe3+ and Fe2+, 0.67Å and 0.80Å, respectively (see Table 1). The Fe2+ ions in the SrM ferrite mostly appears in the form of Fe2O3 [7, 9], and the reticular energy of Fe2O3 is ∼ 1.48× 104 kJmol− 1, a value six time lower than the reticular energy estimated for the pure SrFe12O19 of ∼ 9.02× 104 kJmol− 1.
We want to remark that the reticular energy for the La-doped SrM ferrite is lower considering the reduction of the Fe3+ to Fe2+ ions. The above statement can be verified experimentally by the presence of the Fe2O3 in XRD analysis as reported elsewhere [7, 9, 20, 21]. Also, taken into account the slight decreases of UPOT with increasing the La content (see Fig. 3), the preparation conditions for all samples were the same (see Section 2).
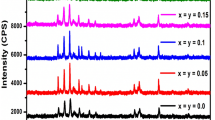
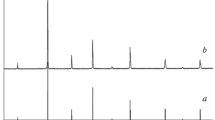
Now, let us check the theoretical findings with the experimental data. Figure 4 shows the X-ray diffraction spectra of Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19 samples with x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6. The obtained results shown that most of the indexed reflections belonging to the pure SrM hexaferrite. However, as La3+ content increases (x ≥ 0.1) some impurities of Fe2O3 and/or Sr2Fe2O5 were detected. The type and the phase content for each value of x are reported in the Table 2. It was found that the amount of Fe2O3 increases from ∼ 7% (x = 0.1) to ∼ 22% (x = 0.6) with increasing the La content. Notice that the presence of this impurity is not an unexpected result as discussed above. In addition, it was not detected La-based impurity or extra phases for any value of x, suggesting the partial substitution of Sr in the crystal structure. The unit-cell parameters were also calculated from X-ray diffraction reflections and by using the Cohen method. The obtained values are listed in the Table 3. In this case, all values of a and c are in line with those reported elsewhere for the same compound [7]. Also, it was not observed appreciable variations of the above mentioned parameters with increasing the La content.
Values of the theoretical density ρmod estimated by using the reticular energy UPOT (see Eq. (8) in Ref. [15]), which in turn is calculated from the cation distribution obtained from the phenomenological model as above mentioned, and those obtained from XRD diffraction patterns, ρXRD, for different La content are listed in the Table 3. It is possible to observe from these values that the changes in the density of the compound do not exceed 2.9%. The experimental volume density of the samples after the SPS consolidation, determined by the Archimedes method was ρexp ∼ 4.75 g/cm3. Thus, the relative density ρexp/ρXRD is ∼ 92 % in all samples. Note that the difference between the experimental density ρexp and the theoretical density ρmod estimated by using the phenomenological model results does not exceeded 5.1%.
On the other hand, the magnetic properties are affected by the presence of the Fe2O3 impurities, as well as by the Fe3+-Fe2+ valence reduction in the iron sites. The room temperature M(H) curves and the magnetic properties of Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19 samples, with (0≤x ≤ 0.6), are shown in Fig. 5. It can be seen that, in general, when the concentration of dopant per unit formula increased, the saturation magnetization and the remanent magnetization decreased from 57.5 emu/g at x = 0 to about 27.1 emu/g at x = 0.6 and from 27.7 emu/g at x = 0 to about 13.4 emu/g at x = 0.6, respectively. According to the results showed in Fig. 5, the lowest values of the magnetic properties (e.g., Ms and Mr) correspond to the samples with x = 0.2 (Ms= 27.5 emu/g and Mr= 10.9 emu/g) and x = 0.6, which may be explained by considering that the amount of Fe2O3 secondary phase was significantly greater in these samples than the rest, as indicated the intensity of peaks on X-ray pattern in Fig. 4.
Also, SrM hexaferrite have a ferrimagnetic structure with the magnetic moments of the Fe3+ cations aligned parallel to each other and to the crystallographic c-axis in 2a, 2b, and 12k, whereas in 4f2 and 4f1 sites, the magnetic moments of the Fe3+ cations point in the opposite direction [7]. Therefore, taking into account the collinear array of the magnetic moments in the unit cell of Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19, the saturation magnetization can be calculated as: \(M_{s}={\sum }_{i=1}^{5}\left ({\mu }^{\text {Fe}^{3+}}Q^{{\text {Fe}^{3+}}}_{i}+{\mu }^{\text {Fe}^{2+}}Q^{\text {Fe}^{2+}}_{i}\right )\).
Due to at T = 0 K the magnetic moment of the Fe3+ (\({\mu }^{\text {Fe}^{3+}}=5.0\mu _{B}\)) and the magnetic moment of the Fe2+ (\({\mu }^{\text {Fe}^{2+}}=4.0\mu _{B}\)), the decreasing of the saturation magnetization may be attributed mainly to the reduction of Fe3+ to Fe2+. As mentioned above (see Fig. 2), such process occurs in 12k spin up sites because the probability of occupation values of the Fe2+ cation in this site is higher than the rest of the iron sites. The theoretical magnetization of saturation calculated from the above-mentioned equation, for x = 0 and x = 0.6, was 20.00 μB and 19.87 μB, respectively. These results indicated that the diminution of the values of the magnetization of saturation do not exceed 0.65%. However, the experimental values of the magnetization of saturation for x = 0 and x = 0.6 were 57.5 emu/g and 27.1 emu/g, respectively, which indicated a diminution of 52.87%, that is much greater than 0.65% obtained from theoretical values above mentioned. It suggests that the decreasing in the magnetization of saturation values was fundamentally result of the presence of the Fe2O3 secondary phase in the samples, although for real samples at room temperature, the assumption of the collinear array of magnetic moments in the unit cell is no longer valid.
On the other hand, values of the coercivity Hc were found to increase from 1 to 3 kOe with the increasing of the La content. A result similar to that was reported elsewhere [6, 7, 10]. Since the coercivity, Hc depends on grain shape, size, distribution, etc [20], the lower value of the coercivity of the sample with x = 0.2 may be related to any substantial change on the microstructure for this sample. Additionally, the coercivity enhancement was reported in other ferrites and related to a microstructure with grains with core/shell morphology [22]. We want to point out that the spark-plasma consolidation promotes this kind of morphology because the process occurs under vacuum [23]. In this case, the core should be comprised of stoichiometric Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19 and the shell could be similar, but oxygen deficiency. However, the existence and a possible relationship between the shell/core morphology and the magnetic properties of these materials must be investigated in details in a future work.
5 Conclusions
High-density samples of Sr1−xLa\(_{\frac {2}{3}x}\)Fe\(_{12}\textit {O}_{19}\thicksim \) 92% were obtained by spark plasma technique. The obtained X-ray diffraction data showed that the presence of the Fe2O3 secondary phase increased with La content of the samples. From the results obtained by using of the phenomenological model, it was found that (i) La3+ ion only occupies the Sr2+ site; (ii) as La content increased, the Fe2+ ions appear as a consequence of the Fe3+ to Fe2+ reduction; and (iii) the Fe2+ ion occupies preferably the 12k and 4f1 iron sites. Such a sequence of processes impacts negatively the magnetic properties of SrM compounds.
References
Pullar, R.C.: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 57, 1191–1334 (2012)
Buzinaro, M.A.P., Ferreira, N.S., Cunha, F., Macêdo, M.A.: Hopkinson effect, structural and magnetic properties of M-type Sm3+-doped SrFe12O19 nanoparticles produced by a proteic solgel process. Ceram. Int. 42, 5865–5872 (2016)
Lechevallier, L., Le Breton, J.M., Morel, A., Tenaud, P.: On the solubility of rare earths in M-type SrFe12O19 hexaferrite compounds. J. Phys.: Condens. Matter. 20, 175203 (2008)
Bhat, B.H., Want, B.: Magnetic behaviour of Neodymium-substituted strontium hexaferrite. Appl. Phys. A 122, 148 (2016)
Berry, F.J., Marco, J.F., Ponton, C.B., Whittle, K.R.: Preparation and characterization of rare earth-doped strontium hexaferrites Sr1−xMxFe12O19 (M=La, Eu). J. Mater. Sci. Lett. 20, 431–434 (2001)
Singh, T., Batra, M.S., Singh, I., Katoch, A.: Composition dependence of the magnetic properties of strontium hexaferrite doped with rare earth ions. J. Phys:. Conf. Series. 534, 012013 (2014)
Liu, X., Zhong, W., Yang, S., Yu, Z., Gu, B., Du, Y.: Influences of La3+ substitution on the structure and magnetic properties of M-type strontium ferrites. J. Magn. Magn. Mater. 238, 207–214 (2002)
Küpferling, M., Grössinger, R., Pieper, M.W., Wiesinger, G., Michor, H.: Structural phase transition and magnetic anisotropy of La-substituted M-type Sr hexaferrite. Phys. Rev. B 73, 144408 (2006)
Seifert, D., Töpfer, J., Langenhorst, F., Le Breton, J.-M., Chiron, H., Lechevallier, L.: Synthesis and magnetic properties of La-substituted M-type Sr hexaferrites. J. Magn. Magn. Mater. 321, 4045–4051 (2009)
Azim, M., Atiq, S., Riaz, S., Naseem, S.: Indexing the structural parameters and investigating the magnetic properties of lanthanum doped strontium hexaferrites. IOP Conf. Series:. Mater. Sci. Eng. 60, 012045 (2014)
Wang, Z., Yang, W., Zhou, Z., Jin, M., Xu, J., Sui, Y.: Preparation and magnetic properties of La-substituted strontium hexaferrite by microwave-assisted Sol-Gel method. J. Supercond. Nov. Magn. 29, 981–984 (2016)
Dixit, V., Kim, S.-G., Park, J., Hong, Y.-K.: Effect of ionic substitutions on the magnetic properties of strontium hexaferrite: A fir st principles study. AIP Adv. 7, 115209 (2017)
Dixit, V., Nandadasa, C.N., Kim, S.-G., Kim, S., Park, J., Hong, Y.-K., Liyanage, L.S.I., Moitra, A.: . J. Appl. Phys. 117, 243904 (2015)
Dixit, V., Nandadasa, C.N., Kim, S.-G., Kim, S., Park, J., Hong, Y.-K.: . J. Appl. Phys. 118, 203908 (2015)
Matilla-Arias, J., Govea-Alcaide, E., Mariño-Castellanos, P., Rosales-Saiz, F.: Phenomenological model for prediction of cation substitution distribution and some physical properties in Mn3+-doped barium hexaferrite. J. Supercond. Nov. Magn. 31(1), 251–256 (2018)
Wohlfarth, E.P.: Handbook of Magnetic Materials, pp 318–323. North-Holland Publishing Company, Netherlands (1982)
Kobayashi, Y., Oda, E., Nishiuchi, T., Nakagawa, T.: Cation distribution analysis of SrLaCo M-type ferrites by neutron diffraction, extended X-ray absorption fine structure and X-ray magnetic circular dichroism. J. Cer. Soc. Jpn. 119, 285–290 (2011)
Mariño-Castellanos, P.A., Moreno, A.C., Orozco, G., Garcia, J.A., Govea-alcaide, E.: Structural and magnetic study of the Ti4+-doped barium hexaferrite ceramic samples: Theoretical and experimental results. Physica. B 406, 3130–3136 (2011)
Chiang, Y.M., Birnie, D.P., Kingery, W.D. (eds.): Physical Ceramics, vol. 522. Wiley, New York (1997)
Bolarín-Miró, A.M., Sánchez-De Jesús, F., Cortés-Escobedo, C.A., Díaz-De la Torre, S., Valenzuela, R.: Synthesis of M-type SrFe12O19 by mechanosynthesis assisted by spark plasma sintering. J. Alloys. Comp. 643, S226–S230 (2015)
Stingaciu, M., Topole, M., McGuiness, P., Christensen, M.: Magnetic properties of ball-milled SrFe12O19 particles consolidated by spark-plasma sintering. Sci. Rep. 5:14112, 1–8 (2015)
Cui, W., Zhang, T., Zhou, X., Wang, Q., Zhao, X., Liu, W., Zhang, Z.: Coercivity enhancement and microstructural optimization in diffusion-processed Ce-Nd-Fe-B-based films. Thin. Solid. Films. 645, 1–4 (2018)
Govea-Alcaide, E., Machado, I.F., Bertolete-Carneiro, M., Muné, P., Jardim, R.F.: . J. Appl. Phys. 112, 113906–13913 (2012)
Funding
The authors received financial support from Brazil’s agencies CNPq (Grant nos. 452657/2015-6 and 303329/2016-5), CAPES/MES (Grant no. 157/2012), and the Petrobras company.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matilla-Arias, J., Govea-Alcaide, E., Mariño-Castellanos, P. et al. Effects of Lanthanum on Structural and Magnetic Properties of Sr1−xLa\(_{\frac {2}{3}x}\)Fe12O19 Compounds: Theoretical and Experimental Results. J Supercond Nov Magn 32, 3671–3678 (2019). https://doi.org/10.1007/s10948-019-5119-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-019-5119-z