Abstract
The current investigation shows the simple and direct sunlight-mediated photocatalytic degradation of methyl orange dye by quasi globular NiFe2O4 nanocrystals synthesized from the high-temperature chemical co-precipitation method. The experiment was carried out under direct sunlight which shows significant degradation results lead to the practical possibility of heterogeneous photocatalysis towards environmental remediation. The as-synthesized quasi globular NiFe2O4 nanocrystals also were characterized by well-known analytical measurements of their structural, morphological, bonding, surface area, band gap and magnetic properties prior to the photocatalytic experiments. The presence of active free radicals formed during the photocatalytic reaction was confirmed from the EPR signals recorded for the solution containing the photocatalyst and dye solution, and accordingly, the photocatalytic degradation mechanism was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heterogeneous photocatalysis has been considered as a promising application towards environmental remediation specifically to dye removal and wastewater treatment [1]. A wide range of investigations has been reported so far in this area using various photocatalysts [2]. However, most studies show the degradation of self-designed photocatalytic reactors [3]. Photocatalytic degradation, an experiment under direct sunlight (a combination of ultraviolet (UV) and visible radiation) with an enhanced efficiency, is currently focused on which can reduce the cost of the equipment, simplify the process and ultimately make a pathway towards bulk-scale reaction. Apart from these perceptions, the choice of the material which can act as an efficient photocatalyst should be given more consideration. In our literature survey [4], we have realized that the magnetically oriented spinel ferrites show excellent catalytic activity towards diversified reactions and extend its applications in numerous fields such as electromagnetic absorbers, sensors, microwave devices, water purification, antibacterial, nanoelectronics, drug delivery and magnetic resonance imaging [5].
The unique structural, electronic, magnetic and optical properties of these materials are the responsible factors of their multiple applications. The magnetic spinel ferrites are generally expressed in the formula AB2O4, where A and B are the representation of divalent and trivalent cations [6]. In our present work, we have opted NiFe2O4, an inverse spinel which has been extensively studied for various applications. For NiFe2O4 in its inverse spinel structure, Fe3+ ions occupy all the tetrahedral sites and half of the octahedral sites, whereas Ni2+ occupies the rest of the octahedral sites of the crystal lattice [7]. NiFe2O4 in nano dimensions exhibit high magnetocrystalline anisotropy and high saturation magnetization with a unique magnetic structure [8]. It possesses a semiconducting band gap of 1.53 eV [9] and so far various methods have been employed to synthesize this material.
The current work utilizes the high-temperature chemical co-precipitation method which is known to be cost-effective, simple handling of reaction, bulk production and high crystalline nature of synthesized nanomaterials [10]. In general, the chemical co-precipitation method was carried out in room temperature, but we believed that the moderately high temperature controlled along with constant stirring has a significant effect on the formation of nanomaterials and hence the reaction temperature was fixed to a moderately high temperature of 100 ∘C in our synthesis. The synthesized sample was evaluated for its photocatalytic activity on methyl orange dye. The methyl orange dye was considered as an important classification of azo-dye-based environmental pollutant [11]. The adverse effects of this dye can be severe threats to the ecosystem, and the elimination of methyl orange dye was carried out successfully by heterogeneous photocatalytic degradation which was reported in several investigations using various catalysts [12]. However, most of the experiments have been carried out under UV or visible light irradiation in a self-designed photocatalytic reactor [13].
Here, in the current investigation, we are reporting the synthesis of NiFe2O4 quasi globular nanocrystals and evaluated its photocatalytic activity on methyl orange dye under direct sunlight. This mode of experiments with significant results will lead to the practical possibility of heterogeneous photocatalysis towards environmental remediation. The as-synthesized quasi globular NiFe2O4 nanocrystals were characterized by the well-known characterization techniques, and the degradation mechanism was also discussed in detail. Also, we promote the utilization of abundantly available solar energy from sunlight, a natural resource for the degradation of dye pollutants in wastewater.
2 Experimental
2.1 Materials
Ferric chloride (FeCl3 ⋅6H2O), nickel chloride (NiCl2 ⋅6H2O) and sodium hydroxide pellets (NaOH) purchased from Merck Chemicals Ltd., India, were employed for the synthesis of quasi globular NiFe2O4 nanocrystals without further purification. Double-distilled water was used throughout the experiments.
2.2 Synthesis of Quasi Globular NiFe2O4 Nanocrystals
A high-temperature chemical co-precipitation method was used in our work for the synthesis of quasi globular NiFe2O4 nanocrystals. Nickel and ferric chloride salt precursor solutions at the ratio of 1:2 were added to an aqueous solution of 0.5 M sodium hydroxide drop by drop at the temperature of 100 ∘C under constant stirring for 1 h. This addition results in the conversion of metal hydroxides from their respective salts and immediately transforms into the ferrite formation. A brown-coloured precipitate was obtained which was filtered and washed repeatedly with distilled water to ensure the removal of water-soluble impurities. Finally, the precipitate was dried at 110 ∘C inside a hot air oven and subjected to calcination in a furnace at 550 ∘C for 4 h.
2.3 Characterization Methods
An X-ray diffractogram of quasi globular NiFe2O4 nanocrystals was recorded using a Rigaku Ultima IV high-resolution X-ray diffractometer with Cu Kα radiation at λ = 0.154 nm. The percentage transmittance of the functional groups present in the sample was measured by using a Perkin Elmer infrared spectrophotometer (model RX l). High-resolution scanning and transmission microscopic analysis (HR-SEM and HR-TEM) with an energy-dispersive X-ray (EDX) spectrum was carried out in a JEOL JSM6360 and JEOL JEM 3010 model electron microscope respectively. Magnetic measurements were carried out at room temperature using the PMC MicroMag3900 model vibrating sample magnetometer (VSM) equipped with a 1-T magnet.
2.4 Photocatalytic Activity Reaction Procedure
Photocatalytic activity of the synthesized quasi globular NiFe2O4 nanocrystals was analysed using methyl orange dye under direct-solar radiation. To 100 mL of methyl orange dye solution, about 50 mg of catalyst was added. Subsequently, the mixture was stirred in the dark for about 30 min to maintain equilibrium between adsorption and desorption. The resulting suspension was irradiated under direct sunlight at the time interval between 10.30 a.m. to 3.30 p.m. by placing on the top roof of our laboratory, Chennai, India (8∘4′− 37∘6′ N latitude) during summer (April–May). The solutions were stirred continuously and open to air environment. The photocatalytic degradation (PCD) efficiency (η) was calculated from the following expression:
where Ci is the initial concentration of methyl orange and Ct is the concentration of methyl orange after t minutes.
3 Results and Discussion
3.1 Structural Information
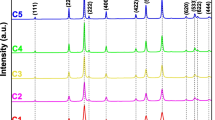
X-ray diffraction patterns were recorded for the synthesized quasi globular NiFe2O4nanocrystals, and well-defined Bragg’s reflections were observed as high-intensity peaks with the absence of any impurity phase. These Bragg’s reflections are shown in Fig. 1, and the lattice constant (ao) was calculated to be 8.27 Å which is in good agreement with the other reported literature [14]. The high-intensity diffraction peaks at 2𝜃 of 30.6, 35.9, 43.7, 57.7 and 63.2 express the high-crystalline nature of quasi globular NiFe2O4 nanocrystals which corresponded to the reflections of (220), (311), (400), (511) and (440) planes respectively. All the peaks are indexed with the standard JCPDS data with card number 74-2081. A force will be generated during the sintering process, which is the cause of the material density. The uniform grain size distribution is mainly due to the driving force of the homogeneous grain. The strong intensities of (220), (440) and (511) planes are more sensitive towards the cations on octahedral and tetrahedral and the oxygen ion parameters. The B sites will be strongly occupied by Ni2+ ions. This shows that the intensities of the planes decline upon the addition of magnesium. The increase in the intensity of the (440) plane is more than that of the (220) plane. The absence of secondary peaks indicates that the as-synthesized quasi globular NiFe2O4 nanocrystals possess a single-phase cubic structure, and in particular, the high-intensity reflection at the (311) plane significantly denoted the single face-centred cubic spinel phase.
The crystallite size was calculated by the Debye–Scherrer formula using the FWHM value of the respective indexed peak.
where λ is the wavelength of the X-ray radiation (λ = 1.5406 Å), β is the full width half maximum of the characteristic peak (in radians), 𝜃 is the Bragg diffraction angle for the hkl plane and D is the grain size (in nanometres). On the basis of the above formula, the average crystallite size (Dave) of the quasi globular NiFe2O4 nanocrystals is calculated to be in the range of 16–20 nm in size.
3.2 Bonding Information
The bonding information for the synthesized NiFe2O4 nanocrystals was analysed through transmittance data obtained from the Fourier transform infrared (FTIR) spectrum in the range of 4000–400 cm− 1 as shown in Fig. 2. It is very clear that high-frequency bands (ν1) lie in the range of 580–620 cm− 1 and 420–435 cm− 1 (ν2) corresponds to the lower-frequency bands. These bands confirm the presence of the ferrite group in all the prepared samples. In addition, a broad signal was observed in the range of 3380–3440 cm− 1 which shows the presence of water symmetric and anti-symmetric stretching. The broadness corresponds to the stretching vibrations of OH groups [17]. The ν1 and ν2 bands show the presence of AB2O4-type ferrites. The ν3 band arises due to the presence of divalent metal ion–oxygen complexes on B sites, and the ν4 band was due to the mass of the divalent tetrahedral cation that appears generally below 400 cm− 1, which was not observed in the present study. In addition, bending vibrations of water molecules appeared around ∼ 1600 cm− 1. The metal–oxygen (Ni–O and Fe–O) intrinsic stretching vibrations in both the octahedral and tetrahedral sites of the crystal lattice are strongly observed in the range 700–400 cm− 1 [15].
3.3 Morphological, Surface Area and Elemental Composition Information
High-resolution scanning electron microscopic (HR-SEM) images of the synthesized NiFe2O4 nanocrystals are shown in Fig. 3a, b, and a compact homogeneous arrangement of quasi globular-shaped nanocrystals is clearly visible in the respective micrographs. The particles have no clear boundaries as they aggregate. It is clear from the images that the grain size and boundaries had a great impact on the magnetic, electrical and dielectric properties of these samples. The grain was found to be 25–45 nm, which was measured using the Image-J software associated with the SEM instrument. The irregular arrangement of crystals with high agglomeration may be due to the choice of the synthesis method, existing defects, annealing temperature and the existence of magnetic interactions between the ions in the crystals [16].
Figure 4 shows the elemental composition and purity of the synthesized NiFe2O4 nanocrystals which confirms both the homogeneity and gradient of the elements Ni, Fe and O present in the sample. The EDX result strongly reveals the single phase formation of the NiFe2O4 structure from the complete utilization of precursors employed in the synthesis with the absence of other impurities in the samples. The result was in good agreement with the XRD results. Also, the relative atomic ratio of metal ions agrees well with the stoichiometric ratio employed in the synthesis. A small peak around 2.1–2.2 keV is due to the presence of gold, which is employed for the processing of samples in SEM analysis for enhanced morphological visibility. Further in-depth morphological analysis of synthesized NiFe2O4 nanocrystals was performed on a high-resolution transmission electron microscope (HR-TEM) as displayed in Fig. 5a, b. The HR-TEM images of NiFe2O4 nanocrystals are self-assembled with high agglomeration, and this could be collectively due to the van der Waals forces and electrostatic interactions between the ions present in the sample [17]. In the current work, a high specific surface area 65.64 m2/g was obtained for the synthesized NiFe2O4 nanocrystals. The pore volume was found to be 3.41 cm3/g. This means that most of the Ni nanoparticles are dispersed on the surface of the ferrite. The corresponding pore size distributions of the nanocatalyst were determined to be 34.74 nm using the Barrett–Joyner–Halenda (BJH) method, indicating that NPs are mesoporous which can be more beneficial for the photocatalytic degradation applications.
3.4 Band Gap Information
To estimate the band gap, the diffuse reflectance spectra (DRS) and photoluminescence spectra of NiFe2O4 nanocrystals are recorded at room temperature as shown in Fig. 6. A graph was plotted between [F(R)hυ]2 versus hυ by following the modified Kubelka–Munk function [18], and the band gap energy for the NiFe2O4nanoparticle is calculated using the formula E = hν = hc/λ. The band gap energy of NiFe2O4 was found to be in the range between 1.68 and 1.70 eV [19, 20].
3.5 Magnetic Information
In order to predict the magnetic behaviour of synthesized NiFe2O4 nanocrystals, the recorded magnetic hysteresis measurements at room temperature are shown in Fig. 7. All the spinel ferrites exhibit hysteresis loops showing the presence of a long-range ferrimagnetic ordering in nickel ferrite nanoparticles. The hysteresis shows that the samples are belonging to a soft ferrite category. The magnetic properties like remanence magnetization (Mr), saturation magnetization (Ms) and coercivity (Hc) were calculated from the hysteresis curves. The saturation magnetization value for bulk nickel ferrite (55 emu/g) is higher than that of the nickel ferrite nanoparticles (38.21 emu/g) [38]. This can be explained based on the spin canting effect and the non-multicollinearity of spin with a magnetic core on the particle surface. The recorded results showed a well-saturated ferromagnetic behaviour with a saturation magnetization (Ms) of 34.91 emu/g and remanent magnetization (Mr) of 0.524 emu/g.
3.6 Photocatalytic Degradation
Photo catalytic activity experiments were performed for the synthesized NiFe2O4 nanocrystals on methyl orange dye under direct-solar radiation. The variation in the absorbance of dye molecules is plotted in regular intervals of time as shown in Fig. 8. We have utilized the direct solar radiation from the sunlight which consists of a mixture of UV and visible light, and hence, no wavelength optimization was required in our experiments. Also, we have directed our experiments in neutral pH medium so that the final medium after the degradation can also be expected to be closer to neutral pH; thereby, the final water after degradation can be further used for other purposes.
A known quantity of NiFe2O4 nanocrystals (200 mg) was fixed as catalyst load, and the concentration of methyl orange dye was taken as 10 mg/L. Our photocatalytic activity experiment results show that there is no significant degradation observed under dark (dye + catalyst only). However, in the presence of NiFe2O4 nanocrystals, the results show a significant degradation ∼ 72.66% of methyl orange dye within 5 h under direct sunlight. This result reveals the high photocatalytic activity of NiFe2O4 nanocrystals for methyl orange dye degradation.
3.7 Mineralization Analysis
The mineralization process during photocatalytic experiments was carried out with chemical oxygen demand (COD) analysis. The estimated COD of the dye solution before the photocatalytic experiments was estimated to be 6012 mg/L, and after the light illumination, it was significantly reduced to 682 mg/L. The decrease in COD values from 6012 to 682 mg/L confirms the mineralization of methyl orange dye with discolouration. In addition, we have observed in our experiments that the blue colour formed initially during the reaction became faint and finally turned colourless after 5 h.
3.8 Methyl Orange Degradation—EPR Approach
The demonstration of the mechanism of photocatalytic degradation was supported by electron paramagnetic resonance (EPR) signals of solution containing synthesized NiFe2O4nanocrystals with the methyl orange dye solution as shown in Fig. 9. In photocatalysis, the electrons are transferred between the reactants and the catalyst; in situ EPR spectroscopy method was used to analyse the electron transfer directly. The value of g ≈ 2.000 is dissymmetrical with g1 = 2.021 (e\(_{\text {CB}}^{\mathrm {-}}\) trapped at O vacancies), g2 = 2.008 (⋅OH) and g3 = 2.001 (\(\cdot \textit {O}_{\mathrm {2}}^{\mathrm {-}})\) and confirms the release of a large number of active free radicals which is required to generate reactive species like super oxide radical anion and hydroxide radicals. As well known, the production of these active species will result in effective degradation and, thus, this material plays a major role in enhancing the degradation by effective release of free radicals upon illumination of light. The steps involved in the mechanism of degradation were summarized in the following (3)–(9) and also given as a pictorial representation in Fig. 10.
Hence, the active free radicals generated from a photocatalytic material have a significant role in degrading the organic pollutants from the wastewater. Similarly, in our results, we have investigated the electron transfer of Fe from the conduction band to the nickel support surface where the electrons are trapped in vacancies in close vicinity of the Ni–Fe2O4 interphase. This in situ EPR spectroscopy is the ideal method for studying the electron-transfer process. The formation of active free radicals was confirmed from the EPR signals during the photocatalytic degradation reaction of methyl orange dye by NiFe2O4 nanocrystals, and hence, this photocatalyst is considered to be a suitable heterogeneous catalyst for the bulk production.
3.9 Estimation of COD
The COD was used to calculate the organic count of the wastewater. The COD of the methyl orange was calculated after and before treatment. The low values in the COD show the mineralization of the dye molecules. The degradation percentage was calculated using the following equation:
4 Conclusion
The NiFe2O4 nanocrystals were synthesized using simple high-temperature chemical co-precipitation method which is evaluated for its photocatalytic activity on the degradation of methyl orange dye under direct sunlight through a simple reaction. Our results show that there is no significant degradation observed under the dark condition (dye + catalyst only). However, in the presence of NiFe2O4 nanocrystals, the results show a significant degradation ∼ 72.66% of methyl orange dye solution within 5 h under direct sunlight. This result reveals the high photocatalytic activity of NiFe2O4 nanocrystals for methyl orange dye degradation. In the mineralization process, the significant decrease in COD values from 6012 to 682 mg/L confirms the mineralization of methyl orange dye with discolouration. The nickel ferrites play a major role in enhancing the degradation by effective release of free radicals upon illumination of light; this was confirmed by the EPR mechanism. Synergy between electron transfer and optical transitions in NiFe2O4 was visualized by the in situ EPR method.
References
Nazir, S., Sami, S., Haider, S., Shahid, M., Sher, M., Warsi, M. F., Nadeem, Q., Khan, M. A.: Structural, spectral, dielectric and photocatalytic studies of Zr-Ni doped MnFe2O4 co-precipitated nanoparticles. Ceram. Int. 42, 13459–13463 (2016)
Dong, P., Hou, G., Xi, X., Shao, R., Dong, F.: WO3-based photocatalysts: morphology control, activity enhancement and multifunctional applications. Environ. Sci.: Nano 4, 539–557 (2017)
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972)
Natarajan, K., Bajaj, H. C., Tayade, R. J.: Direct sunlight driven photocatalytic activity of GeO2/monoclinic-BiVO4 nanoplate composites. Sol. Energ. 148, 87–97 (2017)
Huang, Y., Sudibya, H. G., Chen, P.: Detecting metabolic activities of bacteria using a simple carbon nanotube device for high-throughput screening of anti-bacterial drugs. Biosens. Bioelectron 26, 4257–4261 (2011)
El Moussaoui, H., Mahfoud, T., Habouti, S., El Maalam, K., Ben Ali, M., Hamedoun, M.: Synthesis and magnetic properties of tin spinel ferrites doped manganese. J. Magn. Magn. Mater. 405, 181–186 (2016)
Anandan, S., Selvamani, T., Guru Prasad, G., Asiri, A. M., Wu, J. J.: Magnetic and catalytic properties of inverse spinel CuFe2O4 nanoparticles. J. Magn. Magn. Mater. 432, 437–443 (2017)
Gautam, S., Shandilya, P., Singh, V. P., Raizada, P., Singh, P.: Solar photocatalytic mineralization of antibiotics using magnetically separable NiFe2O4 supported onto graphene sand composite and bentonite. J. Water. Process. Eng. 14, 86–100 (2016)
Lazarova, T., Georgieva, M., Tzankov, D., Voykova, D.: Influence of the type of fuel used for the solution combustion synthesis on the structure, morphology and magnetic properties of nanosized NiFe2O4. J. Alloys Compd. 700, 272–283 (2017)
Peng, Y., Yi, Y., Li, L., Ai, H., Wang, X., Chen, L.: Fe-based soft magnetic composites coated with NiZn ferrite prepared by a co-precipitation method. J. Magn. Magn. Mater. 428, 148–153 (2017)
El Hassani, K., Beakou, B. H., Kalnina, D., Oukani, E., Anouar, A.: Effect of morphological properties of layered double hydroxides on adsorption of azo dye methyl orange: A comparative study. Appl. Clay Sci. 140, 124–131 (2017)
Soltani, T., Lee, B. -K.: Improving heterogeneous photo-Fenton catalytic degradation of toluene under visible light irradiation through Ba-doping in BiFeO3 nanoparticles. J. Mol. Catal. A: Chem. 425, 199–207 (2016)
Peng, Z., Wu, D., Wang, W., Tan, F., Wang, X., Chen, J., Qiao, X.: Effect of metal ion doping on ZnO nanopowders for bacterial inactivation under visible-light irradiation. Powder Technol. 315, 73–80 (2017)
Deraz, N. M., Omar, H.: Abd-Elkader: Preparation and characterization of nanomagnetic Ni-MgFeDeraz, N. M., Omar, H., Abd-Elkader: Preparation and characterization of nanomagnetic Ni-MgFe3O4 system for biological applications. J. Pure. Appl. Microbio. Microbiology 7, 333–339 (2013)
Deraz, N. M., Omar, H.: Abd-Elakader: Preparation and characterization of nano-magnetic Ni0.5Mg0.5Fe2O4 system for biological applications. J. Pure Appl. Microbio. 7, 339 (2013)
Selvam, N. C. S., Kumar, R. T., Kennedy, L. J., Vijaya, J. J.: Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J. Alloys Compd. 509, 9809–9815 (2011)
Maa, K., Zhub, J., Xiea, H., Wanga, H.: Effect of porous microstructure on the elastic modulus of plasma-sprayed thermal barrier coatings: experiment and numerical analysis. Surf. Coat. Technol. 235, 589–595 (2013)
Hays, J., Reddy, K. M., Graces, N. Y., Engelhard, M. H., Shutthanandan, V., Luo, M., Xu, C., Giles, N. C., Wang, C., Thevuthasan, S., Punnoose, A.: Effect of Co doping on the structural, optical and magnetic properties of ZnO nanoparticles. J. Phys. Condens. Matter. 19, 203–206 (2007)
Ren, A. O., Liu, C., Hong, Y., Shi, W., Lin, S., Li, P.: Enhanced visible-light-driven photocatalytic activity for antibiotic degradation using magnetic NiFe2O4/Bi2O3 heterostructures. Chem. Eng. J. 258, 301–308 (2014)
Rahmayeni, Z., Novesar Jamarun, E., Arief, S.: Synthesis of ZnO-NiFe2,O4 magnetic nanocomposites by simple solvothermal method for photocatalytic dye degradation under solar light 32, 1411–1419 (2016). https://doi.org/10.13005/ojc/320315
Acknowledgments
Dr. S. Sendhilnathan gratefully acknowledges the DST (Ref. no. SERC no. 100/IFD/7194/2010-11 dated December 10, 2010) for the financial assistance received through the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirthna, Sendhilnathan, S., Rajan, P.I. et al. Synthesis and Characterization of NiFe2O4 Nanoparticles for the Enhancement of Direct Sunlight Photocatalytic Degradation of Methyl Orange. J Supercond Nov Magn 31, 3315–3322 (2018). https://doi.org/10.1007/s10948-018-4601-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-018-4601-3