Abstract
In this study, we have performed the first-principles investigation of the structural, electronic, magnetic, and optical properties of TbNi5, TbNi3Ru2, and TbNi3Rh2 compounds. The full-potential linearized augmented plane waves with local orbitals method is used in the framework of density functional theory (DFT) employing the generalized gradient approximation (GGA) for the exchange correlation functional as implemented in WIEN2k package. The structural properties are reposed on the evaluation of the equilibrium lattice parameters of these compounds under hexagonal structure such as lattice constants (a and c), bulk modulus (B), and its first pressure derivative \((B^{\prime })\). The spin-polarized electronic structures, including band structure and density of states, are calculated employing the GGA plus band correlated Hubbard parameter (GGA + U) scheme. The results show that density of states and magnetic moment of the pure TbNi5 compound are changed by doping. These changes are observed in the appearance of additional peaks on the spectral density of states (DOS) and in the augmentation of the total magnetic moment of TbNi3 X 2 (X = Ru and Rh) intermetallic compounds. Based on the electronic structure results, the frequency dependents of optical conductivity are estimated in all the spectra and interpreted in the interband optical absorption part.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The RNi5 intermetallic compound family (R is the rare-earth element) has a vast magneto-crystalline anisotropy, where it is caused by the crystal field interaction [1, 2], and large electronic structures; it crystallizes under the hexagonal system of the CaCu5-type structure, which is described by the P6/m m m space group. Actually, various electronic and experimental works have been extensively investigated on RNi5 compounds and their substitutional derivatives, due to the existence of large-spectrum unusual physical properties and also due to the prospects of their exceptional applications in hydrogen storage technology and as adiabatic nuclear cooling agents [3,4,5,6], such as absorption capacity of hydrogen, observed in LaNi5 compound with the formation of a hydride LaNi5 H 6,7 [7], the ERNi5 compound which present a meta-magnetic from its ferromagnetic stable state. In some RNi5 compounds, the magneto-caloric character is referred [8]. Through the litterature, we can mention the availability of the meta-magnetic even in paramagnetic PRNi5 and ferromagnetic ERNi5 compounds [9,10,11,12]. The magnetic properties of the RNi5 compounds are obtained by the summarizing of three kinds of interactions: anisotropic interaction, spin-orbital interaction, and exchange interaction between the ion and the crystal field. The indirect exchange between 4f electrons in the conduction band gives the magnetic aspect within RNi5 systematic compounds. The ferromagnetic temperature ordering T C is estimated in the vicinity around 25 K [13].
The RNi5 compounds have also been excessively studied with the use of experimental techniques like the measurements of the magnetization and the susceptibility [14, 15], heat capacity [16], X-ray magnetic circular dichroism [17], μ SR spectroscopy [18], NMR spin echo [19], and elastic and inelastic neutron scattering [20,21,22]. The substitution of nickel atoms with other p − or d − metals exhibits substantial changes in most physical properties of RNi5intermetallic compounds. For example, non-monotonic concentration dependences of magnetic [23, 24], electronic [24, 25], and crystalline [23, 26] are observed in the TbNi 5−x Al x system. Many works have been referred in the TbNi5 group doping by the replacing of Ni with a nonmagnetic element such as Al, Ga, and Si [27,28,29]. In the other part, the substitution of nickel by a magnetic element (Fe or Co atoms) gives modifications in increasing Curie temperature (T C ≈ 280 K for TbNi 4Fe and T C ≈ 60 K for TbNi 4Co) [30]. Important changes on the electronic structure and optical conductivity due to the influence of nonmagnetic Cu and Al have been deeply studied and referred availably in the literature [31, 32].
In this work, we have investigated the study of structural, electronic, magnetic, and optical properties of the pure TbNi5 and their TbNi3Ru2 and TbNi3Rh2 derivative intermetallic compounds; the goal of this research is to show the role and the effect of the substitute transition metal (TM) on the electronic, magnetic, and optoelectronic properties of TbNi5 compound.
The rest of the paper is ordered as follows: Section 2 exhibits brief descriptions of the calculation method. Section 3 gathers the obtained results and discussion of the structural, electronic, magnetic, and optical properties. In the end, the conclusions taken during this approach are summarized in Section 4.
2 Methodology
In this present approach, the projector full-potential augmented pane wave plus local orbitals (FP-L/APW + lo) [33, 34] has been performed within the first-principles calculations of the framework of density functional theory [35] and implemented in WIEN2k package [36] to give estimated results of structural, electronic, magnetic, and optical properties of pure TbNi5 compound and their novel TbNi3Ru2- and TbNi3Rh2-derived alloys. In this work, we have used the optimized parameters as the muffin-tin sphere radii R MT are equal to 2.50, 2.24, 2.24, and 2.24 a.u. for Tb, Ni, Ru, and Rh, respectively. The plane wave–cutoff R MT × K MAX is chosen as 8, where R MT is the smallest muffin-tin (MT) sphere radius and K MAX is the greatest (maximum) modulus of vectors in the reciprocal lattice \(\vec {K}=\thinspace \vec {k}+\thinspace \vec {G}\) of the first Brillouin zone (BZ). The mesh of 12 × 12 × 12 Monkhorst-Pack scheme is generated by 135 special k −points which serve the integration in the first Brillouin zone. The energy convergence criterion for self-consistency is less than 10 −4 eV. In fact, the valence electron configurations are considered as (6s 2 4f 9) for Tb, (4s 2 3d 8) for Ni, (5s 1 4d 7) for Ru, and (5s 1 4d 8) for Rh. The exchange-correlation potential is defined under the framework of generated gradient approximation of Perdew-Burke-Ernzerhof (GGA-PBE) [37]. The GGA approximation is applied to treat the structural properties whereas the electronic, magnetic, and optical properties of the herein compounds are examined by employing the GGA-PBE with on-site Hubbard term of Coulomb repulsion (PBE-GGA + U) [38,39,40]. The Hubbard correlation of direct and exchange (indirect) terms in the 4f- shell of Tb was previously chosen as U = 3.5 eV and J = 0.7 eV [32]. The substitution of the nickel (Ni) by ruthenium (Ru) and rhodium (Rh), transition elements in TbNi5 compound make the transition element at the crystallographic nonequivalent position of 2c.
3 Results and Discussion
3.1 Structural Properties
The TbNi5 compound is structured according to the hexagonal system of CaCu 5-type with P6/mmm symmetry space group (No. 191); the experimental lattice constants of this compound are found equal to a = 4.892 Å, and c = 3.964 Å [41].
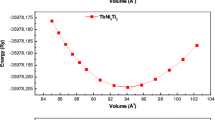
According to the linear Birch-Murnaghan’s equation of state (EOS) [42, 43], enunciated as the following expression, the total energy versus cell volume (E − V ) curve was optimized in the goal to determine the equilibrium structural parameters in the stable state, such as lattice constants (a 0 and c 0), bulk modulus (B), its first pressure derivative (\(B^{\prime })\), and the minimum total energy (E 0).
where V is the primitive cell volume and a, b, c, and d are the optimized parameters.
The obtained results of the fitting equilibrium structural parameters of TbNi 3 X 2 (X = Ni, Ru, and Rh) compounds are regrouped in Table 1, with other available experimental data and previous theoretical values inspired from the literature. The (E − V ) curves for the three-alloys of TbNi 3 X 2 are plotted and shown in Fig. 1, in which their graphs brand clearly the consistent minimum total energy of the system at the equilibrium for each compound. The comparison between our obtained structural results and experimental ones indicates a slight difference in the lattice constants observed for the case of TbNi5 intermetallic compound, with an estimated rate of −0.14% for a 0, and of −0.47% for c 0, compared with measurement data. Furthermore, it is noticeable for the cases of other derivative compounds of TbNi5 (TbNi3Ru2 and TbNi3Rh2) that the absence of the structural parameter data is an asset (advantage) to considering our results as a necessary reference to supplying further projects.
3.2 Electronic Structure
3.2.1 Band Structure
The investigation on the electronic properties is useful to describe the electronic behavior of each material and classify the compounds in their true electronic category; the obtained results through the predicted or experimental methods will serve to give the appropriate potential use of the materials in technological devices. The calculated spin-polarized (spin-up, and spin-down) electronic band structures of hexagonal TbNi5, TbNi3Ru2, and TbNi3Rh2 alloys are predicted under the framework of the PBE-GGA + U parameterization scheme, within their equilibrium lattice parameters presented in Table 1. Figures 2, 3, and 4 depict the band structures of TbNi5, TbNi3Ru2, and TbNi3Rh2 compounds along the high-symmetry points in the first Brillouin zone (BZ) of the hexagonal system. It is obvious from these figures of all TbNi 3 X 2 (X = Ni, Ru, and Rh) compounds that some energy bands go across the Fermi level (E F) in both spin-up and spin-down directions, confirming the metallic nature of these alloys. Therefore, the remarkable effect of the transition element (TM = Ru, and Rh) substitutions is seen which modify the electronic structure of TbNi5 compound by producing residual energy bands. The total densities of states (TDOS) curves are in perfect projection on their corresponding band structures which confirm the metallic behavior founding previously. Based on the TDOS plots of TbNi5, TbNi3Ru2, and TbNi3Rh2 intermetallic compounds, the important N (E) intensities are situated in the range of the valence band below E F (from −3.39 to 0 eV for TbNi5, from −3.75 to 0 eV for TbNi3Ru2, and from −3.95 to 0 eV for TbNi3Rh2), where the specific part of TbNi5 is principally filled by 3d- Ni electrons of (2c) and (3g) sites, whereas the rest of the other two energetic condensed parts of TbNi3 X 2 compounds are contributed by both 3d- Ni electrons of the (3g) site and 3d- TM electrons of the (2c) site.
3.2.2 Density of States
To study the electronic structure of solids in detail, we must take our considerations to investigate on the electron density of states (DOS). The total and partial densities of states (TDOS and PDOS) projectors of the equilibrium TbNi 3 X 2 (X = Ni, Ru, and Rh) alloys are calculated through the spin PBE-GGA + U approximation. The TDOS curves are presented in Figs. 2, 3, and 4, whereas the PDOS curves of TbNi5, TbNi3Ru2, and TbNi3Rh2 are illustrated on Figs. 5, 6 and 7, respectively. The spin-dependent PDOS calculations of TbNi5 show that the states of 3d- Ni identified by the multi-peak structure are mainly located in the region between −4 eV and E F, while the peaks around (−6.5 eV for spin- up projection and + 1.1 eV for spin-down projection) belong to 4f- Tb electrons. Moreover, intensive peaks of 4f- Tb states are spotted at −5.92 and + 0.95 eV for spin-up and spin-down cases, respectively, where this obtained result is agreeing in good matching with the other calculations [24, 25, 32]. According to Figs. 5 and 6 that show the PDOS curves of TbNi3Ru2 and TbNi3Rh2 compounds, the 3d- TM (TM = Ru, and Rh) and 3d- Ni contributions in both of their spin directions dominate the range from −5 to −1 eV for TbNi3Ru2 alloy and in the range from −5 to −2 eV for TbNi3Rh2 alloy, where a strong hybridization appears between these 3d- TM and 3d- Ni states along this filled part. The large exchange splitting between spin-up and spin-down electrons is referred to 4f- Tb states, which gives an increase in the quantity of the magnetic moment, whereas spin exchange splitting corresponding to 3d- Ni and –TM states are in weak evaluation. In both dependence PDOSs of TbNi3Ru2 and TbNi3Rh2, intense narrow peaks formed by 4f- Tb states are situated in the broad energy region from −7 to −5 eV for the spin-up direction and from −2.5 to + 2.15 eV for the spin-down direction. Furthermore, the impact of Ru and Rh substitute impurities for Ni in 2c positions is focused on some deformations in the broad energy part between −5 eV and E F, where their Ru and Rh bands are distributed in different manners comparing to the pure TbNi5. It is noticeable that some summit peaks of 4f- Tb states are reduced in the case of TbNi3Rh2, while some peaks of 4f- Tb states disappear in the case of TbNi3Ru2. Also, it is lucidly observed that the TDOS intensities diminish when the Ru and Rh atoms replace the Ni atom in 2c positions.
3.3 Magnetic Properties
3.3.1 Magnetic Moment
Systematic predictions of the magnetic properties of TbNi 3 X 2 (X = Ni, Ru, and Rh) alloys within the GGA + U parameterization approximation were displayed in their sizes of total magnetic moments (M Tot), local magnetic moments of Tb, Ni (2c), Ru (2c), Rh (2c), and Ni (3g) sites, and interstitial magnetic moments. The obtained values are listed in Table 2, wherein it is evident that the total magnetic moment for each compound mainly comprises the Tb contribution with weak magnetic moment quantities coming from other Ni, Ru, and Rh elements; this phenomenon is due to the wide splitting of 4f- Tb states between both spin-up and spin-down directions. The local magnetic moments of each site have the same sign in parallel ordering, confirming the intrinsic ferromagnetic property of these compounds. Therefore, the hybridization between 3d- Ni (3g) and 3d- TM (2c) states is principally responsible for reducing the atomic magnetic moment of Ni (3g) and TM (2c) atoms from its free space charge value and also producing feeble local magnetic moments on the interstitial zone. The obtained magnetic moment results corresponding to the pure TbNi5 intermetallic compound are in well concordance with the experimental data [45] available in the literature. Furthermore, the effects of the Ru and Rh atom substitutions for the Ni atom at 2c positions within the pure TbNi5 compound are collected to decrease the total magnetic moment of the system.
3.4 Optical Properties
3.4.1 Dielectric Function
The frequency-dependent complex dielectric function ε (ω) is a response of the substance which is useful for the optical properties of each system.
where the real and the imaginary parts are dependent on each other by the Kramers-Kronig (KK) relation [48, 49]. The computed frequency dependent on the real (ε 1) and imaginary parts (ε 2) of TbNi 3 X 2 (X = Ni, Ru, and Rh) in parallel (E x x ) and perpendicular (E z z ) directions of polarization, along the wavelength limit, is illustrated in Figs. 8 and 9, respectively. According to Fig. 2, the static dielectric constant ε 1 (0)values are about 1.00, 1.78, and 1.76 for TbNi5, TbNi3Ru2, and TbNi3Rh2 alloys, respectively. The existence of multi-peaks situated in the energy region between 15 and 35 eV and around 10 eV in the case of the pure TbNi5 compound also can be seen. Due to the impact of Ru and Rh transition metal substitutes, the intensities of these peaks are progressed in their energy ranges. Moreover, other peaks are located at 41 eV for TbNi3Ru2 and for TbNi3Rh2. The increasing of the peak summit is observed in TM-doped TbNi5 alloys, explaining the strong energy absorption character. Additionally, it is evident that the highest peak is referred in ε 1(z z) around 21 eV for the case of TbNi5 compound, whereas the spotting of both ε 1(x x) and ε 1(z z) highest peaks are at 41 eV for TbNi3Ru2 compound and at 46 eV for TbNi3Rh2 compound.
Imaginary parts of the dielectric function of TbNi5, TbNi3Ru2, and TbNi3Rh2 compounds are shown in Fig. 9; in fact, we remark that their curves decrease with the change of the photon energy from lower to higher, where this shift showed pronounced peaks in the cases of TbNi3Ru2 and TbNi3Rh2 compounds comparing to the pure TbNi5 compound, demonstrating the increasing of the absorption ranges of these TM-doped TbNi5 alloys. Moreover, these imaginary part of the curves decreases drastically from the highest values which are situated in the infrared region. Furthermore, the effect of transition metal (TM) doping in 2c positions creates a small peak with low intensity in both parallel (E x x ) and perpendicular (E z z ) polarizations around 43 and 46 eV for TbNi3Ru2 and TbNi3Rh2 compounds, respectively.
3.4.2 Reflectivity
The complex refractive index is defined as N (ω) = n (ω) + i k (ω), where n (ω) and k (ω) are the real and imaginary parts of the complex refractive index, which are related to ε (ω) by the following relations:
With the knowledge of n (ω) and k (ω), it is obvious to determine the percentage of reflectivity of solids at normal incident of electromagnetic wave, which is estimated by the ration of the refracted on the incident energies of photon. It is given as follows:
We have computed the estimated reflectivity R (ω) through the availability of frequency-dependent real and imaginary parts of the dielectric function for both E x x and E z z polarizations. Figure 10 depicts the evolution of R (ω) versus photon energy; we can see that their graphs decrease from the lower (infrared region) to the higher energy regions, where the highest reflectivity values of all TbNi5, TbNi3Ru2, and TbNi3Rh2 compounds are located in the infrared region (E ˜0 eV) about 88, 86, and 89%, respectively; this decrease of R (ω) is explained by the enhancement of the absorption part in the dielectric function. It is noticeable that the reflectivity R (ω) is strongly related to the imaginary part of the dielectric function, because their curves are rigorously similar. In the region from 5 to 35 eV, the reflectivity peaks of TM-doped TbNi5 are more pronounced than those of the pure TbNi5, which are original from the inter-band interactions, where the reflectivity energy intervals of all the three compounds are the same; their highest intensities are focused in the infrared part and in the first fragment of the visible part of the spectrum. Moreover, the TM (Ru and Rh) substitutions on the TbNi5 system present a slight change in the height of peaks, comparing to the pure TbNi5.
3.4.3 Absorption Coefficient
The absorption coefficient is a quantity that translates the response of the system under the effect of excitation. It is given in dependence with the dielectric function according to the following formula:
where it is fully linked to the imaginary part of the dielectric function.
Here, c is the light speed in vacuum and ω is the energy unit.
The absorption coefficient of TbNi 3 X 2 (X = Ni, Ru, and Rh) is reported in Fig. 11 showing the behavior of the both parallel (E x x ) and perpendicular (E z z ) polarization cases. Therefore, it is observed for all the three TbNi5, TbNi3Ru2, and TbNi3Rh2 alloys that the absorption coefficient \(\alpha \thinspace \left (\omega \right )\) of E x x and E z z increases from a less value in the energy part from 0 to 20 eV; after this region, due to the interband interactions, many multi-peaks have been formed in the energy range between 20 and 35 eV; finally, the curves decrease to attain zero intensity in the proximity of 40 eV. The sharp peak is observed for E z z around 27.5 eV for the pure TbNi5, interpreting the top absorption of the light in this frequency point. The highest value of the absorption coefficient of TM-doped TbNi5 is localized at 42.5 eV for E x x of TbNi3Ru2 and at 27 eV for E z z of TbNi3Rh2.
It is noticeable that the energy ranges of absorption of both TM-doped TbNi5 alloys are widely expanded on the full energy spectrum with a width upper than of the pure TbNi5 compound. The major impact of the impurity TM within TbNi5 system is reported in the apparition of isolated sharp peaks in the ultraviolet spectrum around 42.5 and 47.5 eV for TbNi3Ru2 and TbNi3Rh2 compounds, respectively.
3.4.4 Optical Conductivity
The optical conductivity is defined as a factor which describes the nature of the frequency dependence and the intensity of the reflecting medium as optical response. It has been given under the following formula:
where n (ω) and α (ω) are refractive index and absorption coefficient, respectively.
The optical conductivity spectra σ (ω) of the pure TbNi5 compound and their TbNi3Ru2 and TbNi3Rh2 alloys are shown in Fig. 12 along the photon energy. A violent enhancement is observed for the three compounds at the lower energy range of the infrared spectrum, which forms the sharpest peak, explained by the Drude rise (mechanism of interaction between electromagnetic waves and conduction electrons). Above \(\hbar \omega \) ˜0.5 eV, the σ (ω) behavior of all the compounds decreases abruptly versus the increasing of the optical frequency σ (ω); this increasing is accompanied by the creation of multi-peaks which are due to the interband interactions. Then, the σ (ω) curve reaches zero intensity at 40 eV for the TbNi5 compound, while some intense peaks appear in the energy range close to 42.5 eV for TbNi3Ru2 alloy and 46 eV for TbNi3Rh2 alloy, showing the effect of the inter-band absorption of light in these photon frequencies. It is obvious to mention that our obtained σ (ω) results of TbNi5 case agree in well matching with the previous theoretical data [50]. Moreover, the introducing by substitution of the TM (Ru and Rh) element makes shifts in the optical conductivity dispersion σ (ω); in fact, multi-peaks are more pronounced in the range from 25 to 30 eV; other peaks become intensively reduced in the ultraviolet spectrum around 65 eV, comparing to the pure TbNi5 intermetallic compound.
4 Conclusions
To sum up, in this study, we have performed the GGA and GGA + U calculations of the structural, electronic, magnetic, and optoelectronic properties of TM-doped TbNi5 (TM = Ru and Rh) compounds, by using the first-principles FP-L/APW + lo method within the framework of the DFT theory. Therefore, in this approach, we have shown the role of the transition metal TM substitutions for nickel in 2c positions proclaims changes on the electronic, magnetic, and optoelectronic properties of the pure TbNi5 compound. Our obtained results of equilibrium parameters of TbNi5system are consistent with the available experimental data and theoretical values. The effect of doping Ru and Rh elements on the system (TbNi5) depicts more modifications on the electronic structure, which are specified by production of novel reported peaks at the level of density of states (DOS) spectrum, confirming the metallic behavior of the three intermetallic compounds. The total and atomic magnetic moments are also evaluated; their results present fewer differences with those of the TbNi5 parent, where the total magnetic moment is principally contributed by Tb atom. In the optoelectronic properties, the impact of the TM doped TbNi5 system is characterized by great energy of absorption, apparition of isolated sharp peaks of absorption coefficient in the ultraviolet spectra, and pronounced peaks spotted in both reflectivity and absorption spectra, where these rises are significantly explained by the interband interactions. Moreover, the absorption bandwidths of TbNi3Ru2 and TbNi3Rh2 are more broadened on the light spectrum. In the end, the optical conductivity dispersion was predicted in reasons of comparing and interpreting the experimental results of the pure TbNi5 system, demonstrating that the σ (ω) allure of the three alloys is lucidly interpreted by their corresponding calculations of the density of states.
References
Romaka, V.V., Marciniak, B., Romaka, L., Gorelenko, Y., Pavlyuk, V.: J. Alloy. Comp. 493, L12–L14 (2010)
Kuchin, A.G., Ermolenko, A.S., Khrabrov, V.I., Kourov, N.I., Makarova, G.M., Belozerov, Y.V., Lapina, T.P., Kulikov, Y.A.: J. Magn. Magn. Mater. 29, 238 (2002)
Trémolet de Lacheisserie, E., Gignoux, D., Schlenker, M.: Magnetism: Materials and Applications. Springer, Berlin (2005)
Gschneidner, K.A., Pecharsky, V.K., Tsokol, A.O.: Rep. Progr. Phys. 68, 1479 (2005)
Mushnikov, N.V.: Phys. Uspekhi 55, 421 (2012)
Coey, J.M.D.: Magn. IEEE Trans. 47, 4671 (2011)
Senoh, H., Takeichi, N., Takeshita, H.T., Tanaka, H., Kiyobayashi, T., Kuriyama, N.: Hydrogenation properties of RNi5 (R: rare-earth) intermetallic compounds with multi pressure plateaux. Mater. Trans. 44, 1663–1666 (2003)
Ranke, P.J., Mota, M.A., Grangeia, D.F., Magnus, A., Carvalho, G., Gandra, F.C.G., Coelho, A.A., Caldas, A., Oliveira, N.A., Gama, S.: Magnetocaloric effect in the R Ni5 (R = Pr, Nd,Gd, Tb, Dy, Ho, Er) series. Phys. Rev. B: Condens. Matter Mater. Phys. 70, 134428 (2004)
Radwański, R.J., Kim-Ngan, N.H., Kayzel, F.E., Franse, J.J.M., Gignoux, D., Schmitt, D., Zhang, F.Y.: The specific heat of ERNi5 and LaNi5. J. Phys.: Condens. Matter 4, 8853–8862 (1992)
Kayzel, F.E.M., Franse, J.J., Radwański, R.J.: High field magnetization and specific heat of ERNi5. IEEE Trans. Magn. 30, 890–892 (1994)
Gignoux, D., Schmitt, D.: Commensurability versus incommensurability in rare earth intermetallic compounds. J. Magn. Magn. Mater. 129, 53–58 (1994)
Gignoux, D., Schmitt, D.: Metamagnetism and complex magnetic phase diagrams of rare earth intermetallics. J. Alloys Compd. 225, 423–431 (1995)
Kuchin, A.G., Ermolenko, A.S., Khrabrov, V.I., Kourov, N.I., Makarova, G.M., Belozerov, Y.V., Lapina, T.P., Kulikov, Y.A.: J. Magn. Magn. Mater. 238, 29 (2002)
Gignoux, D., NaitSaada, A., de la Bathie, R.P.: Magnetic properties of TbNi and HoNi single crystals. J. Phys. Colloq. 40(C5), 188–190 (1979)
Grechnev, G.E., Desnenko, V.A., Panfilov, A.S., Svechkarev, I.V., Brommer, P.E., Franse, J.J.M., Kayzel, F.E.: Pressure effect on electronic structure and magnetic properties of RNi5. Phys. B 237–238, 532–533 (1997)
Svoboda, P., Vejpravova, J., KimNgan, N.T.H., Kaysel, F.J.: Specific heat study of selected RNi5. J. Magn. Magn. Mater. 272–276, 595–596 (2004)
Galera, R.M., Rogalev, A.: Hard X-ray magnetic circular dichroism in GdNi5 and TbNi5 single crystals. J. Appl. Phys. 85, 4889–4891 (1999)
de Reotier Dalmas, P., Yaouanc, A., Gubbens, P.C.M., Gignoux, D., Gorges, B., Schmitt, D., Hartmann, O., W⋅⋅appingm, R., Weidinger, A.: Effect of Tb3+ crystal field on the positive muon precession frequency in TbNi5. J. Magn. Magn. Mater. 104–107, 1267–1268 (1992)
Carboni, C., Gignoux, D., Li, Y., Ross, J.W., Tary, A.: The field dependence of the hyperfine splitting of terbium in TbNi5. J. Phys.: Condens. Matter 8, 1763–1774 (1996)
Goremychkin, E.A., Muhle, E., Ivanitski, P.G., Krotenko, V.T., Pasechkin, M.V., Slisenko, V.V., Vasilkevich, A.A., Lippold, B., Chistyakov, O.D., Savitski, E.M.: Crystal electric field splitting in TbNi5 and ERNi5 studied by inelastic neutron scattering. Phys. Status Solidi B 121, 623–631 (1984)
Gignoux, D., Rhyne, J.J.: Spin excitations in TbNi5 by inelastic neutron scattering. J. Magn. Magn. Mater. 54–57, 1179–1180 (1986)
Lemaire, R., Paccard, D.: Structure magnétique du composé intermétallique TbNi5. C. R. Acad. Sci. B (Paris) 270, 1131–1133 (1970)
Kuchin, A.G., Ermolenko, A.S., Khrabrov, V.I., Kourov, N.I., Makarova, G.M., Belozerov, Y.V., Lapina, T.P., Kulikov, Y.A.: J. Magn. Magn. Mater. 238, 29 (2002)
Burzo, E.: Rom. Rep. Phys. 59, 337 (2007)
Burzo, E., Takacs, A., Neumann, M., Chioncel, L.: Phys. Status Solidi C 1, 3343 (2004)
Blazina, Z., Sorgi, B., Drǎsner, A.: J. Phys.: Condens. Matter 9, 3099 (1997)
Burzo, E., Takàcs, A., Neumann, M., Chioncel, L.: Phys. Status Solidi (c) 1, 3343 (2004)
Lizárraga, R., Bergman, A., Björkman, T., Liu, H.P., Andersson, Y., Gustafsson, T., Kuchin, A.G., Er_molenko, A.S., Nordström, L., Eriksson, O.: Phys. Rev. B74, 094419 (2006)
Falkowski, M., Andrzejewski, B., Kowalczyk, A.: J. Alloys Compd. 442, 155 (2007)
Haldar, A., Dhiman, I., Das, A., Suresh, K.G., Nigam, A.K.: J. Alloys Compd. 509, 3760 (2011)
Nekrasov, I.A., Kokorina, E.E., Galkin, V.A., Kuzmin, Y.u.I., Knyazev, Y.u.V., Kuchin, A.G.: Phys. B 407, 3600 (2012)
Knyazev, Y.u.V., Lukoyanov, A.V., Kuz′min, Y.u.I., Kuchin, A.G.: Phys. Solid State 55, 385 (2013)
Wong, K.M., Alay-e-Abbas, S.M., Shaukat, A., Fang, Y., Lei, Y.: J. Appl. Phys. 113, 014304 (2013)
Wong, K.M., Alay-e-Abbas, S.M., Fang, Y., Shaukat, A., Lei, Y.: J. Appl. Phys. 114, 034901 (2013)
Hohenberg, P., Kohn, W.: Phys. Rev. 136, B864 (1964)
Blaha, P., Schwarz, K., Sorantin, P., Trickey, S.K.: Comput. Phys. Commun. 59, 339 (1990)
Perdew, J.P., Burke, S., Ernzerhof, M.: Phys. Rev. Lett. 77, 3865 (1996)
Novak, P., Kunes, J., Chaput, L., Pickett, W.E.: Phys. Status Solidi B 243, 563 (2006)
Anisimov, V.I., Solovyev, I.V., Korotin, M.A., Czyzyk, M.T., Sawatzky, G.A.: Phys. Rev. B 48, 16929 (1993)
Petukhov, A.G.: Phys. Rev. B 67, 153106 (2003)
Buschow, K.H.J.: Rep. Prog. Phys. 40, 1179 (1977)
Murnaghan, F.D.: Proc. Natl. Acad. Sci. USA 30, 5390 (1944)
Shang, S.L., Wang, Y., Kim, D., Liu, Z.-K.: Mater. Comput. Sci. 47, 1040 (2010)
Goraus, J., Malankiewicz, P.: Acta Phys. Polon. 121, 1077 (2012)
Haldara, A., Dhimanb, I., Dasb, A., Suresha, K.G., Nigamc, A.K.: J. Alloys Compd. 509, 3760–3765 (2011)
Knyazev, Y.V., Kuz’min, Y.I., Kuchin, A.G., Lukoyanov, A.V., Nekrasov, I.A.: Opt. Spectrosc. 104, 3 (2008)
Knyazev, Y.V., Lukoyanov, A.V., Kuz’min, Y.I., Haldar, A., Suresh, K.G.: Opt. Spectrosc. 117, 3 (2014)
Mahan, G.D.: Many Particle Physics. Plenum Press, New York (1990)
Dressel, M., Gruner, G.: Electrodynamics of Solids. Cambridge University Press, Cambridge (2002)
Knyazev, Y.V., Lukoyanov, A.V., Kuz’min, Y.I., Kuchin, A.G.: Phys. Solid State 55, 2 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amine Monir, M.E., Baltach, H., Mouchaal, Y. et al. The Effects of Ru and Rh Substitutions on the Magneto-electronic and Optical Properties of the TbNi5 Intermetallic Compound: An Ab Initio Investigation. J Supercond Nov Magn 31, 547–559 (2018). https://doi.org/10.1007/s10948-017-4211-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-017-4211-5